Abstract
Background
Ultraviolet A1 (340–400 nm, UVA1) phototherapy is highly effective in sclerotic lesions of systemic sclerosis (SSc). Histological evaluation of skin specimens obtained before and after UVA1 phototherapy revealed loosening of collagen bundles and the appearance of small collagen fibers. We have previously shown that UVA1 irradiation induced collagenase in vitro study by using SSc fibroblasts. The increased levels of mRNA and protein of decorin in SSc fibroblasts were reported. In this study, we focus on the lesional expression of small dermatan sulfate proteoglycan, decorin that has a role of binding to collagen and fibrillogenesis.
Case presentation
We employed immunohistochemical analysis of decorin before and after UVA1 phototherapy. The skin specimens from three patients who were effectively treated with UVA1 phototherapy were analysed. Monoclonal antibody 6B6 as the specific reactivity to decorin was used. The increased decorin was focally accumulated in the newly synthesized collagen fibers in the sclerotic lesion of SSc. After UVA1 phototherapy, decorin was decreased in upper to middle dermis, although decorin was slightly increased in papillary dermis.
Conclusions
These results suggest that decreased and normalized levels of accumulated decorin may relate to the efficacy of sclerotic lesions in UVA1 phototherapy.
Similar content being viewed by others
Background
Decorin is a leucine-rich proteoglycan (PG) with a protein core of 36 kDa and a glycosaminoglycan (GAG) chain attached to the N-terminus [1]. Decorin contributes to fibril stability of several collagen types in vivo, including types I, II, V, VI and XIV by binding through leucine-rich repeat regions or GAG chain [2], and influences cell adhesion by interacting with extracellular adhesive molecules such as fibronectin [3] and thrombospondin [4]. In some cells, decorin activates the epidermal growth factor receptor, thereby triggering a signaling cascade that leads to phosphorylation of mitogen-activated protein kinases, to the induction of p21, and to growth suppression [5]. Decorin also interacts with transforming growth factor (TGF)-β and modulates its activity and transfection of decorin gene prevent fibrosis in kidney [6], or it may increase TGF-β activity on osteoblasts [7].
SSc is a connective tissue disease characterized by fibrosis of the skin, subcutaneous tissue, and various internal organs. The most prominent pathological manifestation of the disease is an abnormal accumulation of extracellular matrix molecules, especially type I and III collagen [8, 9]. Decorin binds to type I collagen and regulates fibrillogenesis, and mRNA of decorin from fibroblast of patients with SSc were previously examined. Decorin mRNA with early stage SSc (less than 1 year duration of disease) was highly expressed than that of normal control [9].
SSc is an autoimmune disorder characterized by excessive deposition of collagen within the skin and other organs. SSc can severely affect the quality of life and may cause significant morbidity. Numerous treatments have been tried but with only limited success. UVA1 phototherapy has proven to be effective for atopic dermatitis [10], cutaneous T cell lymphoma [11], and urticaria pigmentosa [12] because it depletes skin-infiltrating T cells or mast cells. More recently, UVA1 has been shown to clear sclerosis plaques in patients with long-standing widespread morphea, resistant to previous treatments [13, 14] and to be effective for scleroderma in patients with SSc [15]. UVA1 is also effective to other sclerotic disorders such as localized scleroderma [16] and lichen sclerosus even with low dose regimen [17]. We have previously shown that UVA1 phototherapy treated skin lesions were markedly softened after 9–29 exposures in all the SSc patients studied [15]. Clinical improvement was associated with an increase in joint passive range of motion values, skin temperature, and cutaneous elasticity. Histological evaluation of skin specimens obtained before and after UVA1 phototherapy revealed loosening of collagen bundles and the appearance of small collagen fibers. Moreover, we have shown that UVA1 irradiation induced collagenase in vitro study by using SSc fibroblasts [[18] in press]. In this study, we analysed the skin specimen of previous study in which UVA1 phototherapy was highly effective to examine a small dermatan sulfate proteoglycan, decorin that has a role of binding to collagen and fibrillogenesis.
Case Presentation
Patients
Two diffuse type (2 female) and one limited type (1 female) patients with SSc were treated with UVA1 phototherapy after informed consent had been obtained. The patients' profiles are summarized in detail in Table 1 (see additional file 1). Three patients enrolled in this study were identical to those of the previous report [15].
UVA1 Phototherapy
For UVA1 irradiation, a partial body UVA1 Sellamed 2000 System Dr Sellmaier (Sellas, Gevelsberg, Germany) irradiation device was used as previously described [15]. The emission was filtered with a UVA1 filter (Sellas) and an infrared absorbing filter UG1 (Schott, Mainz, Germany) and consisted exclusively of UV wavelength greater than 340 nm and smaller than 450 nm. The UVA1 irradiance was measured with a IL 1700 photometer (International Light, Newburyport, Mass) and found to be 50 mW/cm2 at body distance [19]. To rule out sensitivity to UVA1 irradiation, all patients were phototested before UVA1 phototherapy with increasing doses of UVA1 radiation (10–60 J/cm2) as previously described [15]. For UVA1 phototherapy, all patients were treated with the lesional forearm and hand to single doses of 60 J/cm2 UVA1 daily (Monday-Friday). UVA1 phototherapy was given as monotherapy except for the oral medication which the patients had been given before a phototherapy session. Topical treatment was restricted to the use of emollients.
Immunohistochemical analysis of Decorin
6B6 is a monoclonal antibody prepared with a purified small dermatan sulfate PG from human ovarian fibroma capsule as an antigen [20]. Its epitope region located within a stretch of amino acid residues 50–65, termed the N-terminal cysteine cluster region, and is useful for immunohistochemistry [21].
Biopsy specimens taken from three patients before and at the end point of treatment and three normal skin taken from margin of benign tumor were fixed with 10% buffered formalin for 48 h, followed by embedding in paraffin. Tissue sections (3 μm) were dewaxed in xylene, and rehydrated through decreasing concentrations of ethanol. To block endogenous peroxidase activity, rehydrated sections were treated in a 0.3 % H2O2 in absolute ethanol for 5 min, and were processed for immunostaining with 6B6 (Seikagaku Kogyo, Tokyo, Japan, 500-fold dilution) and a Histofine Simple Stain Max PO (M) (Nichirei, Tokyo, Japan) according to a manufacturer's instruction. For regular histology tissue sections were stained with Elastica van Gieson stain.
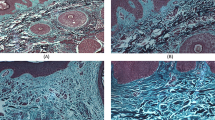
Site-matched biopsy specimens were taken from the forearms of patients before and after UVA1 phototherapy. The histopathology revealed that there were enlarged collagen bundles in the dermis before therapy. After UVA1 irradiation, histological analysis showed a loosening of collagen bundles, the appearance of small collagen fibers in the dermis and a decrease in the thickness of the dermal collagen (Fig. 1). In normal skin, three specimens were stained similarly and decorin localized entire dermis co-distributing with collagen fiber, but epidermis, dermal region facing the basement membrane, and appendage was not stained (Fig. 2G). In SSc patients, decorin was stained entire dermis, especially focally accumulated collagen bundles in upper to middle dermis. Decorin shows remarkable decrease around enlarged collagen bundles in upper to middle dermis according to depth that UVA1 achieve after UVA1 phototherapy, However, decorin was slightly increased around increased small collagen fibers in papillary dermis (Fig. 2A,2B,2C,2D,2E,2F).
Histological evaluation of site-matched skin biopsy specimens before (A, C, E) and after (B, D, F) UVA1 phototherapy Biopsy specimens were taken from sclerotic skin lesions of the forearms of patients 1 (A, B), 2 (C, D), and 3 (E, F). Before UVA1 therapy, there are enlarged collagen bundles in the dermis. After UVA1 irradiation, histological analysis shows a loosening of collagen bundles, the appearance of small collagen fibers in the dermis and a decrease in the thickness of the dermal collagen. (Elastica van Gieson stain, original magnification × 200)
Immunohistochemical analysis of decorin of site-matched skin biopsy specimens before (A, C, E) and after (B, D, F) UVA1 phototherapy Biopsy specimens were taken from sclerotic skin lesions of the forearms of patients 1 (A, B), 2 (C, D), and 3 (E, F), and were stained with monoclonal antibody 6B6 that recognize core protein of small dermatan sulfate proteoglycan, decorin. Decorin is decreased in upper to middle dermis and enlarged collagen bundles are disappeared after UVA1 phototherapy. Decorin is slightly increased in papillary dermis, suggesting newly synthesized collagen. In normal skin (G), decorin is stained entire dermis, but epidermis, dermal region facing the basement membrane, and appendage are not stained.
Its mechanism assumed that degradation of collagen fibers by UVA1-induced matrix metalloproteinase may lead to decrease accumulated decorin in the upper to middle dermis and normalization of decorin may induce normal collagen assembly. This sequence may soften the skin of SSc.
Conclusions
In this study, aberrant localization of decorin was normalized after UVA1 phototherapy. The altered expression of decorin may correlate with the change of collagen fiber. These results suggest that decreased and normalized levels of accumulated decorin may relate to the efficacy of sclerotic lesions in UVA1 phototherapy.
References
Krusius T, Ruoslahti E: Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A 1986, 83: 7683–7687.
Iozzo RV: The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem 1999, 274: 18843–18846. 10.1074/jbc.274.27.18843
Schmidt G, Robenek H, Harrach B, Glossl J, Nolte V, Hormann H, Richter H, Kresse H: Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol 1987, 104: 1683–1691.
Winnemoller M, Schon P, Vischer P, Kresse H: Interactions between thrombospondin and the small proteoglycan decorin: interference with cell attachment. Eur J Cell Biol 1992, 59: 47–55.
Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV: Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest 1998, 101: 406–412.
Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi Y, Pierschbacher MD, Ruoslahti E: Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 1992, 360: 361–364. 10.1038/360361a0
Takeuchi Y, Kodama Y, Matsumoto T: Bone matrix decorin binds transforming growth factor-beta and enhances its bioactivity. J Biol Chem 1994, 269: 32634–32638.
Kuroda K, Shinkai H: Decorin and glycosaminoglycan synthesis in skin fibroblasts from patients with systemic sclerosis. Arch Dermatol Res 1997, 289: 481–485. 10.1007/s004030050225
Kuroda K, Shinkai H: Gene expression of types I and III collagen, decorin, matrix metalloproteinases and tissue inhibitors of metalloproteinases in skin fibroblasts from patients with systemic sclerosis. Arch Dermatol Res 1997, 289: 567–572. 10.1007/s004030050241
Krutmann J, Czech W, Diepgen T, Niedner R, Kapp A, Schopf E: High-dose UVA1 therapy in the treatment of patients with atopic dermatitis. J Am Acad Dermatol 1992, 26: 225–230.
Plettenberg H, Stege H, Megahed M, Ruzicka T, Hosokawa Y, Tsuji T, Morita A, Krutmann J: Ultraviolet A1 (340–400 nm) phototherapy for cutaneous T-cell lymphoma. J Am Acad Dermatol 1999, 41: 47–50.
Stege H, Schopf E, Ruzicka T, Krutmann J: High-dose UVA1 for urticaria pigmentosa. Lancet 1996, 347: 64. 10.1016/S0140-6736(96)91600-1
Stege H, Berneburg M, Humke S, Klammer M, Grewe M, Grether-Beck S, Boedeker R, Diepgen T, Dierks K, Goerz G, et al.: High-dose UVA1 radiation therapy for localized scleroderma. J Am Acad Dermatol 1997, 36: 938–944.
Rook AH, Freundlich B, Jegasothy BV, Perez MI, Barr WG, Jimenez SA, Rietschel RL, Wintroub B, Kahaleh MB, Varga J, et al.: Treatment of systemic sclerosis with extracorporeal photochemotherapy. Results of a multicenter trial. Arch Dermatol 1992, 128: 337–346. 10.1001/archderm.128.3.337
Morita A, Kobayashi K, Isomura I, Tsuji T, Krutmann J: Ultraviolet A1 (340–400 nm) phototherapy for scleroderma in systemic sclerosis. J Am Acad Dermatol 2000, 43: 670–674. 10.1067/mjd.2000.105165
Kerscher M, Volkenandt M, Gruss C, Reuther T, von Kobyletzki G, Freitag M, Dirschka T, Altmeyer P: Low-dose UVA phototherapy for treatment of localized scleroderma. J Am Acad Dermatol 1998, 38: 21–26.
Kreuter A, Gambichler T, Avermaete A, Happe M, Bacharach-Buhles M, Hoffmann K, Jansen T, Altmeyer P, von Kobyletzki G: Low-dose ultraviolet A1 phototherapy for extragenital lichen sclerosus: results of a preliminary study. J Am Acad Dermatol 2002, 46: 251–255. 10.1067/mjd.2002.118552
Yin L, Yamauchi R, Tsuji T, Krutmann J, Morita A: The Expression of Matrix Metalloproteinase-1 mRNA Induced by Ultraviolet A1 (340–400 nm) Is Phototherapy Relevant to the Glutathione (GSH) Content in Skin Fibroblasts of Systemic Sclerosis. J Dermatol 2003, in press.
Morita A, Krutmann J: Ultraviolet A radiation-induced apoptosis. Methods Enzymol 2000, 319: 302–309.
Sobue M, Nakashima N, Fukatsu T, Nagasaka T, Katoh T, Ogura T, Takeuchi J: Production and characterization of monoclonal antibody to dermatan sulfate proteoglycan. J Histochem Cytochem 1988, 36: 479–485.
Sawada H, Shinomura T, Kimata K, Takeuchi J, Tsuji T, Watanabe H: Characterization of an anti-decorin monoclonal antibody, and its utility. J Biochem (Tokyo) 2002, 132: 997–100.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-5945/3/2/prepub
Acknowledgements
This work was in part supported by Gant-in-Aid for Scientific Research (C) from the Ministry of Education, Science and Culture of Japan (12670831, AM), a grant for the Basic Dermatological Research from Shiseido Co. Ltd., and a grant from Ojinkai (Nagoya City University Medical School), Nagoya, Japan. Written consent was obtained from the patient or their relative for publication of the patient's details.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
None declared.
Authors' contributions
H.S. participated in the design of the study, carried out the immunohistochemistry and drafted the manuscript. A.M. conceived of the study. Z.I. participated in the coordination.
All authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sawada, H., Isogai, Z. & Morita, A. Altered decorin expression of systemic sclerosis by UVA1 (340–400 nm) phototherapy: Immunohistochemical analysis of 3 cases. BMC Dermatol 3, 2 (2003). https://doi.org/10.1186/1471-5945-3-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-5945-3-2