Abstract
Background
Primary pancreatic leiomyosarcoma (PLMS) is rare. The clinical characteristics and prognosis is still not completely understood. The aim of the present study is to identify the clinical characteristics and long-term outcomes of PLMS from the existing reported cases in different scientific literature.
Methods
PLMS cases reported in Chinese and English journals were collected and reviewed. Clinical features and long-term outcomes of these cases were summarized and analyzed statistically.
Results
A total of 69 cases reported from both Chinese and English journals were included in the present study. An equal incidence in gender was observed. The mean age was 53.9 ± 14.7 years. The most common symptoms were abdominal mass, abdominal pain, and weight loss. The mean size of the tumor was 11.4 ± 7.1 cm. The incidence of PLMS between the head and body-tail of the pancreas had a similar pattern. Twenty-five percent of patients had distant metastasis and 19% of patients had adjacent organs/vessels invasion at the time of diagnosis. But lymph node metastasis was documented in only one (1.5%) patient. The median survival time was 48 months. The overall 1-, 3-, 5-, and 10-year survival rates were 66.6%, 51.2%, 43.9%, and 29.3%, respectively. Results from the multivariate analysis showed that non-radical resection (P = 0.000; hazard ratio (HR) 5.128; 95% confidence interval (CI) 2.041-12.987) was the independent adverse prognostic factor. Adjacent organs/vessels invasion (yes) may be considered as an another potential independent adverse prognostic factor (P = 0.071; HR 2.708; 95% CI 0.981-7.474).
Conclusions
PLMS is rare without specific clinical features. PLMS is an aggressive tumor and has a poor prognosis. Radical resection can prolong survival time of the patients.
Similar content being viewed by others
Background
Primary leiomyosarcoma is an extremely rare mesenchymal tumor of the pancreas, which accounts for 0.1% of malignant pancreatic tumors [1]. Primary pancreatic leiomyosarcoma (PLMS) was first described by Ross in 1951 [2], and to date 64 cases have been reported [3]. However, most of them have been documented as single cases, with clinical characteristics and prognosis which cannot be completely understood. To understand the characteristics and long-term outcomes of PLMS, the present study reviews earlier reported cases in Chinese and English journals across the globe.
Methods
The systematic literature search was performed via China Knowledge Resource Integrated (CNKI) database to identify the cases reported in Chinese journals, with the last search on 1 March 2013. A similar search was made in MEDLINE, EMBASE, and the Cochrane databases for cases reported in English journals. The primary key words used were ‘pancreas and leiomyosarcoma’, ‘pancreatic and leiomyosarcoma’, ‘pancreas and mesenchymal tumor’, ‘pancreatic and mesenchymal tumor’, ‘pancreas and sarcoma’, and ‘pancreatic and sarcoma’. Only the cases originated from the pancreas were included in the study. Clinical features and long-term outcomes of these cases were summarized and analyzed.
SPSS v.13.0 (SPSS, Inc., Chicago, IL, USA) was used for all analyses. The Pearson χ2 test and Fisher’s exact test were used to compare categorical variables. The Kaplan-Meier method was used to calculated overall survival (OS) using the log-rank test. Cox regression analysis was performed for multivariate survival analysis. A two-sided P value <0.05 was considered statistically significant.
Results
Demographic and clinical data
Twenty cases were reported in Chinese journals (Additional file 1), and 49 cases were reported in English journals [1, 2, 4–35] (Additional file 2). A total of 69 cases were included in the present study. The gender of 68 patients in these 69 cases was documented, which was 35 men and 33 women. An equal incidence in gender was observed (male-female ratio of 1.06) in the data. The mean age was 53.9 ± 14.7 years (median, 53 years; range, 14-87 years).
Symptoms were described in 63 cases and of which 58 patients had symptoms. The most common symptoms were abdominal mass (29 cases), abdominal pain (25 cases), and weight loss (19 cases). Other symptoms like jaundice (8 cases), anemia (6 cases), gastrointestinal bleeding (5 cases), and vomiting (5 cases) were also reported.
The tumor location was documented in 63 cases. The tumors were located in the head of the pancreas in 30 cases, and in the body-tail in 32 cases. One case had tumor in the whole pancreas. The mean size was 11.4 ± 7.1 cm (median, 10 cm; range, 1-30 cm). The gross morphology was recorded in 49 cases. Twenty-six tumors were solid mass, eight tumors were cystic mass, and fifteen were mixed mass.
The status of metastasis and regional invasion at the time of diagnosis were identified in 68 patients. Seventeen (25%) patients had distant metastasis and 13 (19%) patients had adjacent organs/vessels invasion. Lymph node metastasis was documented in only one (1.5%) patient. Men had a higher incidence of metastasis and regional invasion than women (19/35 vs. 11/33; P = 0.082). The surgical treatment method was illustrated in 62 cases, of which 40 cases achieved radical resection, and 22 cases underwent palliative resection or biopsy.
Survival analysis
Forty-nine cases with long-term outcomes data were included in the survival analysis. The median survival time was 48 months. The overall 1-, 3-, 5-, and 10-year survival rates were 66.6%, 51.2%, 43.9%, and 29.3%, respectively. Univariate analysis showed that age ≥55 years, distant metastasis (yes), adjacent organs/vessels invasion (yes), and radical resection (no) were the potential adverse prognostic factors (Table 1). Multivariate analysis of the cases showed that non-radical resection (P = 0.000; hazard ratio (HR) 5.128; 95% confidence interval (CI) 2.041-12.987) was the independent adverse prognostic factor. Adjacent organs/vessels invasion (yes) might be also considered as another independent adverse prognostic factor (P = 0.071; HR 2.708; 95% CI 0.981-7.474) (Table 2).
Discussion
PLMS is rare. Earlier studies by Baylor et al. showed only five leiomyosarcomas among 5,057 pancreatic malignant tumors screened [1]. PLMS is considered to originate either from the smooth muscle region of the pancreatic ducts or the wall of small intra-pancreatic vessels [4]. Leiomyosarcomas originating from the stomach, duodenum, and retroperitoneal organs often invade the pancreas, mimicking PLMS [36]. A definite diagnosis of PLMS is needed to rule out tumors originating from these adjacent organs.
A generally equal incidence of PLMS in gender was observed. There were 35 men and 33 women with a ratio of 1.06. The age of the patients ranged from 14 to 87 years (median, 53 years). The most common symptoms observed in the patients were abdominal mass, abdominal pain, and weight loss, but no specific symptoms were recorded from any of the patients diagnosed with PLMS. Small tumors usually had no symptoms and were found incidentally [36]. The mean size of tumors was 11.4 ± 7.1 cm (range, 1-30 cm). Twenty-three of 49 tumors were mixed or cystic mass. Large tumors with cystic degeneration presented a highly aggressive malignancy, such as adjacent organ invasion and distant metastasis [6]. The tumors were located in the head of the pancreas in 30 cases and in the body-tail in 32 cases. There was a similar incidence between the head and body-tail.
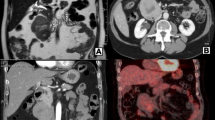
Due to its presence as an obvious mass, the tumor can be found easily by imaging studies. Trans-abdominal ultrasonography (TAUS), computed tomography (CT), positron emission tomography and computed tomography (PET/CT), magnetic resonance imaging (MRI), angiography, and endoscopic ultrasonography (EUS) were all effective in diagnosing the PLMS, but there are no specific imaging features [7]. PLMS appears as a heterogeneous, hypervascular mass on CT scan. As the tumor volume increases, hemorrhagic, necrotic, and cystic changes can be observed [5, 8]. Tumors with cystic lesions can be confused with pseudocysts of the pancreas [6, 9]. PLMS appears as a high incidence of distant metastasis and regional invasion with rare lymphatic involvement, which could be the key point to differential diagnosis. It is difficult to do preoperative qualitative diagnosis by non-invasive diagnostic method. CT or US guided fine-needle aspiration biopsy (FNAB) is an alternative diagnostic method for PLMS. Three of four cases showed a definite diagnosis through US-guided FNAB reported in literature [3].
The accurate diagnosis of PLMS depends mainly on the histological examinations and immunohistochemical staining. Histologically, PLMS showed well-formed fascicles of spindle cells with blunt-ended nuclei intersecting at vertical angles, varying degrees of pleomorphism, and a substantial number of mitosis [10, 11]. However, it is difficult to histologically differentiate PLMS from inflammatory myofibroblastic tumors and non-myogenic spindle cell sarcomas, namely fibrosarcomas, malignant fibrous histiocytomas, and malignant peripheral nerve sheath tumors [10]. Therefore, an immunohistochemical staining is crucial for an accurate diagnosis. Immunohistochemical examinations have an important value in evaluating spindle cell tumors which were supposed to be of smooth muscle origin. These tumors are positive for muscle markers (such as α-smooth muscle actin, MSA, and desmin), with negative expression of epithelial (such as cytokeratin, EMA, and CEA) and neural (such as S100 protein) markers [10].
PLMS is an aggressive tumor. In the present systematic review, 25% of patients had distant metastasis and 19% of patients had adjacent organs/vessels invasion at the time of diagnosis. Lymph node metastasis was not common which was documented only in one patient (1.5%). This feature helps us to differentiate PLMS from other pancreatic malignant tumors and more surgeons prefer to perform a local radical resection rather than extensive node dissection based on this feature. Gender may be related to the degree of malignancy. Men seemingly had a higher incidence of metastasis and regional invasion than women (P = 0.082).
Survival analysis showed that median survival time of PLMS was 48 months. The overall 1-, 3-, 5-, and 10-year survival rates were 66.6%, 51.2%, 43.9%, and 29.3%, respectively. From univariate analysis, we showed that age ≥55 years, distant metastasis (yes), adjacent organs/vessels invasion (yes), and radical resection (no) were the potential adverse prognostic factors of PLMS. Multivariate analysis indicated that non-radical resection was the independent adverse prognostic factor. Radical resection was associated with prolonged overall survival time. Adjacent organs/vessels invasion (yes) might be another independent adverse prognostic factor, but statistical difference was not observed (P = 0.071). Although the dimension of tumors is an important indicator with regard to resectability, large tumor size was not an effective predictor of adverse prognosis in the current study. Mitotic counts >10 mitoses per 10 high-powered fields (HPFs) was reported as another adverse predictor [10]. Since data of this retrospective study extracted from the literature were not in detail, a comprehensive prognosis analysis could not be performed.
Conclusion
PLMS is a very rare malignant tumor of the pancreas. Clinical features and imaging examinations are no specific. The accurate diagnosis of PLMS depends on histological examinations and immunohistochemical staining. PLMS is an aggressive tumor with poor prognosis. Radical resection can prolong survival time of the patient.
Abbreviations
- CEA:
-
Carcinoembryonic antigen
- CI:
-
Confidence interval
- CNKI:
-
China Knowledge Resource Integrated database
- CT:
-
Computed tomography
- EMA:
-
Epithelial membrane protein
- EUS:
-
Endoscopic ultrasonography
- FNAB:
-
Fine-needle aspiration biopsy
- HPFs:
-
High-powered fields
- HR:
-
Hazard ratio
- MSA:
-
Muscle-specific actin
- MRI:
-
Magnetic resonance imaging
- OS:
-
Overall survival
- PET/CT:
-
Positron emission tomography and computed tomography
- PLMS:
-
Primary pancreatic leiomyosarcoma
- TAUS:
-
Trans-abdominal ultrasonography.
References
Baylor SM, Berg JW: Cross-classification and survival characteristics of 5,000 cases of cancer of the pancreas. J Surg Oncol. 1973, 5: 335-358.
ROSS CF: Leiomyosarcoma of the pancreas. Br J Surg. 1951, 39: 53-56.
Du X, Cheng Z, Zhou ZG, Zhang MM, Chen ZX: Primary pancreatic leiomyosarcoma: a retrospective analysis of clinical characteristics and prognosis of this rare disease. Hepatogastroenterol. 2012, 59: 2644-2649.
Feinberg SB, Margulis AR, Lober P: Roentgen findings in leiomyosarcoma of the pancreas. Minn Med. 1957, 40: 505-506.
Izumi H, Okada K, Imaizumi T, Hirabayashi K, Matsuyama M, Dowaki S, Tobita K, Makuuchi H: Leiomyosarcoma of the pancreas: report of a case. Surg Today. 2011, 41: 1556-1561.
Aihara H, Kawamura YJ, Toyama N, Mori Y, Konishi F, Yamada S: A small leiomyosarcoma of the pancreas treated by local excision. HPB (Oxford). 2002, 4: 145-148.
Hur YH, Kim HH, Park EK, Seoung JS, Kim JW, Jeong YY, Lee JH, Koh YS, Kim JC, Kim HJ, Cho CK: Primary leiomyosarcoma of the pancreas. J Korean Surg Soc. 2011, Suppl 1: S69-S73.
Srivastava DN, Batra A, Thulkar S, Julka PK: Leiomyosarcoma of pancreas: imaging features. Indian J Gastroenterol. 2000, 19: 187-188.
Sato T, Asanuma Y, Nanjo H, Arakawa A, Kusano T, Koyama K, Shindo M: A resected case of giant leiomyosarcoma of the pancreas. J Gastroenterol. 1994, 29: 223-227.
Nesi G, Pantalone D, Ragionieri I, Amorosi A: Primary leiomyosarcoma of the pancreas: a case report and review of literature. Arch Pathol Lab Med. 2001, 125: 152-155.
Komoda H, Nishida T, Yumiba T, Nishikawa K, Kitagawa T, Hirota S, Ito T, Matsuda H: Primary leiomyosarcoma of the pancreas–a case report and case review. Virchows Arch. 2002, 440: 334-337.
Berman JK, Levene N: Sarcoma of the pancreas. AMA Arch Surg. 1956, 73: 894-896.
Becker WF, Welsh RA, Pratt HS: Cystadenoma and cystadenocarcinoma of the pancreas. Ann Surg. 1965, 161: 845-863.
Ishikawa O, Matsui Y, Aoki Y, Iwanaga T, Terasawa T, Wada A: Leiomyosarcoma of the pancreas. Report of a case and review of the literature. Am J Surg Pathol. 1981, 5: 597-602.
Lakhoo K, Mannell A: Pancreatic leiomyosarcoma. A case report. S Afr J Surg. 1991, 29: 59-60.
Russ PD: Leiomyosarcoma of the pancreatic bed detected on CT scans. AJR Am J Roentgenol. 1993, 161: 210-
de Alava E, Torramade J, Vazquez JJ: Leiomyosarcoma of the pancreas. Virchows Arch A Pathol Anat Histopathol. 1993, 422: 419-422.
Ishii H, Okada S, Okazaki N, Nose H, Yoshimori M, Aoki K, Tsuda H, Hirohashi S: Leiomyosarcoma of the pancreas: report of a case diagnosed by fine needle aspiration biopsy. Jpn J Clin Oncol. 1994, 24: 42-45.
Peskova M, Fried M: Pancreatic tumor of mesenchymal origin–an unusual surgical finding. Hepatogastroenterol. 1994, 41: 201-203.
Aranha GV, Simples PE, Veselik K: Leiomyosarcoma of the pancreas. Int J Pancreatol. 1995, 17: 95-97.
Shimizu M, Hirokawa M, Matsumoto T, Iwamoto S, Manabe T: Fatty replacement of the pancreatic body and tail associated with leiomyosarcoma of the pancreatic head. Pathol Int. 1997, 47: 633-636.
Owen CH, Madden JF, Clavien PA: Spindle cell stromal tumor of the pancreas: treatment by pancreatoduodenectomy. Surgery. 1997, 122: 105-111.
Chawla S, Gairola M, Nachiappan PL, Deshpande A, Rathi AK, Rath GK: Pancreatic leiomyosarcoma in a middle-aged lady. Trop Gastroenterol. 1998, 19: 118-119.
Paciorek ML, Ross GJ: MR imaging of primary pancreatic leiomyosarcoma. Br J Radiol. 1998, 71: 561-563.
Zalatnai A, Kovacs M, Flautner L, Sipos B, Sarkady E, Bocsi J: Pancreatic leiomyosarcoma. Case report with immunohistochemical and flowcytometric studies. Virchows Arch. 1998, 432: 469-472.
Ferlan-Marolt V, Vladislav P, Alojz P: Pancreatic leiomyosarcoma: clinicopathohistological presentation of a rare tumor. Hepatogastroenterol. 2000, 47: 556-559.
Machado MC, Cunha JE, Penteado S, Bacchella T, Jukemura J, Costa AC, Halpern-Salomon I: Preoperative diagnosis of pancreatic leiomyosarcoma. Int J Pancreatol. 2000, 28: 97-100.
Deveaux PG, Aranha GV, Yong S: Leiomyosarcoma of the pancreas. HPB (Oxford). 2001, 3: 175-177.
Maarouf A, Scoazec JY, Berger F, Partensky C: Cystic leiomyosarcoma of the pancreas successfully treated by splenopancreatectomy: a 20-year follow-up. Pancreas. 2007, 35: 95-98.
Muhammad SU, Azam F, Zuzana S: Primary pancreatic leiomyosarcoma: a case report. Cases J. 2008, 1: 280-
Riddle ND, Quigley BC, Browarsky I, Bui MM: Leiomyosarcoma arising in the pancreatic duct: a case report and review of the current literature. Case Rep Med. 2010, 2010: 252364-
Zhang H, Jensen MH, Farnell MB, Smyrk TC, Zhang L: Primary leiomyosarcoma of the pancreas: study of 9 cases and review of literature. Am J Surg Pathol. 2010, 34: 1849-1856.
Zhang QY, Shen QY, Yan S, Zheng SS: Primary pancreatic pleomorphic leiomyosarcoma. J Int Med Res. 2011, 39: 1555-1562.
Vanderpuye V, Clegg-Lamptey JN, Yarney J, Aryeetey N: Metastatic primary leiomyosarcoma of the pancreas to the liver: report of a surgically treated case. J Gastrointest Cancer. 2012, 43: 70-72.
Moletta L, Sperti C, Beltrame V, Gruppo M, Blandamura S, Pasquali C, Pedrazzoli S: Leiomyosarcoma of the pancreas with liver metastases as a paradigm of multimodality treatment: case report and review of the literature. J Gastrointest Cancer. 2012, 43: 246-250.
Nordback I, Mattila J, Tarkka M: Resectable leiomyosarcoma of inferior vena cava presenting as carcinoma of the pancreas. Case report. Acta Chir Scand. 1990, 156: 577-580.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
All the authors declare no competing interests.
Authors’ contributions
Xu drafted the manuscript. Zhang and Zhao designed the study and helped in drafting the manuscript. Xu, Wang, and You collected the data and performed the statistical analysis. All authors have read and approved the final manuscript.
Jianwei Xu, Taiping Zhang contributed equally to this work.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Xu, J., Zhang, T., Wang, T. et al. Clinical characteristics and prognosis of primary leiomyosarcoma of the pancreas: a systematic review. World J Surg Onc 11, 290 (2013). https://doi.org/10.1186/1477-7819-11-290
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7819-11-290