Abstract
Background
Poor control of type 2 diabetes results in substantial long-term consequences. Studies of new diabetes treatments are rarely designed to assess mortality, complication rates and costs. We sought to estimate the long-term consequences of liraglutide and rosiglitazone both added to glimepiride.
Methods
To estimate long-term clinical and economic consequences, we used the CORE diabetes model, a validated cohort model that uses epidemiologic data from long-term clinical trials to simulate morbidity, mortality and costs of diabetes. Clinical data were extracted from the LEAD-1 trial evaluating two doses (1.2 mg and 1.8 mg) of a once daily GLP-1 analog liraglutide, or rosiglitazone 4 mg, on a background of glimepiride in type 2 diabetes. CORE was calibrated to the LEAD-1 baseline patient characteristics. Survival, cumulative incidence of cardiovascular, ocular and renal events and healthcare costs were estimated over three periods: 10, 20 and 30 years.
Results
In a hypothetical cohort of 5000 patients per treatment followed for 30 years, liraglutide 1.2 mg and 1.8 mg had higher survival rates compared to the group treated with rosiglitazone (15.0% and 16.0% vs. 12.6% after 30 years), and fewer cardiovascular, renal, and ocular events. Cardiovascular death rates after 30 years were 69.7%, 68.4% and 72.5%, for liraglutide 1.2 mg, 1.8 mg, and rosiglitazone, respectively. First and recurrent amputations were lower in the rosiglitazone group, probably due to a 'survival paradox' in the liraglutide arms (number of events: 565, 529, and 507, respectively). Overall cumulative costs per patient, were lower in both liraglutide groups compared to rosiglitazone (US$38,963, $39,239, and $40,401 for liraglutide 1.2 mg, 1.8 mg, and rosiglitazone, respectively), mainly driven by the costs of cardiovascular events in all groups.
Conclusion
Using data from LEAD-1 and epidemiologic evidence from the CORE diabetes model, projected rates of mortality, diabetes complications and healthcare costs over the long term favor liraglutide plus glimepiride over rosiglitazone plus glimepiride.
Trial registration
LEAD-1 NCT00318422; LEAD-2 NCT00318461; LEAD-3 NCT 00294723; LEAD-4 NCT00333151; LEAD-5 NCT00331851; LEAD-6 NCT00518882.
Similar content being viewed by others
Background
Type 2 diabetes is a chronic disease associated with insulin resistance and a progressive failure of the pancreatic beta cells. [1–3]. Type 2 diabetes is believed to account for about 90% of all cases of diabetes [4]. The American Diabetes Association (ADA) reported that, in the USA in 2007, 17.5 million people were diagnosed with diabetes. Estimates from the Centers for Disease Control and Prevention (CDC), which include persons with both diagnosed and undiagnosed diabetes, place the number of Americans with diabetes at 23.6 million [5]. The number of people with diagnosed diabetes is growing at a rate of 1 million per year [6], and is projected to reach over 48 million by 2050 [7]. The impact of diabetes on the US economy is alarming, with a total estimated cost of US$174 billion in 2007. A majority of the economic burden, $116 billion, can be attributed to expenditures for medical care [6]. A majority of these costs are for treatment of complications of the disease [8–11].
Large population-based studies have established that diabetes is associated with increased rates of cardiovascular morbidity and death [12–15]. Clinical trials have shown the benefits of intensive glucose lowering therapies to reduce the risk of microvascular disease [1], cardiovascular events and death [16, 17], or the combined risk of micro- and macrovascular events [18], in diabetic patients. Diabetes-related complications greatly diminish patients' health-related quality of life [19–21]. More recently, new evidence suggest that intensive treatment and extreme reductions in HbA1c below 6.5%, may have no effect, or (in one study) even increase the rate of cardiovascular events and death in high risk patients with diabetes [18, 22]. Thus, until this new evidence can be completely understood and supported by large longitudinal studies, it seem plausible that an intervention targeting reduction in glycemia levels to current guidelines, as well as improving concomitant risk factors, such as blood pressure, lipid levels and bodyweight might prevent and reduce the risk of micro- and macro-vascular complications. This intervention has recently been endorsed by a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association [23].
Liraglutide is a new once-daily human glucagon-like peptide (GLP)-1 analog. GLP-1 is a natural glucose-regulating peptide that enhances insulin secretion and reduces glucagon secretion, both in a glucose-dependent manner. Naturally occurring GLP-1 would require continuous infusion because of its short half-life, and so is impractical for routine therapeutic use; therefore, GLP-1 receptor agonists with an extended duration of action have been developed. The efficacy and safety of liraglutide treatment has been investigated both as monotherapy [24], and in combination with a number of currently approved therapies (metformin, sulfonylurea, thiazolidinediones) for type 2 diabetes in a large phase 3a trial program with extensive use of active comparators (the Liraglutide Effect and Action in Diabetes [LEAD 1–6] trial program) [25–30].
Our objective was to model the long-term outcomes of adding either liraglutide or rosiglitazone to glimepiride in patients with type 2 diabetes using data from the LEAD-1 clinical trial and a validated simulation model (CORE) of type 2 diabetes.
Methods
Background
Data on subject characteristics at baseline and treatment effects were extracted from the LEAD-1 study, which compared the efficacy and safety of three different doses of the once-daily human GLP-1 analog liraglutide (0.6 mg, 1.2 mg and 1.8 mg once daily, OD) added to glimepiride (2–4 mg OD), versus glimepiride alone (placebo) and rosiglitazone (4 mg) in combination with glimepiride, in 1041 type 2 diabetic patients. Patients were stratified based on previous oral antidiabetic drug (OAD) monotherapy or combination therapy and randomly allocated to any of the five arms and followed for 26 weeks. The results of the study showed that all doses of liraglutide plus glimepiride were associated with an improvement in HbA1c and fasting plasma glucose (FPG) levels compared to placebo, and that higher doses of liraglutide (1.2 mg and 1.8 mg) resulted in significantly greater reductions in HbA1c and greater bodyweight loss compared to rosiglitazone. Rates of all hypoglycemic events and nocturnal hypoglycemic events did not significantly differ across treatment arms. For the purposes of this analysis, we focused only on the two highest doses of liraglutide (1.2 mg and 1.8 mg) compared to rosiglitazone, all in combination with glimepiride. The 0.6 mg dose of liraglutide was omitted because it is mainly to be utilized as an escalation dose.
Model
The CORE Diabetes Model (CDM) has been described in detail previously [31–33]. This interactive computer simulation model has been used to determine the long-term health outcomes and economic consequences of interventions in type 1 or type 2 diabetes using surrogate clinical endpoints, such as HbA1c, systolic blood pressure, lipids, serum cholesterol, and body mass index (BMI) [34–37]. The model has a Markov structure combined with Monte Carlo simulation and the use of tracker variables, which allows for the development and progression of multiple complications in an individual patient over time, improving the limitations of traditional Markov models. The CDM predicts the progression of diabetes type 2 over long-term horizons using the most relevant published epidemiological and clinical data, including studies such as the United Kingdom Prospective Diabetes Study (UKPDS) [38]. The CDM includes 15 sub-models to simulate the most frequent diabetes complications, such as angina, cataracts, congestive heart failure, foot ulcer and amputation, hypoglycemia, ketoacidosis, lactic acidosis, macular edema, myocardial infarction, nephropathy, neuropathy, peripheral vascular disease, retinopathy, stroke, and non-specific mortality. These sub-models run in parallel to allow the hypothetical subjects to develop concomitant complications as appropriate. Cohorts can be defined using demographic characteristics in terms of age, gender, baseline risk factors and pre-existing complications. This model has been validated against 66 published studies, including external (third-order) validation of simulations of type 2 diabetes [32].
Interventions
Data on the treatment effects of liraglutide 1.2 mg and 1.8 mg or rosiglitazone added to glimepiride were extracted from the LEAD-1 study (Table 1).
Simulation cohorts
An analytic cohort of 5000 simulated patients was assembled using the treatment-specific baseline demographics and risk factors from the LEAD-1 study (Table 2) [18, 25, 39, 40]. The LEAD-1 study was conducted in 21 countries throughout Europe and Asia. Subjects had a mean duration of diagnosed diabetes of 7.9 years, were 56.1 years old, and had an average BMI of 29.9 kg/m2. This trial is described greater in detail by Marre et al. [25]. Treatment specific changes in glycemic control, blood pressure, BMI, and lipids were used to determine the incidence and time to onset of complications, predicted survival, and cost of complications.
Analysis
A US healthcare payer perspective was used for the cost analysis. Only direct medical costs of complications are included in the analysis and a discount rate of 3% annually was applied to costs beyond year 1. Table 3 displays the cost inputs used in the simulation [10, 41–45]. Drug costs were not applied for the three treatment groups, including the cost of glimepiride, as the price of liraglutide is unknown and applying only the rosiglitazone price would bias the findings in favor of liraglutide. Three analytic time horizons (10, 20 and 30 years) were selected for simulation. Longer-term horizons permit a more complete estimation of complication rates and predicted survival. For sensitivity analysis, the lower and upper limits of the 95% confidence intervals (CI) reported for the changes in HbA1c for each of the three treatment groups were used.
Results
Table 4 reports the predicted survival, cardiovascular mortality, event rates for complications and costs. These results are reported for the three treatment groups in LEAD-1 and for the three analytic time horizons.
As expected, predicted overall survival declined and complication rates increased for all three treatments as the analytic horizon was extended from 10 to 30 years. Overall survival in both liraglutide-treated groups was higher than in the rosiglitazone-treated group at all three time points. After 30 years the differences in survival were 2.4% and 3.6% higher in the group treated with liraglutide 1.2 mg and 1.8 mg respectively, compared to rosiglitazone. Complication rates were higher at all three time points for the rosiglitazone group compared to the two liraglutide groups.
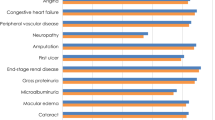
Applying the unit cost data in Table 3 to the event rate predictions from Table 4 produced an estimate of total costs of complications during the follow-up up to 30 years excluding the costs of liraglutide and rosiglitazone as the cost for the former is presently unknown since the medication is not presently FDA approved or marketed. Total cumulative costs per patient, defined as the management costs, costs of ongoing disease complications and costs of acute events related to the disease, during the 30 years of follow-up were $276 dollars lower in the group treated with liraglutide 1.2 mg compared to liraglutide 1.8 mg, and $1438 dollars lower compared to the rosiglitazone group (Table 4). As expected, the costs related to cardiovascular events were the main factor in all groups, representing 57.4% of the total costs per patient for liraglutide 1.2 mg, 58.5% for liraglutide 1.8 mg, and 59.1% for rosiglitazone. Management costs and costs related to the treatment of ulcers, amputations and neuropathies were lower in the rosiglitazone group (Figure 1).
We used the upper and lower limits of the 95% CI of the reported changes in HbA1c for each of the three treatment groups to evaluate the sensitivity of our findings to uncertainty in the treatment benefit. The absolute survival and event rates changed slightly across all three time periods: less than 5% in either direction depending on whether the simulations were run using the upper or lower bound of the CI. In neither case did the model produce predicted outcomes for the rosiglitazone group that were better than either liraglutide group.
Discussion
As there are no long-term follow-up studies of liraglutide or rosiglitazone measuring mortality as the primary endpoint, reliance must be placed on simulation models that have reproduced accurately the outcomes of long-term cohorts of patients with diabetes [46]. Our modelling study has shown that, in patients with type 2 diabetes treated with glimepiride, adding liraglutide 1.8 mg or 1.2 mg, compared to adding rosiglitazone 4 mg, may lead to improved survival and reductions in complications over a 10 to 30-year period. Additionally, the groups treated with liraglutide had a higher projected survival rate and lower cumulative medical costs, compared to rosiglitazone. These differences increase over time but are noticeable even after the first 10 years of follow-up. The lower number of complications related to ulcers and amputations in the rosiglitazone treatment group, compared to the two groups with liraglutide, may be explained in part by the lower survival time, as there is less chance of this type of complication with the shorter exposure time.
Other events, such as visual disorders, are influenced by additional factors, especially changes in blood pressure, hence the effects of the therapies on blood pressure should be considered and could support an explanation of these differences; in the LEAD-1 study, liraglutide 1.8 mg and 1.2 mg showed a higher reduction of systolic blood pressure compared to rosiglitazone (-2.81 mmHg, -2.56 mmHg, and -0.93 mmHg, respectively).
As expected, cardiovascular events were the leading cause of death across all groups; nevertheless the survival rate was relatively higher than that usually expected in these patients. This may be caused by a study effect, as the population from the LEAD-1 study, used for this simulation study, could be considered 'healthier' than the average type 2 diabetic patient after 8 years of diagnosis. Most patients were recruited in European and Asian countries, with mean near normal levels of total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglycerides. Further, a very low proportion of patients reported a previous cardiovascular event or renal impairment at baseline.
Cardiovascular disease was also the main contributor to the cumulative costs for all groups, and explains in great part the overall higher costs of the rosiglitazone group, despite the lower survival time of this group, and lower costs in management and complications related to neuropathy, ulcers, and amputations. The safety of liraglutide and rosiglitazone, specifically regarding minor and major hypoglycemic events, was also projected from the LEAD-1 study, probably underestimating the real effect in events and costs for a longer follow-up. Treatment switching, dose adjustments and adherence were not considered to have any effect in the simulation, thus providing an ideal scenario that may be more optimistic than actual practice, but the effect could be non-differential across treatments and therefore keep the trend in differences as reported here, probably with a higher number of events earlier in the follow-up.
We would like to point out a few additional limitations of the research. Although the model uses data from epidemiologic and clinical trials, some recently published studies have called into question the cardiovascular benefits of intensive glycemic control in patients with longer duration of diabetes and/or existing diabetes complications [18, 22]. It is important to note that, at the time of writing, these newer data have not yet been incorporated into the CORE diabetes model, so the potentially negative effect of more intensive treatments is not considered; only information from the ADVANCE trial was used as the reference for the current management of diabetic patients in the use of aspirin, statins, and angiotensin receptor blocker (ARB)/angiotensin-converting enzyme (ACE) inhibitors. The effect of rosiglitzaone on systolic blood pressure in LEAD-1 may be underestimated when compared to evidence from other trials [47]. One important caution when interpreting the results of the cumulative costs is that the costs of adding liraglutide or rosiglitazone to glimepiride treatment are not included because liraglutide is not on the market and the price is not known. More specific research will be required to determine the cost-effectiveness of the treatments.
In the sensitivity analysis, only changes in HbA1c were considered, using the lower and upper limits of the 95% CI for every treatment as this was the primary endpoint of the LEAD-1 study. Nevertheless, other significant changes in the study, that is, blood pressure, lipids and weight, could have been included, thereby increasing the uncertainty of estimates in the simulation but assessing a more comprehensive effect of these therapies.
Finally, the utility of diabetes models to predict life expectancy and other disease outcomes with precision is open to criticism. Models are imperfect instruments of real world outcomes. Nevertheless, attempts to correlate diabetes model predictions of outcomes with the results of long-term trials have been undertaken. These studies have shown that models can produce findings broadly consistent with long-trials under specified conditions [48].
Conclusion
This study represents one of the first uses of a disease simulation model to examine the long-term clinical effects of a GLP-1 by incorporating data from a head-to-head active comparator clinical trial. This study represents an important advance relative to previously published works, which were based on modelling data from placebo-controlled clinical trials [39]. Notably, the availability of head-to-head clinical trial data and the incorporation of active-comparator designs as part of the registration study program provide valuable additional therapeutic information for healthcare decision-makers during the immediate post-launch experience.
This study shows that in patients with type 2 diabetes treated with glimepiride, adding liraglutide 1.8 mg or 1.2 mg, compared to adding rosiglitazone 4 mg, may improve survival rates and reductions in complications over a 10- to 30-year period. The liraglutide 1.2 mg and 1.8 mg groups had higher projected survival rates and lower cumulative costs, compared to rosiglitazone 4 mg.
Improvements in the cardiovascular event rates are important as these events are the main contributor to death and increased cost of treating type 2 diabetes.
Authors' information
SDS and ACR are health economic researchers based at the University of Washington, Seattle, USA. CC work as senior health economist in Novo Nordisk Inc, USA. MH is principal scientist in health economic of Novo Nordisk A/S, Denmark. LB is a diabetologist working in clinical practice in USA.
References
UKPDS Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998, 352: 837-853. 10.1016/S0140-6736(98)07019-6.
Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G, ADOPT Study Group: Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006, 355: 2427-2443. 10.1056/NEJMoa066224.
Kasuga M: Insulin resistance and pancreatic beta cell failure. J Clin Invest. 2006, 116: 1756-1760. 10.1172/JCI29189.
World Health Organization: 2008, [http://www.who.int/mediacentre/factsheets/fs312/en/]
CDC National Center for Chronic Disease Prevention and Health Promotion: 2007 National Diabetes Fact Sheet. [http://www.cdc.gov/diabetes/pubs/factsheet07.htm]
American Diabetes Association: Economic costs of diabetes in the US in 2007. Diabetes Care. 2008, 31 (Suppl 1): S1-S2. 10.2337/dc08-S001.
Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ: Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006, 29: 2114-2116. 10.2337/dc06-1136.
Caro JJ, Ward AJ, O'Brien JA: Lifetime costs of complications resulting from type 2 diabetes in the US. Diabetes Care. 2002, 25: 476-481. 10.2337/diacare.25.3.476.
Nichols GA, Brown JB: The impact of cardiovascular disease on medical care costs in subjects with and without type 2 diabetes. Diabetes Care. 2002, 25: 482-486. 10.2337/diacare.25.3.482.
O'Brien JA, Patrick AR, Caro J: Estimates of direct medical costs for microvascular and macrovascular complications resulting from type 2 diabetes mellitus in the United States in 2000. Clin Ther. 2003, 25: 1017-1038. 10.1016/S0149-2918(03)80122-4.
Gilmer TP, O'Connor PJ, Rush WA, Crain AL, Whitebird RR, Hanson AM, Solberg LI: Predictors of health care costs in adults with diabetes. Diabetes Care. 2005, 28: 59-64. 10.2337/diacare.28.1.59.
Kannel WB, D'Agostino RB, Wilson PW, Belanger AJ, Gagnon DR: Diabetes, fibrinogen, and risk of cardiovascular disease: the Framingham experience. Am Heart J. 1990, 120: 672-676. 10.1016/0002-8703(90)90026-T.
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M: Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998, 339: 229-234. 10.1056/NEJM199807233390404.
Stamler J, Vaccaro O, Neaton JD, Wentworth D: Diabetes, other risk factors, and 12-year cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993, 16: 434-444. 10.2337/diacare.16.2.434.
Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A: Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000, 102: 1014-1019.
Gaede P, Lund-Andersen H, Parving HH, Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008, 358: 580-591. 10.1056/NEJMoa0706245.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008, 359: 1577-1589. 10.1056/NEJMoa0806470.
ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008, 358: 2560-2572. 10.1056/NEJMoa0802987.
Clarke P, Gray A, Holman R: Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. 2002, 22: 340-349. 10.1177/027298902400448902.
Lloyd A, Sawyer W, Hopkinson P: Impact of long-term complications on quality of life in patients with type 2 diabetes not using insulin. Value Health. 2001, 4: 392-400. 10.1046/j.1524-4733.2001.45029.x.
Huang ES, Brown SE, Ewigman BG, Foley EC, Meltzer DO: Patient perceptions of quality of life with diabetes-related complications and treatments. Diabetes Care. 2007, 30: 2478-2483. 10.2337/dc07-0499.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, the VADT Investigators: Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009, 360: 129-139. 10.1056/NEJMoa0808431.
Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS, American Diabetes Association; American College of Cardiology Foundation; American Heart Association: Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009, 32: 187-192. 10.2337/dc08-9026.
Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courreges J-P, Verhoeven R, Buganova I, Madsbad S: Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007, 30: 1608-1610. 10.2337/dc06-2593.
Marre M, Shaw J, Brandle M, Wan Bebakar W, Kamaruddin N, Strand J, Zdravkovic M, Le-Thi TD, Colagiuri S, on behalf of the LEAD-1 SU study group: Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009, 26 (3): 268-278. 10.1111/j.1464-5491.2009.02666.x.
Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, During M, Zdravkovic , Matthews D: Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin in type 2 diabetes. Diabetes Care. 2009, 32: 84-90. 10.2337/dc08-1355.
Garber A, Henry R, Ratner R, Garcia-Hernadez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovc M, Bode B, for the LEAD-3 (Mono) Study Group: Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009, 373: 473-481. 10.1016/S0140-6736(08)61246-5.
Zinman B, Gerich J, Buse J, Lewin A, Schwartz SL, Raskin P, Hale PM, Zdravkovic M, Blonde L: Effect of the GLP-1 analog liraglutide on glycemic control and weight reduction in patients on metformin and rosiglitazone: a randomized double-blind placebo-controlled trial [abstract]. Diabetologia. 2008, 51 (Suppl 1): S359.
Russell-Jones D, Vaag A, Schmitz O, Sethi B, Lalic NM, Antic S, Zdravkovic M, Ravn GM, Simo R: Significantly better glycaemic control/weight reduction with human GLP-1 analogue liraglutide, than with insulin glargine: all as add-on to metformin + sulphonylurea in type 2 diabetes [abstract]. Diabetologia. 2008, 51 (Suppl 1): S68.
Blonde L, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett J, Zychma M, Buse J: Liraglutide: superior glycemic control vs exenatide when added to metformin and/or SU in type 2 diabetes [abstract]. Can J Diabetes. 2008, 32 (Suppl): A107.
Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, Lammert M, Spinas GA: The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004, 20 (Suppl 1): S5-S26. 10.1185/030079904X1980.
Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, Lammert M, Spinas GA: Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004, 20 (Suppl 1): S27-S40. 10.1185/030079904X2006.
Brändle M, Herman WH: The CORE Diabetes Model. Curr Med Res Opin. 2004, 20 (Suppl 1): S1-S3. 10.1185/030079904X1962.
Palmer AJ, Roze S, Valentine WJ, Minshall ME, Hayes C, Oglesby A, Spinas GA: Impact of changes in HbA1c, lipids and blood pressure on long-term outcomes in type 2 diabetes patients: an analysis using the CORE Diabetes Model. Curr Med Res Opin. 2004, 20 (Suppl 1): S53-S58. 10.1185/030079903125002611.
Palmer AJ, Roze S, Valentine WJ, Minshall ME, Lammert M, Oglesby A, Hayes C, Spinas GA: What impact would pancreatic beta-cell preservation have on life expectancy, quality-adjusted life expectancy and costs of complications in patients with type 2 diabetes? A projection using the CORE Diabetes Model. Curr Med Res Opin. 2004, 20 (Suppl 1): S59-S66. 10.1185/030079904X2024.
Roze S, Valentine WJ, Zakrzewska KE, Palmer AJ: Health-economic comparison of continuous subcutaneous insulin infusion with multiple daily injection for the treatment of Type 1 diabetes in the UK. Diabet Med. 2005, 22: 1239-45. 10.1111/j.1464-5491.2005.01576.x.
Watkins JB, Minshall ME, Sullivan SD: Application of economic analyses in U.S. managed care formulary decisions: a private payer's experience. J Manag Care Pharm. 2006, 12: 726-35.
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective, observational study. BMJ. 2000, 321: 405-412. 10.1136/bmj.321.7258.405.
Saaddine JB, Cadwell B, Gregg EW, Engelgau MW, Vinicor F, Imperatore G, Narayan V: Improvements in Diabetes Processes of Care and Intermediate Outcomes: United States, 1988–2002. Ann Intern Med. 2006, 144: 465-474.
Minshall ME, Oglesby AK, Wintle ME, Valentine WJ, Roze S, Palmer AJ: Estimating the long-term cost-effectiveness of exenatide in the United States: an adjunctive treatment for type 2 diabetes mellitus. Value Health. 2008, 11: 22-33.
Tarn T, Smith M: Pharmacoeconomic Guidelines Around the World. ISPOR Connections. 2004, 1: 5-15.
Fleming T, Ed: 2006 Drug Topics Red Book. 2006, Thomson PDR, Montvale, NJ, USA, 11
Kantor J, Margolis DJ: Treatment options for diabetic neuropathic foot ulcers: A cost-effectiveness analysis. Dermatologic Surgery. 2001, 27: 347-351. 10.1046/j.1524-4725.2001.00280.x.
DRG Guidebook: A Comprehensive Resource to the DRG Classification System. 2001, St Anthony Press, 17
Shearer A, Scuffham P, Gordois A, Oglesby A: Predicted costs and outcomes from reduced vibration detection in people with diabetes in the U.S. Diabetes Care. 2003, 26: 2305-2310. 10.2337/diacare.26.8.2305.
American Diabetes Association Consensus Panel: Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004, 27: 2262-2265. 10.2337/diacare.27.9.2262.
Komajda M, Curtis P, Hanefeld M, Beck-Nielsen H, Pocock SJ, Zambanini A, Jones NP, Gomis R, Home PD, for The RECORD Study Group: Effect of the addition of rosiglitazone to metformin or sulfonylureas versus metformin/sulfonylurea combination therapy on ambulatory blood pressure in people with type 2 diabetes: A randomized controlled trial (the RECORD study). Cardiovascular Diabetology. 2008, 7: 10-10.1186/1475-2840-7-10.
The Mt Hood 4 Modeling Group: Computer Modeling of Diabetes and Its Complications. Diabetes Care. 2007, 30: 1638-1646. 10.2337/dc07-9919.
Acknowledgements
Novo Nordisk A/S Bagsvaerd, Denmark provided research funding for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
SDS has received research support from Novo Nordisk. He has received consulting fees from Novo Nordisk, Amylin and Novartis. RA-C worked for Merck Sharp & Dohme, Colombia to 2004, then for sanofi-aventis Latin America (Panama), until August 2007, and has acted as a consultant for sanofi-aventis Latin America thereafter. CC and MH are employees of Novo Nordisk. LB has acted as a consultant for, has attended speakers' bureaux for, and is a shareholder in Amylin Pharmaceuticals, Inc., is a share holder in and has attended speakers bureaux for Eli Lilly & Co.
Authors' contributions
All authors contributed equally to the design, analysis and interpretation of study findings. However, decisions regarding final analysis and interpretation of findings were the responsibility of the first author, SDS. All authors contributed to the writing process and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Sullivan, S.D., Alfonso-Cristancho, R., Conner, C. et al. Long-term outcomes in patients with type 2 diabetes receiving glimepiride combined with liraglutide or rosiglitazone. Cardiovasc Diabetol 8, 12 (2009). https://doi.org/10.1186/1475-2840-8-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-8-12