Abstract
Background
Previous reports have clearly indicated a significant relationship between hemoglobin (Hb) A1c change from one visit to the next and microvascular complications, especially nephropathy (albuminuria and albuminuric chronic kidney disease, CKD). In contrast, data on macrovascular disease are less clear. This study was aimed at examining the association of HbA1c variability with cardiovascular disease (CVD) in the large cohort of subjects with type 2 diabetes from the Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicenter Study.
Methods
Serial (3–5) HbA1c values obtained during the 2-year period preceding recruitment, including that obtained at the enrolment, were available from 8,290 subjects from 9 centers (out of 15,773 patients from 19 centers). Average HbA1c and HbA1c variability were calculated as the intra-individual mean (HbA1c-MEAN) and standard deviation (HbA1c-SD), respectively, of 4.52±0.76 values. Prevalent CVD, total and by vascular bed, was assessed from medical history by recording previous documented major acute events. Diabetic retinopathy (DR) was assessed by dilated fundoscopy. CKD was defined based on albuminuria, as measured by immunonephelometry or immunoturbidimetry, and estimated glomerular filtration rate, as calculated from serum creatinine.
Results
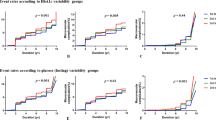
HbA1c-MEAN, but not HbA1c-SD, was significantly higher (P<0.0001) in subjects with history of any CVD (n. 2,133, 25.7%) than in those without CVD (n. 6,157, 74.3%). Median and interquartile range were 7.78 (7.04-8.56) and 7.49 (6.81-8.31), respectively, for HbA1c-MEAN, and 0.47 (0.29-0.75) and 0.46 (0.28-0.73), respectively, for HbA1c-SD. Logistic regression analyses showed that HbA1c-MEAN, but not HbA1c-SD (and independent of it), was a significant correlate of any CVD. Similar findings were observed in subjects with versus those without any coronary or cerebrovascular event or myocardial infarction. Conversely, none of these measures were associated with stroke, whereas both correlated with any lower limb vascular event and HbA1c-SD alone with ulceration/gangrene. All these associations were independent of known CVD risk factors and microvascular complications (DR and CKD).
Conclusions
In patients with type 2 diabetes, HbA1c variability has not a major impact on macrovascular complications, at variance with average HbA1c, an opposite finding as compared with microvascular disease, and particularly nephropathy.
Trial registration
ClinicalTrials.Gov NCT00715481
Similar content being viewed by others
Background
Glycemic exposure, i.e. the extent and duration of hyperglycemia, is a major risk factor for both micro and macrovascular complications of diabetes. In fact, data from subjects with type 1 and type 2 diabetes from the Diabetes Control and Complications Trial (DCCT) and the UK Prospective Diabetes Study, respectively, have shown a strong association between exposure to hyperglycemia and diabetic complications [1, 2] as well as a significant benefit of intensive glycemic control on the development and progression of microvascular disease [3, 4]. In addition, post-trial, prolonged follow-up has uncovered a beneficial effect of prior intensive regimen on cardiovascular disease (CVD) in both studies [5, 6]. Though difference in average hemoglobin (Hb) A1c was found to explain virtually all of the difference in risk of complications between the intensive and conventional groups in the DCCT [7], it is still under debate whether or not variability of glycemic control has also a role.
Glycemic variability comprises “glucose variability” and “HbA1c variability”. Glucose variability relates to within-day fluctuations of glycemia, especially as a consequence of post-prandial hyperglycemia [8], which may eventually reflect in HbA1c levels above the normal range. Conversely, HbA1c variability relates to changes in glycemia over longer periods of time, which result in change in HbA1c from one visit to the next [9]. Several observational studies have shown that post-prandial glucose, even in the range of impaired glucose tolerance, is a strong independent predictor of CVD [10]. However, three retrospective analyses of the DCCT found that within-day glucose variability does not contribute to the development of microvascular complications [11–13]. Moreover, a prospective study specifically addressing this issue showed no effect of either post-prandial glucose [14] or glucose variability [15] on CVD events, though a post hoc subgroup analysis demonstrated lower CVD risk in older patients targeting postprandial versus fasting/pre-meal glycemia [16] and continuous glucose monitoring showed that intraday glycemic variability is associated with the presence and severity of arterial lesions in subjects with type 2 diabetes undergoing coronary angiography for chest pain [17].
Conversely, robust evidence links HbA1c variability to the risk of microvascular complications in both type 1 and type 2 diabetes. In fact, retrospective analyses of the DCCT [18] and the Finnish Diabetic Nephropathy (FinnDiane) Study [19] have shown that change in HbA1c from one visit to the next is an independent risk factor for the development of both diabetic retinopathy (DR) and nephropathy (DN) in individuals with type 1 diabetes. In addition, HbA1c variability was shown to be an independent variable that added to the effect of HbA1c on the risk of microalbuminuria in adolescent patients with type 1 diabetes from the Oxford Regional Prospective Study and the Nephropathy Family Study [20]. Very recently, two prospective cohort studies from Japan and Taiwan, the Tsukuba Kawai Diabetes Registry 2 [21] and the Diabetes Management through an Integrated Delivery System project [22], have shown that HbA1c variability is associated with microalbuminuria in patients with type 2 diabetes even after adjustment for known predictors of albuminuria. These data are consistent with our recent report from the Renal Insufficiency And Cardiovascular Events (RIACE) Italian Multicenter Study that HbA1c variability is an independent correlate of albuminuria and the albuminuric phenotypes of chronic kidney disease (CKD), but not of non-albuminuric CKD and DR [23].
However, data are less stringent with respect to the association of HbA1c variability with CVD. In type 1 diabetes, HbA1c variability was predictive of incident CVD events in the FinnDiane Study [19], whereas HbA1c variation (not variability) over time showed a stronger association with coronary artery disease than baseline values in the Pittsburgh Epidemiology of Diabetes Complications Study [24]. Recently, a prospective analysis of the Hong Kong Diabetes Registry has shown that HbA1c variability is associated with incident CKD and CVD, independent of average HbA1c and other confounding variables [25].
This analysis was aimed at assessing the independent association of HbA1c variability with CVD, either total or by vascular bed, in the large cohort of Caucasian subjects with type 2 diabetes from the RIACE Italian Multicenter Study.
Methods
Study cohort
We used the data collected at the baseline visit for the RIACE Italian Multicenter Study (registered with ClinicalTrials.gov, NCT00715481; URL http://www.clinicaltrials.gov/ct2/show/NCT00715481), an observational, prospective cohort study on the impact of estimated glomerular filtration rate (eGFR) on morbidity and mortality from CVD in type 2 diabetes.
The RIACE cohort consisted of 15,933 Caucasian patients with type 2 diabetes (defined by the American Diabetes Association criteria), attending consecutively 19 hospital-based Diabetes Clinics of the National Health Service throughout Italy (see Additional file 1) in years 2007–2008. Exclusion criteria were dialysis or renal transplantation. The study protocol was reviewed by the locally appointed ethics boards. The quality and completeness of data were controlled and 160 patients were excluded due to missing or implausible values and the remaining 15,773 subjects were subsequently analyzed. Multiple HbA1c values (3 to 5, mean±SD: 4.52±0.76) serially measured at intervals of 6–12 months during the 2-year period preceding the enrolment were available from 9 centers for a total 8,290 patients (52.6% of the entire cohort). CVD risk factors and complications were determined as part of the baseline assessment using standardized protocols across study centers.
CVD risk factors
All patients underwent a structured interview in order to collect the following information: age, smoking status, known diabetes duration, current glucose-, blood pressure (BP)- and lipid-lowering treatments and anti-platelet and anti-coagulant therapy, with indication of the class of drug. Weight and height were assessed with calculation of body mass index (BMI); BP was measured with a sphygmomanometer after a 5 min of rest. Triglycerides, total and HDL cholesterol were determined by standard analytical methods; LDL cholesterol was calculated by the Friedwald formula. Hypertension was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg and/or anti-hypertensive treatment. Dyslipidemia was defined as high (≥100 mg/dl) LDL cholesterol and/or lipid-lowering treatment.
Complications
Prevalent CVD at baseline was assessed from medical history by recording previous documented major acute CVD events, including acute myocardial infarction (AMI), stroke, foot ulcer or gangrene, amputation, coronary, carotid, and lower limb revascularization, and surgery for aortic aneurysm. CVD events were adjudicated based on hospital discharge records or specialist visits by an ad hoc committee in each center [26].
The presence of CKD at baseline was assessed by albuminuria and serum creatinine. As previously reported in detail [27], albumin excretion rate (AER) was obtained from timed (24 hour) urine collections or calculated from albumin/creatinine ratio in early-morning, first-voided urine samples, in the absence of symptoms and signs of urinary tract infection or other interfering clinical conditions. Albuminuria was measured in one-to-three fresh urine samples for each patient by immunonephelometry or immunoturbidimetry and, in case of multiple measurements, the geometric mean was used for analysis. In subjects with multiple measurements (4,062 with at least two and 2,310 with three values), concordance rate between the first value and the geometric mean was >90% for all classes of albuminuria [27]. Patients were then assigned to one of the following categories of albuminuria (mg/24 hours): normoalbuminuria (AER <30), microalbuminuria (AER 30–299), or macroalbuminuria (AER ≥300). Serum (and urine) creatinine was measured by the modified Jaffe method. One to three measurements were obtained for each patients and eGFR was calculated by the four-variable Modification of Diet in Renal Disease (MDRD) Study equation, using the mean serum creatinine value in case of multiple measures, as reported in previous publications [27–29]. Patients were then assigned to one of the following categories of eGFR (mL/min/1.73 m2): 1 (≥90); 2 (60–89); 3 (30–59); 4 (15–29); and 5 (<15). Finally, subjects were classified as having no CKD or CKD stages 1–5, based on the presence or absence of micro or macroalbuminuria and the value of eGFR calculated by the MDRD Study equation, according to the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative [30]. Patients assigned to CKD stages (and GFR classes) 4 and 5 were pooled together. As previously reported [29], CKD patients were further classified as having one of the following CKD phenotypes: albuminuria alone (stages 1–2 CKD), reduced eGFR alone (stage ≥3 CKD without albuminuria), or both (stage ≥3 CKD with albuminuria).
The presence of DR at baseline was assessed by dilated fundoscopy. In each center, an expert ophthalmologist was asked to fill in a standardized report format and to classify DR into absent, mild, moderate or severe non-proliferative DR, proliferative DR, and maculopathy, according to the Global Diabetic Retinopathy Project Group [31]. Patients were classified based on the actual fundus appearance or the retinal disease condition which had eventually required a previous photocoagulation or surgical treatment. Based on the worst eye, patients with non-proliferative DR of mild or moderate degree were classified as having non-advanced DR, whereas those with severe non-proliferative DR or pre-proliferative DR, proliferative DR, maculopathy, or blindness were grouped into the advanced DR category [32].
HbA1c variability
HbA1c was measured in each center by high performance liquid chromatography using DCCT-aligned methods. Average HbA1c and HbA1c variability were calculated for each patient as the intra-individual mean (HbA1c-MEAN) and SD (HbA1c-SD), respectively, for HbA1c values obtained during the 2-year period preceding recruitment, including that obtained at the enrolment. The inter-individual difference in the number of HbA1c assessments (a few values would make the SD apparently greater than many values) was adjusted according to the formula: adj-HbA1c-SD = SD/√[n/(n-1)] [9, 11]. Furthermore, as a normalized measure of variability, the coefficient of variation of HbA1c (HbA1c-CV) was calculated as the ratio of HbA1c-SD and HbA1c-MEAN to correct for larger SD due to higher absolute values of HbA1c-MEAN [19].
Statistical analysis
Data are expressed as median (interquartile range, IQR) and/or mean±SD, for continuous variables, and number of cases and percentage for categorical variables. Patients were stratified by previous major acute CVD event(s). Continuous variables were compared by Student’s t test or one-way ANOVA, for normally distributed variables, and by Mann–Whitney U test or Kruskall-Wallis test, in case of variables with a skewed distribution. Pearson chi-square was applied to categorical variables.
Logistic regression analyses with backward variable selection (probability for removal >0.10) were performed to assess whether increments in HbA1c-MEAN (Model 1), increments of HbA1c-MEAN and HbA1c-SD (Model 2), and quartiles of both variables (Model 3) are independent correlates of CVD complications as compared with no CVD. Covariates were, age, BMI, gender, known disease duration, smoking habits, triglycerides, HDL cholesterol, hypertension, dyslipidemia, albuminuria and eGFR categories, CKD phenotype and DR stage, specific treatments and either eGFR and albuminuria categories if DR was the dependent variable, or DR categories if renal parameters were the dependent variable. Results of these analyses were expressed as odd ratios (ORs) with their 95% confidence intervals (CIs). Logistic regression analyses s were repeated entering adj-HbA1c-SD (or HbA1c-CV) instead of HbA1c-SD as a measure of HbA1c variability.
All p values were two-sided, and a p value of less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Patients’ characteristics
As previously reported [23], participants included in this analysis, i.e. those having multiple (3–5) HbA1c values, had a median (IQR) age and duration of diabetes at enrolment of 68 (61–74) and 14 (7–23) years, respectively, and a male/female ratio of 57/43. Likely due to longer disease duration, these subjects showed a worse CVD risk profile and a higher prevalence of any CVD event and were more frequently on glucose-, lipid- and BP-lowering drug treatment, than those excluded from the analysis due to unavailability of serial HbA1c measurements. HbA1c-MEAN of participants was 7.57% (6.86-8.38), HbA1c-SD was 0.46 (0.29-0.74) and adj-HbA1c-SD was 0.40 (0.25-0.65). Both variability measures, i.e. HbA1c-SD and adj-HbA1c-SD, were closely related to HbA1c-MEAN and between each other.
One or more major acute CVD events were adjudicated in 2,133 of the 8,290 patients analyzed (25.7%). Clinical characteristics of subjects with or without prior CVD event(s) are shown in Tables 1 and 2. As compared with individuals without CVD, patients with prior event(s) were older, predominantly male, and had a longer diabetes duration, a worse metabolic control, a significantly higher rate of insulin treatment, and a slightly but significantly higher number of HbA1c measures (4.63±0.70 versus 4.48±0.78, P<0.0001). Concerning the other CVD risk factors, these patients were more frequently treated for dyslipidemia and/or hypertension and had higher triglycerides and lower HDL cholesterol, but also lower total, LDL and non-HDL cholesterol and (marginally) BP levels. Finally, subjects with prior CVD event(s) showed a higher prevalence of DR, albuminuria, reduced eGFR and CKD.
Average HbA1c and HbA1c variability
As previously reported [23], increasing HbA1c-MEAN and HbA1c-SD (or HbA1c-CV) were associated with longer and shorter diabetes duration, respectively, whereas both measures correlated with a more adverse CVD risk profile, more severe complications, and more intensive treatment, with a few differences.
Values for current HbA1c, HbA1c-MEAN, HbA1c-SD, HbA1c-CV and Adj-HbA1c-SD in subjects with or without any major acute CVD event are given in Tables 1 (total) and 3 (by vascular bed, i.e. coronary, carotid and lower limb). As compared with men, women showed significantly higher values of current HbA1c (7.75±1.40 versus 7.58±1.32, P<0.0001), HbA1c-MEAN (7.80±1.24 versus 7.62±1.17, P<0.0001) and lower levels of HbA1c-SD (0.59±0.49 versus 0.60±0.47, P=0.049), HbA1c-CV (7.39±5.50 versus 7.65±5.38, P=0.001), and, non-significantly, Adj-HbA1c-SD (0.52±0.43 versus 0.52±0.40, P=0.060), as compared with men. When stratified by presence or absence of any CVD event, total and by vascular bed, data in men and women were almost identical to those from the whole cohort presented in Table 3 (Additional file 2: Tables S1-S2). Current HbA1c and HbA1c-MEAN were higher in patients with history of any CVD, either total or by vascular bed, whereas HbA1c-SD and Adj-HbA1c-SD were significantly increased in subjects with AMI, any lower limb vascular event and ulceration/gangrene and HbA1c-CV only in those with this latter event. Current HbA1c and HbA1c-MEAN values, but not measures of HbA1c variability, increased with the number of major acute CVD events and with the number of vascular beds affected (P<0.0001). However, while these values did not change significantly with increasing number of events (from 1 to ≥5), they were higher in subjects with 2 or 3 vascular beds affected than in those with involvement of only one site.
Multiple logistic regression models
We used logistic models as multivariate regression analyses because of several potential confounding factors for the association between HbA1c variability and prevalence of macrovascular complications. Even after adjusting for CVD risk factors and complications, HbA1c-MEAN, but not HbA1c-SD (and independent of it in Models 2 and 3) was a significant correlate of any CVD (Table 4).
When CVD was examined by vascular bed, similar findings were observed in subjects with versus those without any coronary event (AMI and/or coronary revascularization) or any cerebrovascular event (stroke and/or carotid revascularization) in all 3 models (Tables 5 and 6) and in those with versus without AMI in Model 3 only (Table 5). Conversely, none of these measures were associated with stroke (Table 6), whereas both correlated with any lower limb vascular event (ulceration/gangrene and/or lower limb revascularization; Hb-A1c-SD only in Model 2) and HbA1c-SD alone with ulceration/gangrene (Table 7).
In all models, expressing HbA1c variability as HbA1c-CV or adj-HbA1c-SD instead of HbA1c-SD did not change the results.
Discussion
Recently, microvascular complications were shown to be predicted by HbA1c variability from one visit to the next, independently of average HbA1c and known risk factors for microangiopathy, both in type 1 [18–20] and type 2 [21–23] diabetes. Less clear is the relation of HbA1c variability with CVD. This cross-sectional analysis did not show a significant association of CVD with HbA1c variability, whereas events were independently associated with average HbA1c. However, we did also analyze acute major CVD events occurring in different vascular beds and found significant differences in their relation with HbA1c variability versus average HbA1c.
The correlation of average HbA1c with CVD is consistent with data from large trials [5, 6]; this correlation was observed in both sexes, though women had higher average HbA1c values than men, in keeping with previous reports [33, 34]. Conversely, the lack of association of HbA1c variability with total CVD events (as well as with any coronary or cerebrovascular events, AMI or stroke) is at variance with results of previous longitudinal studies in Finnish individuals with type 1 diabetes [19] and subjects with type 2 diabetes from the Hong Kong Diabetes Registry [25]. Though this discrepancy might be explained by differences in type of diabetes or ethnicity, it is more likely attributable to the much lower HbA1c variability observed in our cohort, as compared with these previous reports. In fact, HbA1c-SD values were 0.47 and 0.46 in subjects without and with CVD, respectively, versus 0.79 and 0.87 in the type 1 Finnish cohort [19] and 1.1 and 1.4 in the type 2 Chinese cohort [25]. Conversely, HbA1c-MEAN values in our cohort (7.5 and 7.8, respectively) were similar to those found in subjects from the Hong Kong Diabetes Registry (7.4 and 7.8, respectively) [25]. Thus, it might be hypothesized that the contribution of small changes in HbA1c values from one visit to the next is negligible and that the impact of average HbA1c would be predominant under these conditions, with values above 7.5% conferring an increased risk. In this view, the results of our study may not be in contrast with those of the above-mentioned reports [19, 25]; however, if we consider that such a small variability of HbA1c was significantly associated with CKD (especially albuminuric), but not DR [23], we might conclude that DN is the most sensitive complication to changes in HbA1c, whereas CVD and DR are influenced by wider fluctuations of this parameter over time.
The diverging correlation of acute major CVD events occurring in different vascular beds with HbA1c variability versus average HbA1c might reflects distinct characteristics of vascular disease at these levels. In particular, multiple mechanisms contribute to various extent to the development of ulceration/gangrene, including macroangiopathy, but also microvascular disease and neuropathy. This might explain the fact that both average Hb-A1c and Hb-A1c variability were associated with lower limb vascular event(s) (including also lower limb revascularization) and particularly the correlation of Hb-A1c variability, but not average Hb-A1c, with ulceration/gangrene alone. This finding is also consistent with our previous report that the independent association of reduced eGFR with coronary events is stronger than that with peripheral events, which are related predominantly with the albuminuric CKD phenotypes reflecting predominantly microvascular disease [26].
Strengths of this study include the large size of the cohort, the completeness of data, the analysis of a contemporary dataset, the adjustment for treatments, and, as mentioned above, the analysis by vascular bed. The main limitation is the cross-sectional design for the assessment of prevalent CVD, which did not allow to examine the effect of HbA1c.variability on the development of macrovascular complications in uncomplicated individuals, as in the studies of Waden et al. [19] and Luk et al. [25]. Another limitation might be that the RIACE participants who had serial (3–5) HbA1c measures differed significantly (i.e. longer diabetes duration, worse CVD risk profile, higher prevalence of any CVD event, and higher rate of treatment) from those who did not and were therefore excluded from this analysis. Thus, though virtually all subjects from the 9 centers which made available these data had more than two HbA1c measures, independently of their HbA1c variability, we cannot rule out a selection bias. Other possible limitations concerning HbA1c values are that they were performed in each center as a part of the patient’s standard care with no pre-specified intervals between HbA1c measurements, and that the number of measures per individual varied from 3 to 5. However, non-centralized measurements did not affect intra-individual variability, intervals between measurements ranged from 6 to 9 months, and adj-HbA1c-SD was used to account for difference in the number of measures. Furthermore, though the number of measurements was not as large as in the study of Sugawara et al. [21] and the period analyzed was not as long as in the study of Hsu et al. [22], re-analyses of these surveys showed that using 3-monthly (i.e. 4 to 5) HbA1c measurements [21] or a series of 2-year HbA1c values [22], as in our study, did not change the results. Finally, potential limitations concerning non-centralized assessment of DN and DR have been addressed in previous RIACE reports [27–29, 32].
In conclusion, in patients with type 2 diabetes, HbA1c variability has not a great impact on macrovascular complications, at variance with average HbA1c, an opposite finding as compared with microvascular disease, and particularly DN and especially albuminuric CKD phenotypes.
Abbreviations
- DCCT:
-
Diabetes Control and Complications Trial
- CVD:
-
Cardiovascular disease
- HbA1c:
-
Hemoglobin A1c
- FinnDiane:
-
Finnish Diabetic Nephropathy
- DR:
-
Diabetic retinopathy
- DN:
-
Diabetic nephropathy
- RIACE:
-
Renal Insufficiency And Cardiovascular Events
- CKD:
-
Chronic kidney disease
- eGFR:
-
Estimated glomerular filtration rate
- BP:
-
Blood pressure
- BMI:
-
Body mass INDEX
- AMI:
-
Acute myocardial infarction
- AER:
-
Albumin excretion rate
- MDRD:
-
Modification of Diet in Renal Disease
- HbA1c-MEAN:
-
Average HbA1c
- HbA1c-SD:
-
HbA1c variability
- adj-HbA1c-SD:
-
Adjusted HbA1c-SD
- HbA1c-CV:
-
Coefficient of variation of HbA1c
- IQR:
-
Interquartile range
- ORs:
-
Odd ratios
- CIs:
-
Confidence intervals.
References
The DCCT Research Group: The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996, 45: 1289-1298.
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR: Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000, 321: 405-412. 10.1136/bmj.321.7258.405.
DCCT Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993, 329: 977-986.
UKPDS The Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998, 352: 837-853.
The DCCT/EDIC Study Research Group: Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005, 353: 2643-2653.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008, 359: 1577-1589. 10.1056/NEJMoa0806470.
Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN for the DCCT/EDIC Research Group: Effect of glycemic exposure on the risk of microvascular complications in the Diabetes Control and Complications Trial—Revisited. Diabetes. 2008, 57: 995-1001. 10.2337/db07-1618.
Home PD: Contributions of basal and post-prandial hyperglycaemia to micro- and macrovascular complications in people with type 2 diabetes. Curr Med Res Opin. 2005, 21: 989-998. 10.1185/030079905X49662.
Kilpatrick ES: The rise and fall of HbA1c as a risk marker for diabetes complications. Diabetologia. 2012, 55: 2089-2091. 10.1007/s00125-012-2610-5.
Standl E, Schnell O, Ceriello A: Postprandial hyperglycemia and glycemic variability. Diabetes Care. 2011, 34 (Suppl 2): S120-S127.
Kilpatrick ES, Rigby AS, Atkin SL: The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006, 29: 1486-1490. 10.2337/dc06-0293.
Kilpatrick ES, Rigby AS, Atkin SL: Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care. 2009, 32: 1901-1903. 10.2337/dc09-0109.
Siegelaar SE, Kilpatrick ES, Rigby AS, Atkin SL, Hoekstra JB, Devries JH: Glucose variability does not contribute to the development of peripheral and autonomic neuropathy in type 1 diabetes: data from the DCCT. Diabetologia. 2009, 52: 2229-2232. 10.1007/s00125-009-1473-x.
Raz I, Wilson PW, Strojek K, Kowalska I, Bozikov V, Gitt AK, Jermendy G, Campaigne BN, Kerr L, Milicevic Z, Jacober SJ: Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care. 2009, 32: 381-386. 10.2337/dc08-1671.
Siegelaar SE, Kerr L, Jacober SJ, Devries JH: A decrease in glucose variability does not reduce cardiovascular event rates in type 2 diabetic patients after acute myocardial infarction: a reanalysis of the HEART2D study. Diabetes Care. 2011, 34: 855-857. 10.2337/dc10-1684.
Raz I, Ceriello A, Wilson PW, Battioui C, Su EW, Kerr L, Jones CA, Milicevic Z, Jacober SJ: Post hoc subgroup analysis of the HEART2D trial demonstrates lower cardiovascular risk in older patients targeting postprandial versus fasting/premeal glycemia. Diabetes Care. 2011, 34: 1511-1513. 10.2337/dc10-2375.
Su G, Mi S, Tao H, Li Z, Yang H, Zheng H, Zhou Y, Ma C: Association of glycemic variability and the presence and severity of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2011, 10: 19-10.1186/1475-2840-10-19.
Kilpatrick ES, Rigby AS, Atkin SL: A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care. 2008, 31: 2198-202. 10.2337/dc08-0864.
Wadén J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH, Finnish Diabetic Nephropathy Study Group: A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. 2009, 58: 2649-2655. 10.2337/db09-0693.
Marcovecchio ML, Dalton RN, Chiarelli F, Dunger DB: A1C variability as an independent risk factor for microalbuminuria in young people with type 1 diabetes. Diabetes Care. 2011, 34: 1011-1013. 10.2337/dc10-2028.
Sugawara A, Kawai K, Motohashi S, Saito K, Kodama S, Yachi Y, Hirasawa R, Shimano H, Yamazaki K, Sone H: HbA(1c) variability and the development of microalbuminuria in type 2 diabetes: Tsukuba Kawai Diabetes Registry 2. Diabetologia. 2012, 55: 2128-2131. 10.1007/s00125-012-2572-7.
Hsu CC, Chang HY, Huang MC, Hwang SJ, Yang YC, Lee YS, Shin SJ, Tai TY: HbA(1c) variability is associated with microalbuminuria development in type 2 diabetes: a 7-year prospective cohort study. Diabetologia. 2012, 55: 3163-3172. 10.1007/s00125-012-2700-4.
Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Morano S, Cavalot F, Lamacchia O, Laviola L, Nicolucci A, Pugliese G, Renal Insufficiency And Cardiovascular Events (RIACE) Study Group: HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: The Renal Insufficiency And Cardiovascular Events (RIACE) Italian Multicenter Study. Diabetes Care. 2013, epub Mar 14
Prince CT, Becker DJ, Costacou T, Miller RG, Orchard TJ: Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes mellitus: findings from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC). Diabetologia. 2007, 50: 2280-2288. 10.1007/s00125-007-0797-7.
Luk AO, Ma RC, Lau ES, Yang X, Lau WW, Yu LW, Chow FC, Chan JC, So WY: Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: Prospective analysis of the Hong Kong Diabetes Registry. Diabetes Metab Res Rev. 2013, e-pub Mar 6
Solini A, Penno G, Bonora E, Fondelli C, Orsi E, Arosio M, Trevisan R, Vedovato M, Cignarelli M, Andreozzi F, Nicolucci A, Pugliese G, Renal Insufficiency And Cardiovascular Events (RIACE) Study Group: Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian Multicenter Study. Diabetes Care. 2012, 35: 143-149. 10.2337/dc11-1380.
Pugliese G, Solini A, Fondelli C, Trevisan R, Vedovato M, Nicolucci A, Penno G, Renal Insufficiency And Cardiovascular Events (RIACE) Study Group: Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the Renal Insufficiency And Cardiovascular Events (RIACE) Study. Nephrol Dial Transpl. 2011, 26: 3950-3954. 10.1093/ndt/gfr140.
Pugliese G, Solini A, Bonora E, Orsi E, Zerbini G, Giorgino F, Cavalot F, Pontiroli AE, Baroni MG, Morano S, Nicolucci A, Penno G: The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation provides a better definition of cardiovascular burden associated with CKD than the Modification of Diet in Renal Disease (MDRD) Study formula in subjects with type 2 diabetes. Atherosclerosis. 2011, 218: 194-199. 10.1016/j.atherosclerosis.2011.04.035.
Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Trevisan R, Vedovato M, Gruden G, Cavalot F, Cignarelli M, Laviola L, Morano S, Nicolucci A, Pugliese G, Renal Insufficiency And Cardiovascular Events (RIACE) Study Group: Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens. 2011, 29: 1802-1809. 10.1097/HJH.0b013e3283495cd6.
National Kidney Foundation: KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002, 39: S1-S266.
Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT, Global Diabetic Retinopathy Project Group: Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003, 110: 1677-1682. 10.1016/S0161-6420(03)00475-5.
Penno G, Solini A, Zoppini G, Orsi E, Zerbini G, Trevisan R, Gruden G, Cavalot F, Laviola L, Morano S, Nicolucci A, Pugliese G, Renal Insufficiency And Cardiovascular Events (RIACE) Study Group: Rate and determinants of association between advanced retinopathy and chronic kidney disease in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2012, 35: 2317-2123. 10.2337/dc12-0628.
Martins RA, Jones JG, Cumming SP, Coelho e Silva MJ, Teixeira AM, Veríssimo MT: Glycated hemoglobin and associated risk factors in older adults. Cardiovasc Diabetol. 2012, 11: 13-10.1186/1475-2840-11-13.
Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Trevisan R, Vedovato M, Gruden G, Laviola L, Nicolucci A, Pugliese G, Renal Insufficiency And Cardiovascular Events (RIACE) Study Group: Gender differences in cardiovascular disease risk factors, treatments and complications in patients with type 2 diabetes: the RIACE Italian multicentre study. J Intern Med. 2013, 274: 176-191. 10.1111/joim.12073.
Acknowledgments
The Authors thank the RIACE Investigators for participating in this study (see the complete list as Additional file 2). This work was supported by the Research Foundation of the Italian Society of Diabetology (Fo.Ri.SID) and the Diabetes, Endocrinology and Metabolism (DEM) Foundation, and by unconditional grants from Eli-Lilly, Takeda, Chiesi Farmaceutici and Boehringer-Ingelheim. The sponsors had no role in design and conduct of the study; collection, management, and interpretation of the data; or preparation, review, and approval of the manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All Authors have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; have been involved in drafting the manuscript or revising it critically for important intellectual content; and have given final approval of the version to be published. All authors read and approved the final manuscript.
Electronic supplementary material
12933_2013_698_MOESM2_ESM.doc
Additional file 2: Table S1: HbA1c-values and measures of HbA1c-variability in female subjects (n. 3,564). Current HbA1c, HbA1c-MEAN, HbA1c-SD, HbA1c-CV, and Adj-HbA1c-SD values in patients with any major acute CVD event by vascular bed (coronary, carotid and lower limb) versus subjects without CVD. Table S2: HbA1c-values and measures of HbA1c-variability in male subjects (n. 4,726). Current HbA1c, HbA1c-MEAN, HbA1c-SD, HbA1c-CV, and Adj-HbA1c-SD values in patients with any major acute CVD event by vascular bed (coronary, carotid and lower limb) versus subjects without CVD. (DOC 66 KB)
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Penno, G., Solini, A., Zoppini, G. et al. Hemoglobin A1c variability as an independent correlate of cardiovascular disease in patients with type 2 diabetes: a cross-sectional analysis of the Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicenter Study. Cardiovasc Diabetol 12, 98 (2013). https://doi.org/10.1186/1475-2840-12-98
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2840-12-98