Abstract
Background
The carboxy-terminal 42 kDa region of Plasmodium vivax merozoite surface protein-1 (PvMSP-142) is a leading candidate antigen for blood stage vaccine development. However, this region has been observed to be highly polymorphic among filed isolates of P. vivax. Therefore it is important to analyse the existing diversity of this antigen in the field isolates of P. vivax. In this study, the genetic diversity and natural selection in PvMSP-142 among P. vivax Korean isolates were analysed.
Methods
A total of 149 P. vivax- infected blood samples collected from patients in Korea were used. The region flanking PvMSP-142 was amplified by PCR, cloned into Escherichia coli, and then sequenced. The polymorphic characteristic and natural selection of PvMSP-142 were analysed using the DNASTAR, MEGA4 and DnaSP programs.
Results
A total of 11 distinct haplotypes of PvMSP-142 with 40 amino acid changes, as compared to the reference Sal I sequence, were identified in the Korean P. vivax isolates. Most of the mutations were concentrated in the 33 kDa fragment (PvMSP-133), but a novel mutation was found in the 19 kDa fragment (PvMSP-119). PvMSP-142 of Korean isolates appeared to be under balancing selection. Recombination may also play a role in the resulting genetic diversity of PvMSP-142.
Conclusions
PvMSP-142 of Korean P. vivax isolates displayed allelic polymorphisms caused by mutation, recombination and balancing selection. These results will be useful for understanding the nature of the P. vivax population in Korea and for development of a PvMSP-142 based vaccine against P. vivax.
Similar content being viewed by others
Background
Merozoite surface protein-1 (MSP-1) is a high molecular mass protein abundantly expressed on the surface of the merozoite of malaria parasites and it plays a critical role in the erythrocyte invasion of the parasites [1]. It is synthesized as a large precursor during schizogony and is subsequently processed via proteolytic cleavage into four major polypeptides of approximately 83, 30, 38, and 42 kDa from the N-terminus to C-terminus [1, 2]. During the invasion process, the C-terminal 42 kDa fragment (MSP-142) is further processed into 33 kDa (MSP-133) and 19 kDa (MSP-119) fragments, and the latter remains on the merozoite surface and is carried into the invaded erythrocytes, but all the other fragments are released from the merozoite surface [3, 4]. Individuals naturally infected with Plasmodium vivax acquire humoral immune responses against the C-terminal part of MSP-1, MSP-119 or MSP-142[5–10]. Antibodies that recognize the C-terminal region of Plasmodium falciparum MSP-1 inhibit invasion of the merozoites into host erythrocytes in vitro[11–14], and immunization of experimental animals with MSP-119 confers protective immunity [15, 16]. These findings demonstrate that MSP-142 is a promising candidate antigen for blood stage vaccine development [1, 17–19]. However, genetic polymorphisms encoding this region, within and among the P. vivax population, are one of the important factors impeding vaccine development.
Vivax malaria had been endemic on the Korean Peninsula for centuries [20], but was eradicated in South Korea by 1979 as a result of intensive efforts led by the World Health Organization and Korean National Malaria Eradication Programme. However, vivax malaria re-emerged in South Korea in 1993 and the outbreak has continued with fluctuating numbers of annual indigenous cases with the total number of cases up to 23,000 [21]. During the early period of the re-emergence, most malaria cases were restricted to military personnel and veterans who served near the Demilitarized Zone (DMZ), and the geographic distribution was limited to the DMZ and adjacent areas where no civilians reside [22]. In spite of the significant decrease in the number of malaria cases among military personnel since the re-emergence, mainly resulting from aggressive chemoprophylaxis, the number of malaria cases in the civilian population has increased and the geographic distribution is expanding into southward cities and counties nearby the DMZ, a pattern indicating the establishment of local transmission in South Korea [21, 23].
Although genetic polymorphisms in the central repeat region of MSP-1 in Korean P. vivax isolates has been previously analysed [24–26], little information is available regarding the genetic polymorphism of MSP-142 among Korean P. vivax population. In this study, the genetic polymorphisms and natural selection in MSP-142 among P. vivax Korean isolates were analysed to gain in-depth understanding of the nature of Korean P. vivax population. The results suggested that a significant level of genetic diversity exists in the MSP-142, particularly in MSP-133, among Korean P. vivax isolates and the region is undergoing a natural selection process.
Methods
Blood samples
A total of 149 blood samples were collected from Korean patients infected with P. vivax in Korea between 1999 and 2010. Plasmodium vivax infection was identified by microscopic examination of thin and thick blood smears and confirmed by polymerase chain reaction (PCR) [27]. All the patients have a febrile illness and have not been abroad at least in recent two years when their blood samples were collected. About 5 ml of blood was collected from each individual. The blood was separated into packed cells and plasma and then stored at −80°C until use. Blood collections performed for this study were conducted following informed consent of the patients and adhering to the institutional ethical guidelines reviewed and approved by either the Ethics committee of Gachon University of Medicine and Science or Inha University School of Medicine.
Genomic DNA extraction and amplification of PvMSP-142
Genomic DNA was extracted from 200 μl of blood sample using a QIAamp Blood Kit (Qiagen, Valencia, CA, USA). Amplification of PvMSP-142 was performed using two rounds of PCR with primers described previously [28]. The first round PCR primers were 5'-ACGTAGCAGCAAAAGCGCAG-3' and 5'-GCAACATGAGCAACAAGAAGG-3' and the primers for nested PCR were 5'-ACTACGCCGAGGACTACGAC-3' and 5'-AGGACAAGCTTAGGAAGCTGG-3'. The amplification reaction for each round of PCR was performed using the following thermal cycling conditions: 94°C for 5 min, 30 cycles at 94°C for 1 min, 50°C for 1 min, and 72°C for 1.5 min, and followed by a final extension at 72°C for 10 min. Ex Taq DNA polymerase (Takara, Otsu, Japan) was used in all PCR reactions in order to eliminate any possible nucleotide misincorporation. The PCR product was analysed on a 1.2% agarose gel, purified from the gel, and then ligated into the T&A cloning vector (Real Biotech Cooperation, Banqiaa City, Taiwan). Each ligation mixture was transformed into Escherichia coli DH5α competent cells and positive clones with the appropriate insert were selected. The nucleotide sequences of the cloned insert were analysed by automatic DNA sequencing. In order to verify the sequences, at least two clones from each isolate were sequenced in both directions. Some isolates underwent three-fold sequence coverage to confirm the existence of rare polymorphisms. The nucleotide sequences reported in this study have been deposited in the GenBank database under the accession numbers JQ446312-JQ446322.
Sequence and phylogenetic analysis
Nucleotide and deduced amino acid sequences of PvMSP-142 were analysed using EditSeq and SeqMan in the DNASTAR package (DNASTAR, Madison, WI, USA). The phylogeny tree was constructed using the neighbour-joining method in MEGA4 [29]. Bootstrap proportions were used to assess the robustness of the tree with 1,000 bootstrap replications.
DNA sequence polymorphism analysis
DNA sequence polymorphism analysis was performed on the 149 PvMSP-142 sequences. The number of segregating sites (S), haplotypes (H), haplotype diversity (Hd), nucleotide diversity (π), and the average number of pair-wise nucleotide differences within the population (K) were estimated using DnaSP ver. 5.10.00 [30]. The π was calculated on a sliding window of 100 bases with a step size of 25 bp to estimate the stepwise diversity across PvMSP-142. The rates of synonymous (dS) and non-synonymous (dN) substitutions were estimated and compared by the Z-test (P < 0.05) in MEGA4 program [29] using the Nei and Gojobori’s method [31] with the Jukes and Cantor correction. Tajima’s D test [32] was performed with DnaSP ver. 5.10.00 to evaluate the neutral theory of evolution. Fu and Li’s D and F statistics [33] were also analysed using DnaSP ver. 5.10.00 [30].
Recombination parameters and linkage disequilibrium
The recombination parameter (R), which included the effective population size and probability of recombination between adjacent nucleotides per generation, and the minimum number of recombination events (Rm) were measured using DnaSP ver. 5.10.00 [30]. Linkage disequilibrium (LD) between different polymorphic sites was computed in terms of the R2 index.
Results
Genetic polymorphisms and amino acid changes
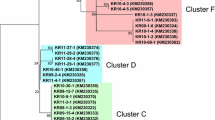
The region corresponding to PvMSP-142 was amplified from the 149 P. vivax Korean isolates. Nucleotide sequence analysis of the 149 PvMSP-142 sequences revealed that there was no size variation between the sequences but they showed polymorphic characteristics. A sequence analysis of the 149 PvMSP-142 sequences resulted in their classification into 11 different haplotypes (haplotypes 1–11) with amino acid changes at 40 positions as compared to the reference Sal I sequence (Figure 1). Of the 40 amino acid changes, three were tetramorphic (N1517S/A/V, D1520T/A/N and I1527M/T/N), nine were trimorphic (T1494E/A, K1505A/T, Q1506E/H, V1516D/E, S1518T/N, S1521T/A, N1524T/A, E1525Q/K and F1526S/L), and the others were dimorphic (Figure 1). Most of the amino acid substitutions were found in the PvMSP-133 region and only one dimorphic substitution (N1692K) was identified in PvMSP-119. Interestingly, seven amino acid changes (N1343Y, N1427Y, L1447W, K1486R, E1603V, L1613V, and N1692K) were unique and had not been identified previously, and represented novel haplotypes. Sequence analysis of the 11 haplotypes of PvMSP-142 based on the hypervariable region also revealed that haplotypes 1–5 were essentially similar to the Belem type. However, the others (haplotypes 6–11) were recombinant forms between Sal I and Belem, which had at least one possible recombination site in their sequences. Phylogenetic analysis revealed that the Korean PvMSP-142 haplotypes were clustered into five clades, a Belem-type and four scattered clades (Figure 2). Upon analysis of the distribution of each MSP-142 haplotype in each year, an interesting finding was observed. In the isolates collected in 1999–2000, only the Belem type haplotypes of PvMSP-142 were identified. However, a recombinant type haplotype (haplotype 7) was first identified among the isolates collected in 2001, and both Belem and recombinant haplotypes were identified thereafter with a prevalence of recombinant haplotypes (Figure 3).
Sequence polymorphism of PvMSP-1 42 in Korean Plasmodium vivax isolates. Sequence analysis revealed that a total of 11 distinct haplotypes of PvMSP-142 were identified in 149 P. vivax Korean isolates. Polymorphic amino acids compared to the reference sequence, Sal I (AF435593), are listed for each haplotype. The dimorphic, trimorphic and tetramorphic amino acid changes are marked in yellow, blue and red, respectively. The total numbers of sequences for each haplotype are listed in the right panel.
Annual distribution of PvMSP-1 42 haplotypes during the study period. The 149 PvMSP-142 sequences from Korean isolates were analysed by year of collection. Only Belem type haplotypes of PvMSP-142 were identified during 1999–2000. Recombinant types began to be identified in 2001, and both Belem-type and recombinant type haplotypes were identified thereafter.
Nucleotide diversity and natural selection of PvMSP-142
DNA sequence analyses were performed to determine the nucleotide diversity and genetic differentiation at PvMSP-142 among the Korean P. vivax isolates. The average number of pair-wise nucleotide differences (K) for the 1,234 bp PvMSP-142 region was 19.570 (Table 1). The overall haplotype diversity (Hd) and nucleotide diversity (π) for all 149 sequences was 0.876 ± 0.009 and 0.01586 ± 0.00047, respectively (Table 1). Analysis of the genetic diversity of the PvMSP-133 and PvMSP-119 fragments revealed that the PvMSP-119 fragment is more highly conserved than the PvMSP-133 fragment, indicating that most of the nucleotide diversity was concentrated in PvMSP-133. The overall haplotype diversity (Hd) and nucleotide diversity (π) for PvMSP-133 was 0.873 ± 0.009 and 0.02051 ± 0.00063, respectively. To examine whether or not natural selection contributed to the diversity observed in PvMSP-142 within the Korean P. vivax population, the average difference of dN-dS for all PvMSP-142 sequences was analysed. The estimated dN-dS was 0.0067, indicating that positive natural selection may be occurring in the PvMSP-142 of Korean isolates (Table 1). PvMSP-133 and PvMSP-119 also showed positive dN-dS values of 0.0085 and 0.0016, respectively. In order to more closely explore natural selection in the PvMSP-142, Tajima’s D test was applied and the value was estimated to be 3.0268 (P < 0.01), indicating that PvMSP-142 is under positive selection pressure (Table 1). The Tajima’s D values for PvMSP-133 and PvMSP-119 also showed positive values of 3.0556 (P < 0.01) and 0.5504 (P > 0.1), respectively. The Fu and Li’s D and F values for PvMSP-142 were 2.0839 (P < 0.02) and 2.9904 (P < 0.02), respectively. Analysis of the sliding window plot (window length 100 bp, step size 25 bp) using the DnaSP package revealed that π ranged from 0 to 0.1301 and supported our observations that most of the variations were concentrated between nucleotide positions 400–675, corresponding to the middle region of PvMSP-142 (Figure 4A).
Natural selection and recombination event of PvMSP-1 42 . (A) Sliding window plot of nucleotide diversity per site (π) comparing the level of genetic diversity at PvMSP-142. The π values were calculated using DnaSP with a window length of 100 bp and step size of 25 bp. (B) The linkage disequilibrium (LD) plot showing non-random association between nucleotide variants in the 149 Korean P. vivax isolates at different polymorphic sites. The R2 values were plotted against nucleotide distance using a two-tailed Fisher’s exact test for significance.
Recombination
For PvMSP-142, the minimum number of recombination events between adjacent polymorphic sites (Rm) was five, whereas the R between adjacent sites (Ra) and per gene (Rb) was 0.0065 and 8.0, respectively. Similar results were obtained when PvMSP-133 was analysed (Rm: 5, Ra: 0.0080, and Rb: 7.5). These high recombination parameter values suggested that meiotic recombination may be occurring between sites, resulting in genetic diversity of the PvMSP-142. The LD index, R2, also declined across the analysed region, suggesting that intragenic recombination may also be a possible factor contributing to the diversity observed in PvMSP-142 (Figure 4B).
Discussion
A blood stage malaria vaccine ideally aims to prevent or considerably reduce blood stage parasitaemia either by inhibiting merozoite invasion into erythrocytes or by targeted destruction of parasitized erythrocytes [19, 34]. Following this approach, several merozoite surface proteins (MSPs) have been considered promising candidate antigens for malaria vaccine development due to their accessibility by antibodies and their essential roles in erythrocyte invasion [19]. However, the genetic diversity of the MSPs identified within and among global isolates has resulted in a major obstacle hampering the development of an effective malaria vaccine. Of the MSPs, MSP-142 is the most outstanding vaccine candidate antigen, which is currently at an advanced stage of clinical evaluation [34–37]. But, its polymorphic nature suggests that routine changes to the vaccine and continuous surveillance of the antigen diversity in field isolates would be required.
In this study, the genetic diversity and natural selection on PvMSP-142 in the 149 P. vivax Korean isolates were analysed. The 149 sequences were classified into 11 distinct haplotypes with amino acid changes at 40 positions as compared to the Sal I sequence. Most of the amino acid substitutions were concentrated in the PvMSP-133 fragment and only a dimorphic change (N1692K) was found in PvMSP-119. It is known that PvMSP-119 is highly conserved, as observed in field isolates obtained from different geographic regions, and only one amino acid change (K1709E) has been reported thus far [38–41]. The amino acid change was not observed in any of the Korean isolates, but the emergence of a new amino acid change in PvMSP-119 in Korean isolates suggest that PvMSP-119 could contribute to the diversity of PvMSP-142. Of the 39 amino acid changes found in PvMSP-133 of Korean isolates, six (N1343Y, N1427Y, L1447W, K1486R, E1603V, and L1613V) were unique and had not been reported previously. These unique changes resulted in the generation of six novel haplotypes that had not been reported so far. The sequence and phylogenetic analyses revealed that none of the Korean haplotypes were identical to either the Sal I or Belem sequences, but haplotypes 1–5 were essentially Belem types, and the others were recombinant types between Belem and Sal I, in which at least one recombination may occur at the hypervariable region of PvMSP-133. Recently, PvMSP-142 was differentiated into 12 distinctive groups (group 1–12) based on sequence differences observed in hypervariable region, but there was no evidence of geographic clustering of global isolates [42]. Phylogenetic analysis of Korean PvMSP-142 haplotypes suggested they were clustered into five distinct clades with the majority belonging to the Belem type, but no clear geographic relationship was also identified. Interestingly, the isolates collected in 1999–2000 showed only limited haplotypes which were closely related to the Belem type. However, a recombinant haloptype was first observed among isolates collected in 2001. Both Belem and recombinant types of the PvMSP-142 haplotypes were identified thereafter, with a prevalence of the recombinant types. These results coincided with several previous studies based on the genetic diversity of several major antigens including circumsporozoite protein, MSP-1, and MSP-3α, as well as microsatellite loci, suggesting that the Korean P. vivax isolates had been genetically homologous until 2000, but the genetic diversity was rapidly disseminated thereafter [26, 43]. It is currently unclear why Korean P. vivax isolates showed such diverse genetic profiles and this issue should be elucidated. PvMSP-119 was found to be more highly conserved than PvMSP-133 in the Korean isolates, but the nucleotide diversity (π) of PvMSP-119 was considerably higher than those found in previous studies [28, 42, 44]. This was due to a non-synonymous substitution (N1692K) in PvMSP-119 of the Korean isolates. Meanwhile, the π values for PvMSP-142 and PvMSP-133 found in the Korean isolates were lower than those observed in other global isolates [28, 42, 44]. This suggests that PvMSP-142 of Korean isolates showed limited genetic diversity as compared to isolates from other endemic regions including India and Sri Lanka.
The rate of non-synonymous and synonymous mutations (dN-dS) is widely used as an indicator of the action of natural selection in gene sequences. An excess of dN relative to dS is a clear signal of positive selection, whereas a lack of dN relative to dS suggests a negative or purifying selection imposed by functional constraints [31, 45]. The positive value of dN-dS (0.0067) observed in the 149 Korean PvMSP-142 sequences suggested that PvMSP-142 in the Korean P. vivax isolates is under the influence of positive natural selection. The observation that PvMSP-133 had a higher dN-dS than PvMSP-142 also suggested that PvMSP-133 is under stronger positive natural selection pressure than the entire PvMSP-142, and this finding was comparable to observations found in P. vivax isolates from different areas [28, 42]. The positive values of Tajima’s D (3.0268, P < 0.01) and Fu and Li’s D (2.0839, P < 0.02) and F (2.9904 < 0.02) statistics indicated that the PvMSP-142 alleles occurred at more intermediate frequencies than expected and that few alleles were rare or near fixation, which is consistent with the action of the balancing selection that maintains allelic variation in a population. These results collectively suggested that strong balancing selection, presumably by host immune pressure [28, 45, 46], occurred at PvMSP-142 in the Korean isolates, and the host immune responses likely played a role in generation and maintenance of the MSP-142 polymorphism.
The diversity of plasmodial antigens is also likely to be generated by genetic recombination during the sexual stage of the parasites in the mosquito [45, 46]. The results obtained in this study indicated that recombination events occurred within the PvMSP-142 sequences in Korean isolates. This was supported by the observation of decline of LD index R2 with increasing nucleotide distance coupled with a high level of haplotype diversity (Hd = 0.876 ± 0.009). Indeed, all recombinant types of the Korean PvMSP-142 haplotypes had putative recombination sites that concentrated in PvMSP-133 rather than being evenly distributed across the entire PvMSP-142, which consistent with previous reports [28, 41, 42]. Considering the first appearance of the recombinant haplotype PvMSP-142 in 2001 and the subsequent prevalence of recombinant types from 2003 to recent years, new PvMSP-142 haplotypes are actively being generated in Korean isolates by recombination events in recent years even though the country with low malaria transmission rate.
Conclusion
This study provided the first in-depth analysis of the genetic diversity and natural selection of PvMSP-142 in Korean P. vivax isolates. PvMSP-142 showed polymorphic characteristics that resulted in 11 distinct haplotypes of the Belem or recombinant types. Most of the observed amino acid changes were identified in PvMSP-133, but a novel amino acid change that had not been reported in global isolates was identified in PvMSP-119. Considering the low transmission rate and unstable malaria conditions in Korea, both interallelic and intragenic recombinations are likely to play roles in the generation and maintenance of the diversity of PvMSP-142. Furthermore, balancing selection in response to host immune responses may also contribute to the diversity of PvMSP-142 in Korean isolates. Theses results will be helpful in understanding the nationwide parasite heterogeneity and the implementation of malarial control programmes in Korea, as well as for the development of a PvMSP-1 based vaccine against P. vivax.
References
Holder AA, Guevara Patino JA, Uthaipibull C, Syed SE, Ling IT, Scott-Finnigan T, Blackman MJ: Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria. Parasitologia. 1999, 41: 409-414.
Holder AA: The precursor to major merozoite surface antigens: Structure and role in immunity. Prog Allergy. 1988, 41: 72-97.
Blackman MJ, Ling LT, Nicholls SC, Holder AA: Proteolytic processing of the Plasmodium falciparum merozoite surface protein-1 produces a membrane-bound fragment containing two epidermal growth factor-like domains. Mol Biochem Parasitol. 1991, 49: 29-34. 10.1016/0166-6851(91)90127-R.
Blackman MJ, Holder AA: Secondary processing of the Plasmodium falciparum merozoite surface protein-1 (MSP1) by a calcium-dependent membrane-bound serine protease: shedding of MSP-133 as a noncovalently associated complex with other fragments of the MSP1. Mol Biochem Parasitol. 1992, 50: 307-315. 10.1016/0166-6851(92)90228-C.
Soares IS, Levitus G, Souza JM, Del Portillo HA, Rodrigues MM: Acquired immune responses to the N- and C-terminal regions of Plasmodium vivax merozoite surface protein 1 in individuals exposed to malaria. Infect Immun. 1997, 65: 1606-1614.
Park JW, Moon SH, Yeom JS, Lim KJ, Sohn MJ, Jung WC, Cho YJ, Jeon KW, Ju W, Ki CS, Oh MD, Choe K: Naturally acquired antibody responses to the C-terminal region of merozoite surface protein 1 of Plasmodium vivax in Korea. Clin Diagn Lab Immunol. 2001, 8: 14-20.
Sachdeva S, Ahmad G, Malhotra P, Mukherjee P, Chauhan VS: Comparison of immunogenicities of recombinant Plasmodium vivax merozoite surface protein 1 19- and 42-kiloDalton fragments expressed in Escherichia coli. Infect Immun. 2004, 72: 5775-5782. 10.1128/IAI.72.10.5775-5782.2004.
Pitabut N, Panichakorn J, Mahakunkijcharoen Y, Hirunpetcharat C, Looareesuwan S, Khusmith S: IgG antibody profile to c-terminal region of Plasmodium vivax merozoite surface protein-1 in Thai individuals exposed to malaria. Southeast Asian J Trop Med Public Health. 2007, 38: 1-7.
Yeom JS, Kim ES, Lim KJ, Oh JH, Sohn MJ, Yoo SB, Kim E, Bae I, Jung YJ, Park JW: Naturally acquired IgM antibody response to the C-terminal region of the merozoite surface protein 1 of Plasmodium vivax in Korea: use for serodiagnosis of vivax malaria. J Parasitol. 2008, 94: 1410-1414. 10.1645/GE-1484.1.
Zeyrek FY, Babaoglu A, Demirel S, Erdogan DD, Ak M, Korkmaz M, Coban C: Analysis of naturally acquired antibody responses to the 19-kd C-terminal region of merozoite surface protein-1 of Plasmodium vivax from individuals in Sanliurfa, Turkey. Am J Trop Med Hyg. 2008, 78: 729-732.
Pirson PJ, Perkins ME: Characterization with monoclonal antibodies of a surface antigen of Plasmodium falciparum merozoites. J Immunol. 1985, 134: 1946-1951.
Blackman MJ, Heidrich HG, Donachie S, McBridge JS, Holder AA: A single fragment of a malaria merozoite surface protein remains on the parasite during red blood cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med. 1990, 172: 379-382. 10.1084/jem.172.1.379.
Chang SP, Gibson HL, Lee NC, Barr PJ, Hui GS: A carboxyl-terminal fragment of Plasmodium falciparum gp 195 expressed by a recombinant baculovirus induces antibodies that completely inhibit parasite growth. J Immunol. 1992, 149: 548-555.
Chappel JA, Holder AA: Monoclonal antibodies that inhibit Plasmodium falciparum invasion in vitro recognize the first growth factor-like domain of merozoite surface protein-1. Mol Biochem Parasitol. 1993, 60: 303-312. 10.1016/0166-6851(93)90141-J.
Burns JM, Parke LA, Daly TM, Cavacini LA, Weidanz WP, Long CA: A protective monoclonal antibody recognizes a variant-specific epitope in the precursor of the major merozoite surface antigen of the rodent malarial parasite Plasmodium yoelii. J Immunol. 1989, 142: 2835-2840.
Devi YS, Mukherjee P, Yazdani SS, Shakri AR, Mazumdar S, Pandey S, Chitnis CE, Chauhan VS: Immunogenicity of Plasmodium vivax combination subunit vaccine formulated with human compatible adjuvants in mice. Vaccine. 2007, 25: 5166-5174. 10.1016/j.vaccine.2007.04.080.
Dutta S, Kaushal DC, Ware LA, Puri SK, Kaushal NA, Narula A, Upadhyaya DS, Lanar DE: Merozoite surface protein 1 of Plasmodium vivax induces a protective response against Plasmodium cynomolgi challenge in rhesus monkeys. Infect Immun. 2005, 73: 5936-5944. 10.1128/IAI.73.9.5936-5944.2005.
Galinski BR, Barnwell JW: Plasmodium vivax : who cares?. Malar J. 2008, 7: (Suppl:S9)-
Holder A: Malaria vaccines: where next?. PLoS Pathog. 2009, 5: e1000638-10.1371/journal.ppat.1000638.
World Health Organization: Synopsis of the world Malaria situation in 1979. Wkly Epidemiol Rec. 1981, 56: 145-149.
Park JW, Jun G, Yeom JS: Plasmodium vivax malaria: status in the Republic of Korea following reemergence. Korean J Parasitol. 2009, 47 (Suppl): S39-S50. 10.3347/kjp.2009.47.S.S39.
Park JW, Klein TA, Lee HC, Pacha LA, Ryu SH, Yeom JS, Moon SH, Kim TS, Chai JY, Oh MD, Choe KW: Vivax malaria: A continuing health threat to the Republic of Korea. Am J Trop Med Hyg. 2003, 69: 159-167.
Yeom JS, Kim TS, Oh S, Sim JB, Barn JS, Kim HJ, Kim YA, Ahn SY, Shin MY, Yoo JA, Park JW: Plasmodium vivax malaria in the Republic of Korea during 2004–2005: Changing patterns of infection. Am J Trop Med Hyg. 2007, 76: 865-868.
Lim CS, Kim SH, Kwon SI, Song JW, Song KJ, Lee KN: Analysis of Plasmodium vivax merozoite surface protein-1 gene sequences from resurgent Korean isolates. Am J Trop Med Hyg. 2000, 62: 261-265.
Kim SH, Hwang SY, Shin JH, Moon CS, Kim DW, Kho WG: Molecular genetic characterization of the merozoite surface protein 1 gene of Plasmodium vivax from reemerging Korean isolates. Clin Vaccine Immunol. 2009, 16: 733-738. 10.1128/CVI.00493-08.
Choi YK, Choi KM, Park MH, Lee EG, Kim YJ, Lee BC, Cho SH, Rhie HG, Lee HS, Yu JR, Lee JS, Kim TS, Kim JY: Rapid dissemination of newly introduced Plasmodium vivax genotypes in South Korea. Am J Trop Med Hyg. 2010, 82: 426-432. 10.4269/ajtmh.2010.09-0245.
Moon SU, Lee HW, Kim JY, Na BK, Cho SH, Lin K, Sohn WM, Kim TS: High frequency of genetic diversity of Plasmodium vivax field isolates in Myanmar. Acta Trop. 2009, 109: 30-36. 10.1016/j.actatropica.2008.09.006.
Thakur A, Alam MT, Sharma YD: Genetic diversity in the C-terminal 42 kDa region of merozoite surface protein-1 of Plasmodium vivax (PvMSP-142) among Indian isolates. Acta Trop. 2008, 108: 58-63. 10.1016/j.actatropica.2008.08.011.
Tamura K, Dudley J, Nei M, Kumar S: MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007, 24: 1596-1599. 10.1093/molbev/msm092.
Librado P, Rozas J: DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009, 25: 1451-1452. 10.1093/bioinformatics/btp187.
Nei M, Gojobori T: Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986, 3: 418-426.
Tajima F: Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989, 123: 585-595.
Fu YX, Li WH: Statistical tests of neutrality of mutations. Genetics. 1993, 133: 693-709.
Ellis RD, Sagara I, Doumbo O, Wu Y: Blood stage vaccines for Plasmodium falciparum: current status and the way forward. Hum Vaccin. 2010, 6: 627-634. 10.4161/hv.6.8.11446.
Malkin E, Long CA, Stowers AW, Zou L, Singh S, MacDonald NJ, Narum DL, Miles AP, Orcutt AC, Muratova O, Moretz SE, Zhou H, Diouf A, Fay M, Tierney E, Leese P, Mahanty S, Miller LH, Saul A, Martin LB: Phase 1 study of two merozoite surface protein 1 (MSP 142) vaccines for Plasmodium falciparum malaria. PLoS Clin Trial. 2007, 2: e12-10.1371/journal.pctr.0020012.
Huaman MC, Martin LB, Malkin E, Narum DL, Miller LH, Mahanty S, Long CA: Ex vivo cytokine and memory T cell responses to the 42-kDa fragment of Plasmodium falciparum merozoite surface protein-1 in vaccinated volunteers. J Immunol. 2008, 180: 1451-1461.
Ogutu BR, Apollo OJ, McKinney D, Okoth W, Siangla J, Dubovsky F, Tucker K, Waitumbi JN, Diggs C, Wittes J, Malkin E, Leach A, Soisson LA, Milman JB, Otieno L, Holland CA, Polhemus M, Remich SA, Ockenhouse CF, Cohen J, Ballou WR, Martin SK, Angov E, Stewart VA, Lyon JA, Heppner DG, Withers MR, MSP-1 Malaria Vaccine Working Group: Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in western Kenya. PLoS One. 2009, 4: e4708-10.1371/journal.pone.0004708.
Pasay MC, Cheng Q, Rzepczyk C, Saul A: Dimorphism of the C-terminus of the Plasmodium vivax merozoite surface protein-1. Mol Biochem Parasitol. 1995, 70: 217-219. 10.1016/0166-6851(95)00015-S.
Soares IS, Barnwell JW, Ferreira MU, Gomes Da Cunha M, Laurino JP, Laurino JP, Castilho BA, Rodrigues MM: A Plasmodium vivax vaccine candidate displays limited allele polymorphism, which does not restrict recognition by antibodies. Mol Med. 1999, 5: 459-470.
Putaporntip C, Jongwutiwes S, Seethamchai S, Kanbara H, Tanabe K: Intragenic recombination in the 30 portion of the merozoite surface protein-1 gene of Plasmodium vivax. Mol Biochem Parasitol. 2000, 109: 111-119. 10.1016/S0166-6851(00)00238-3.
Putaporntip C, Jongwutiwes S, Sakihama N, Ferreira MU, Kho WG, Kaneko A, Kanbara H, Hattori T, Tanabe K: Mosaic organization and heterogeneity in frequency of allelic recombination of the Plasmodium vivax merozoite surface protein-1 locus. Proc Natl Acad Sci USA. 2002, 99: 16348-16353. 10.1073/pnas.252348999.
Dias S, Longacre S, Escalante AA, Udagama-Randeniya PV: Genetic diversity and recombination at the C-terminal fragment of the merozoite surface protein-1 of Plasmodium vivax (PvMSP-1) in Sri Lanka. Infect Genet Evol. 2011, 11: 145-156. 10.1016/j.meegid.2010.09.007.
Honma H, Kim JY, Palacpac NM, Mita T, Lee W, Horii T, Tanabe K: Recent increase of genetic diversity in Plasmodium vivax population in the Republic of Korea. Malar J. 2011, 10: 257-10.1186/1475-2875-10-257.
Pacheco MA, Poe AC, Collins WE, Lal AA, Tanabe K, Kariuki SK, Udhayakumar V, Escalante AA: A comparative study of the genetic diversity of the 42 kDa fragment of the merozoite surface protein-1 in Plasmodium falciparum and P. vivax. Infect Genet Evol. 2007, 7: 180-187. 10.1016/j.meegid.2006.08.002.
Escalante AA, Cornejo OE, Rojas A, Udhayakumar V, Lal AA: Assessing the effect of natural selection in malaria parasites. Trends Parasitol. 2004, 20: 388-395. 10.1016/j.pt.2004.06.002.
Chen Q, Schlichtherle M, Wahlgren M: Molecular aspects of severe malaria. Clin Microbiol Rev. 2000, 13: 439-450. 10.1128/CMR.13.3.439-450.2000.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (2011–0028135).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JMK, HLJ, YMK, and DHL performed all the experiments and analysed the sequence data. SUM performed sequence and phylogenetic analyses. JWP and TSK collected the blood samples. BKN and TSK designed the study and supervised the study process. BKN wrote the paper. TSK and WMS assisted in writing and editing the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kang, JM., Ju, HL., Kang, YM. et al. Genetic polymorphism and natural selection in the C-terminal 42 kDa region of merozoite surface protein-1 among Plasmodium vivax Korean isolates. Malar J 11, 206 (2012). https://doi.org/10.1186/1475-2875-11-206
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2875-11-206