Abstract
Background
Deficits in the mismatch negativity (MMN) and P3a components are the most reliable and robust findings in schizophrenia. These abnormalities have also been recently documented in individuals clinically at risk for psychosis, indicating that the MMN may be a potential biomarker for psychosis. However, the at risk samples included in MMN studies are characterised by pre-existing clinical symptomatology and significant functional decline which are related to MMN amplitude. These factors may be potential confounds in determining whether deficient MMN is present prior to clinical manifestation of the disorder. Therefore, investigating the MMN in the extended psychosis phenotype comprising adolescents with psychotic symptoms from the general population may provide important information on whether abnormal MMN is apparent in the earliest stages of risk.
Methods
Thirty six adolescents completed a duration deviant MMN task. Fourteen adolescents with psychotic symptoms comprised the at risk group and 22 with no psychotic symptoms comprised the Controls. The task consisted of 85% standard tones (25 ms) and 15% deviant tones (50 ms). The groups were compared on MMN and P3a amplitude and latency across frontocentral and temporal electrodes.
Results
Adolescents with psychotic symptoms were characterised by a reduction in MMN amplitude at frontal and temporal regions compared to the controls.
Conclusions
This is the first study to demonstrate impaired auditory discrimination for duration deviant tones in nonclinical adolescents with psychotic symptoms. These findings suggest that MMN amplitude may be a possible biomarker for vulnerability to psychosis.
Similar content being viewed by others
Background
Disturbances in sensory information processing are considered to be important pathophysiological mechanisms underlying the development of psychosis [1]. Electrophysiological markers of these impairments have been proposed as potential biomarkers for schizophrenia. In particular, reduced amplitude of the duration deviant mismatch negativity (MMN) component is one of the most robust and reliable findings in schizophrenia [2].
Mismatch negativity is an automatic neurophysiological response to a deviant auditory stimulus that is interspersed with a series of standard auditory stimuli [3, 4]. A deviant stimulus may differ on a range of auditory dimensions, such as frequency (pitch), duration, intensity or location [5, 6]. The MMN waveform is an early negative ERP that is maximal over frontocentral areas of the scalp and peaks between 100-200 ms. MMN tasks are usually passive and undemanding, and the MMN can be elicited without the participant consciously attending to the auditory stimuli [7, 8]. MMN is therefore an index of the brain’s ability to extract potentially relevant information from an irrelevant background [9]. Multiple MMN generators have been reported in the auditory cortices and in the prefrontal cortex as part of a distributed fronto-temporal network subserving change detection and attentional switching [10–13].
Studies examining the MMN in chronic patients with schizophrenia [2, 14, 15], first episode patients [9, 16], prodromal individuals [1, 17, 18] and those genetically at risk [19–23] have reported reductions in MMN amplitude, particularly to duration deviants. Bodatsch et al. [1] found that reduced duration MMN amplitude distinguished between at risk individuals who converted to psychosis from those who did not. Shin et al. [18] also reported the presence of duration MMN deficits in at risk individuals, regardless of conversion. However, not all studies have found deficient MMN as normal mismatch negativity has been reported in unaffected family relatives of patients with schizophrenia [24, 25] and also in first episode patients [25, 26]. MMN latency findings are inconsistent in schizophrenia studies as some have found delayed latency [27], whereas others have reported shortened [28] or normal MMN latency [29].
At frontocentral electrodes, the MMN wave is often followed by a positive-going ERP component peaking between 250-300 ms [7]. This early P300 component (i.e. P3a) is an automatic process which is thought to reflect an involuntary attention orienting response to a novel stimulus [7, 19, 30, 31]. P3a amplitude in MMN studies is reduced in patients with schizophrenia and also in first episode psychosis [9].
Epidemiological research over the past decade has demonstrated that psychotic symptoms are far more prevalent in the population than actual psychotic disorder [32]. A recent meta analysis of population studies showed that 17% of children and 7.5% of adolescents report psychotic symptoms [33]. These young people have been shown to share a wide range of risk factors with schizophrenia patients [34] and longitudinal research has demonstrated an increased risk of psychotic disorder in adulthood [35, 36]. For these reasons, this population has been considered to represent an ‘extended psychosis phenotype’ [37] and researchers have advocated studying aetiological and risk factors for psychosis in this wider phenotype [38, 39] including underlying genetic and biological factors [40], as opposed to the ‘narrow’ clinical phenotype of schizophrenia. This approach allows investigation of the earliest risk factors associated with psychosis while excluding potential confounds such as disease chronicity, medication effects and other illness related factors.
While there has been a great deal of clinical research on the extended psychosis phenotype to date, there has been limited neurobiological research on this population. Jacobson et al. [41] demonstrated a fronto-temporal dysfunction in adolescents with psychotic-like experiences compared to a control group and Laurens et al. [42], meanwhile, found electrophysiological evidence for disrupted error monitoring in adolescents with psychotic-like experiences from a community sample. This is the first study to investigate the MMN component in adolescents with psychotic symptoms in a community based setting. As the MMN possesses a relatively high specificity for schizophrenia, the investigation of this component in at risk individuals may increase our understanding of the sensitivity of the MMN as an indicator of vulnerability to psychosis.
Methods
Participants
Participants were recruited from the larger Adolescent Brain Development Study (see Kelleher et al. [43] for details). In brief, adolescents aged 11-13 years were recruited from primary (junior) schools in counties Kildare and Dublin and invited to compete a symptom survey in the classroom and attend for clinical interview. Written consent was obtained from both the participant and their parent or guardian and assent was obtained from the adolescents before the study commenced. Following the clinical interview adolescents with psychotic symptoms and control adolescents were sampled from the population-based study and invited to attend National University of Ireland, Maynooth for the Mismatch Negativity Study.
Thirty six adolescents aged 11-13 years agreed to take part in this EEG study comprising 14 with psychotic symptoms (the At-Risk group) and 22 without psychotic symptoms (the control group). All participants had normal hearing and had no previous brain injuries or neurological disorders. No participants were on any medication at the time of testing or had any family history of psychotic illness. Ethics approval was obtained from the ethics committees in the National University of Ireland, Maynooth and in Beaumont Hospital, Dublin 9 (ref no. 06/21).
Assessment of psychotic symptoms
Psychotic symptoms were assessed using the Schedule for Affective Disorders and Schizophrenia for School Aged Children, Present and Lifetime versions (K-SADS-PL) [43]. The K-SADS-PL is designed to measure the severity of symptomatology reported by children and adolescents, and to assess current and past episodes of psychopathology according to DSM-III-R and DSM-IV criteria. Children and parents were interviewed separately, both answering the same questions about the child. The psychosis section of the K-SADS was used to assess the participants’ psychotic symptoms (hallucinations and delusions) [44]. All interviewers recorded extensive notes of potential psychotic phenomena in this section of the interview and a clinical consensus meeting was held following the interviews. Auditory hallucinations that were deemed as clinically significant included voices commenting on behaviour, a voice giving commands, voices conversing, whispering voices and voices at varying volumes where the words cannot clearly be distinguished by the individual. Clinically significant delusions included feelings of being watched, unfounded ideas that others are saying negative things about the individual (which are distinguished from paranoia and self-consciousness), and a belief that ghost is communicating directly with the individual.
MMN paradigm
Participants were presented with 1200 auditory stimuli, of which 1020 (85%) were standard tones of 1000 Hz presented at 25 ms (5 ms rise/fall) and 180 (15%) deviant tones of 1000 Hz at 50 ms (5 ms rise/fall time). The interstimulus interval was 300 ms. Stimuli were presented through the computer speakers at 80 dB, while participants were asked to ignore the sounds and look at a fixation point located in the centre of the computer screen. The task consisted of three experimental blocks of 400 stimuli each with a break of 5 seconds between each block. No motor response or stimulus evaluation was required for the task.
ERP recording
EEG data were recorded using silver/silver chloride electrodes (Brain Vision©) at 64 electrode sites positioned according to the extended international 10-20 system of electrode placement. Electrodes were attached to an elastic electrode cap (Easy Cap©) which was fastened with a chin strap. Horizontal eye movements (HEOG) were recorded from electrodes positioned at the outer canthus of each eye. Vertical eye movements (VEOG) were recorded from electrodes located above and below the left eye. The reference electrode was placed on the nasion of the nose. Skin was lightly abraded to maintain an impedance level below 10kΩ. The EEG signal was amplified (Brain Vision©) with a bandpass filter of 0.16-100 Hz and a gain of 1000. EEG data were digitised at a sampling rate of 500. The conversion rate was 2000 Hz per channel and the range was 150 mV. Filters were set at a low cut off of 0.53 Hz and a high cut off of 30 Hz. Recordings were notch filtered offline at 50 Hz.
ERP data
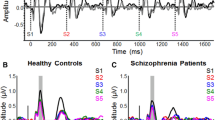
Blinks were averaged offline and an EOG automatic artifact rejection algorithm was applied to the data [45, 46]. Epochs of 50 ms prestimulus to 500 ms post-stimulus were analysed and baseline corrected to the interval −50 to 0 ms. Mismatch negativity was measured from a difference waveform obtained by subtracting the standard tone ERP waveforms from the deviant tone ERP waveforms. MMN amplitude and latency were measured as the most negative data point within the 80-130 ms latency window, post-stimulus onset. Based on visual inspection of grand averaged waveforms (see Figure 1), MMN amplitude and latency was obtained for each group (Controls, At risk) and condition (Standard, Deviant) over 12 frontocentral electrode sites (FP1, FPz, FP2, F1, Fz, F2, FC1, FCz, FC2, C3, Cz, C4). As the MMN is also detected at mastoid areas with reversed polarity in a nose referenced montage, ERPs were recorded over left and right temporal electrode sites (TP9 and TP10) which were positioned over the mastoid areas (see Table 1). Mean amplitude of the subsequent P3a component was also measured over a latency of 150-290 ms at central electrodes Fz and Cz. The average number of trials (M, SD) accepted for the standard and deviant tones, respectively, were 996.68 (53.67); 171.81 (9.56) for the Controls and 918.78 (136.59); 163.00 (24.05) for the at risk. A 2×2 analysis of variance (ANOVA) verified that there were no group differences in the number of trials accepted [F (1, 34) =2.303, p=.138].
Statistical analyses
Statistical analyses were conducted with SPSS for Windows 17.0. Chi-square was employed to compare the at risk and control groups on demographic variables, including age, sex and socioeconomic status (SES). The participants’ socioeconomic status was defined by their parent’s occupations, assessed with the Irish Social Class Scale obtained from the national Central Statistics Office. Mixed factorial ANOVA compared the groups on MMN amplitude and latency. A 2×4 analysis of variance (ANOVA) was conducted where the between groups factor was Group (At risk, Control), and Region (Frontal polar, Frontal; Frontocentral and Central) served as the within groups factor. A subsequent 2×2 ANOVA was also conducted where the between groups factor was Group (at-risk, controls) and the within groups factor was Laterality (left TP9, right TP10). A 2×2 ANOVA compared the groups on P3a amplitude and latency at electrode sites Fz and Cz. An alpha value of p=0.05 was used for statistical significance and Greenhouse Geisser correction was reported when the assumption of sphericity was violated. Bonferroni corrected t-tests were performed to further examine any main and interaction effects. Cohen’s d effect sizes were also calculated to describe the magnitude of any group differences.
Results
The MMN/P3a waveforms at frontocentral and temporal electrodes for each group are displayed. The at risk and control groups did not differ significantly on demographic variables age, sex, handedness and socioeconomic status (see Table 2). For mean amplitude measures, a 2×4 ANOVA with Group (At risk, Controls) as the between subjects factor with Region (frontal polar, frontal, frontocentral and central) as the within groups factor revealed a main effect of Group [F (1, 34) = 6.235, p = .018]. An interaction effect of Region*Group [F (3, 102) = 5.091, p= .014] was also found. Follow up analyses compared the groups at each region and revealed reduced mean MMN amplitude in the at risk group at the frontal polar region [t (34) = −3.086, p= .004]. A 2×2 ANOVA with Group as the between subjects factor and Laterality (TP9, TP10) as the within groups factor also revealed a significant group difference over temporo-parietal areas [F (1, 34) = 6.323, p=.017]. Follow up independent t-tests revealed reduced MMN amplitude in the at risk group over the right temporo-parietal electrode TP10 in comparison to the Control group [t (34) = 2.660, p=.012].
For latency measures, a 2×4 ANOVA with Group (At risk, Controls) as the between subjects factor with Region (frontal polar, frontal, frontocentral and central) as the within groups factor was also conducted. A main effect of Region [F (3, 102) = 5.085, p = .006] was found which demonstrated delayed processing over frontal polar regions. The groups did not differ in MMN latency in any region. A 2×2 ANOVA was conducted for Laterality (TP9, TP10) and Group which revealed no significant differences. A 2×2 ANOVA was conducted on P3a mean amplitude comparing Group (At risk, Controls) and Region (Frontal (Fz) and Central (Cz)). A main effect of Region [F (1, 34) = 14.252, p = .001] revealed increased frontal activity compared to central regions. P3a latency was also analysed and a main effect of Region [F (1, 34) = 6.228, p = .018] showed shorter latency in the frontal region in comparison to the central region. The groups did not differ on P3a amplitude or latency.
Discussion
Adolescents reporting psychotic symptoms from a community sample exhibit deficits in preattentive auditory change detection as indexed by reduced MMN amplitude compared to a control group. This finding is consistent with studies of patients with chronic schizophrenia [2, 15, 47], first episode patients [9, 16] and individuals in the ultra high risk or prodromal stage [1, 17, 19, 48] which have reported reduced MMN amplitude over frontocentral regions. MMN amplitude was also reduced at mastoid electrode sites which is consistent with a previous study in first episode patients [9]. However, this finding is the first to be reported in individuals at risk for psychosis.
The results of the current study support the evidence suggesting that reduced MMN in response to duration deviants may represent a potential biomarker candidate for psychosis. Duration deviant paradigms yield more consistent results and appear to be more sensitive to deficits than frequency deviants. Umbricht and Krljes [49] revealed that the effect size between patients and controls for duration deviants was 40% larger than that for frequency deviants. Deficient MMN in response to a frequency deviant is usually observed in chronic patients and is associated with illness progression and functional status, whereas impaired duration MMN has been proposed as a possible trait marker for schizophrenia [9, 50].
P3a amplitude or latency was unaffected in the at-risk group as no group differences were found for this component. Impairments in MMN accompanied by an unaffected P3a may suggest a subtle disruption in the initial automatic detection of change in the auditory environment that is not sufficiently impaired to affect the subsequent attention orienting response. There were no group differences in MMN latency, which supports previous studies reporting a lack of MMN latency differences in chronic patients with schizophrenia compared to controls [29].
Some limitations of this study must be addressed. In view of the relatively small sample size replication of our findings in larger samples of individuals with psychotic symptoms or prodromal syndromes will be required. The lack of longitudinal follow up data limits the ability to draw conclusions regarding the predictive value of reduced MMN amplitude as a marker of vulnerability to psychosis. Follow up studies will elucidate whether the MMN can distinguish between those who develop psychosis or another psychiatric disorder from those who do not. Nonetheless, this study provides important information regarding the utility of the mismatch negativity as a potential biomarker for a broader psychosis phenotype and demonstrates the value of this population in terms of understanding the underlying pathophysiology of psychosis in pre-disease, pre-medication, community based group. The community based approach of the current work also helps to address limitations of clinic based research, including clinically presenting ultra high risk patients, which may be confounded by pre-existing illness related biological changes, with resultant identification of state rather than trait markers for psychosis Belger et al., [51].
Conclusions
In summary, our results demonstrate that adolescents with psychotic symptoms from the general population are characterised by a reduction in duration MMN amplitude at frontal and temporal areas compared to a control group. These findings are the first to be shown in adolescents from a community sample experiencing nonclinical psychotic symptoms indicating that neurobiological changes, particularly impairments in preattentive auditory processing, are evident long before illness onset during the risk period.
References
Bodatsch M, Ruhrmann S, Wagner M, Muller R, Schultze-Lutter F, Frommann I: Prediction of psychosis by mismatch negativity. Biol Psychiatry. 2011, 69 (10): 959-966. 10.1016/j.biopsych.2010.09.057.
Umbricht D, Koller R, Schmid L, Skrabo A, Grubel C, Huber T: How specific are deficits in mismatch negativity generation to schizophrenia?. Biol Psychiatry. 2003, 53 (12): 1120-1131. 10.1016/S0006-3223(02)01642-6.
Naatanen R, Tiitinen H: Auditory Information Processing as Indexed by the Mismatch Negativity. Advances in Psychological Science Biological and Cognitive Aspects. Edited by: Sabourin M, Craik F, Robert M. 1998, Hove, UK: Psychology Press/Erlbaum (UK) Taylor and Francis, 145-170.
Winkler I, Karmos G, Naatanen R: Adaptive modeling of the unattended acoustic environment reflected in the mismatch negativity event-related potential. Brain Res. 1996, 742 (1-2): 239-252. 10.1016/S0006-8993(96)01008-6.
Cheour M: Development of Mismatch Negativity (MMN) during Infancy. Infant EEG and Event-Related Potentials: Studies in Developmental Psychology. Edited by: de Haan M. 2008, London: Psychology Press
Naatanen R, Alho K: Mismatch negativity-a unique measure of sensory processing in audition. Int J Neurosci. 1995, 80 (1-4): 317-337. 10.3109/00207459508986107.
Light GA, Swerdlow NR, Braff DL: Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007, 19 (10): 1624-1632. 10.1162/jocn.2007.19.10.1624.
Michie PT: What has MMN revealed about the auditory system in schizophrenia?. Int J Psychophysiol. 2001, 42 (2): 177-194. 10.1016/S0167-8760(01)00166-0.
Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB: Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010, 34 (6): 822-829. 10.1016/j.pnpbp.2010.03.019.
Javitt DC: When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009, 5: 249-275. 10.1146/annurev.clinpsy.032408.153502.
Stephan KE, Baldeweg T, Friston KJ: Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006, 59 (10): 929-939. 10.1016/j.biopsych.2005.10.005.
Waberski TD, Kreitschmann-Andermahr I, Kawohl W, Darvas F, Ryang Y, Gobbele R: Spatio-temporal source imaging reveals subcomponents of the human auditory mismatch negativity in the cingulum and right inferior temporal gyrus. Neurosci Lett. 2001, 308 (2): 107-110. 10.1016/S0304-3940(01)01988-7.
Rinne T, Alho K, Ilmoniemi RJ, Virtanen J, Naatanen R: Separate time behaviors of the temporal and frontal mismatch negativity sources. Neuroimage. 2000, 12 (1): 14-19. 10.1006/nimg.2000.0591.
Catts SV, Shelley AM, Ward PB, Liebert B, McConaghy N, Andrews S: Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995, 152 (2): 213-219.
Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N: Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biol Psychiatry. 1991, 30 (10): 1059-1062. 10.1016/0006-3223(91)90126-7.
Kaur M, Battisti RA, Ward PB, Ahmed A, Hickie IB, Hermens DF: MMN/P3a deficits in first episode psychosis: Comparing schizophrenia-spectrum and affective-spectrum subgroups. Schizophr Res. 2011, 130 (1): 203-209. 10.1016/j.schres.2011.03.025.
Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R: Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol Psychiatry. 2008, 64 (5): 376-384. 10.1016/j.biopsych.2008.02.006.
Shin KS, Kim JS, Kang DH, Koh Y, Choi JS, O'Donnell BF: Pre-attentive auditory processing in ultra-high-risk for schizophrenia with magnetoencephalography. Biol Psychiatry. 2009, 65 (12): 1071-1078. 10.1016/j.biopsych.2008.12.024.
Atkinson RJ, Michie PT, Schall U: Duration Mismatch Negativity and P3a in First-Episode Psychosis and Individuals at Ultra-High Risk of Psychosis. Biol Psychiatry. 2012, 71: 98-104. 10.1016/j.biopsych.2011.08.023.
Jessen F, Fries T, Kucharski C, Nishimura T, Hoenig K, Maier W: Amplitude reduction of the mismatch negativity in first-degree relatives of patients with schizophrenia. Neurosci Lett. 2001, 309 (3): 185-188. 10.1016/S0304-3940(01)02072-9.
Michie PT, Innes-Brown H, Todd J, Jablensky AV: Duration mismatch negativity in biological relatives of patients with schizophrenia spectrum disorders. Biol Psychiatry. 2002, 52 (7): 749-758. 10.1016/S0006-3223(02)01379-3.
Schreiber H, Stolz-Born G, Kornhuber HH, Born J: Event-related potential correlates of impaired selective attention in children at high risk for schizophrenia. Biol Psychiatry. 1992, 32 (8): 634-651. 10.1016/0006-3223(92)90294-A.
Shaikh M, Valmaggia L, Broome MR, Durtt A, Lappin J, Day F: Reduced mismatch negativity predates the onset of psychosis. Schizophr Res. In press
Bramon E, Croft RJ, McDonald C, Virdi GK, Gruzelier JG, Baldeweg T: Mismatch negativity in schizophrenia: a family study. Schizophr Res. 2004, 67 (1): 1-10.
Magno E, Yeap S, Thakore JH, Garavan H, De Sanctis P, Foxe JJ: Are auditory-evoked frequency and duration mismatch negativity deficits endophenotypic for schizophrenia? High-density electrical mapping in clinically unaffected first-degree relatives and first-episode and chronic schizophrenia. Biol Psychiatry. 2008, 64 (5): 385-391. 10.1016/j.biopsych.2008.03.019.
Valkonen-Korhonen M, Purhonen M, Tarkka IM, Sipila P, Partanen J, Karhu J: Altered auditory processing in acutely psychotic never-medicated first-episode patients. Brain Res Cogn Brain Res. 2003, 17 (3): 747-758. 10.1016/S0926-6410(03)00199-X.
Kathmann N, Wagner M, Rendtorff N, Engel RR: Delayed peak latency of the mismatch negativity in schizophrenics and alcoholics. Biol Psychiatry. 1995, 37 (10): 754-757. 10.1016/0006-3223(94)00309-Q.
Shutara Y, Koga Y, Fujita K, Takeuchi H, Mochida M, Takemasa K: An event-related potential study on the impairment of automatic processing of auditory input in schizophrenia. Brain Topogr. 1996, 8 (3): 285-289. 10.1007/BF01184786. Spring
Javitt DC: Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol Neurootol. 2000, 5 (3-4): 207-215. 10.1159/000013882.
Muller-Gass A, Macdonald M, Schroger E, Sculthorpe L, Campbell K: Evidence for the auditory P3a reflecting an automatic process: elicitation during highly-focused continuous visual attention. Brain Res. 2007, 1170: 71-78. Sep 19
Polich J: Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007, 118 (10): 2128-2148. 10.1016/j.clinph.2007.04.019.
van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L: A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009, 39 (2): 179-195. 10.1017/S0033291708003814.
Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M: Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med. 41 (1): 1-6. In Press
Kelleher I, Cannon M: Psychotic-like experiences in the general population: characterizing a high-risk group for psychosis. Psychol Med. 1-6. In Press
Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H: Children's self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000, 57 (11): 1053-1058. 10.1001/archpsyc.57.11.1053.
Welham J, Scott J, Williams G, Najman J, Bor W, O'Callaghan M: Emotional and behavioural antecedents of young adults who screen positive for non-affective psychosis: a 21-year birth cohort study. Psychol Med. 2009, 39 (4): 625-634. 10.1017/S0033291708003760.
Wigman JT, Vollebergh WA, Raaijmakers QA, Iedema J, van Dorsselar S, Ormel J, Verhulst FC, van Os J: The structure of the extended psychosis phenotype in early adolescence—a cross-sample replication. Schizophr Bull. 2011, 37 (4): 850-860. 10.1093/schbul/sbp154.
Polanczyk G, Moffitt TE, Arsenault L, Cannon M, Ambler A, Keefe RS, Houts R, Odgers CL, Caspi A: Etiological and clinical features of childhood psychotic symptoms: results from a birth cohort. Arch Gen Psychiatry. 2010, 67 (4): 328-338. 10.1001/archgenpsychiatry.2010.14.
Laurens KR, Hodgins S, Maughan B, Murray RM, Rutter ML, Taylor EA: Community screening for psychotic-like experiences and other putative antecedents of schizophrenia in children aged 9-12 years. Schizophr Res. 2007, 90 (1-3): 328-338.
Kelleher I, Jenner JA, Cannon M: Psychotic symptoms in the general population-an evolutionary perspective. Br J Psychiatry. 2010, 197 (3): 130-146.
Jacobson S, Kelleher I, Harley M, Murtagh A, Clarke M, Blanchard M: Structural and functional brain correlates of subclinical psychotic symptoms in 11-13 year old schoolchildren. Neuroimage. 2010, 49 (2): 1875-1885. 10.1016/j.neuroimage.2009.09.015.
Laurens KR, Hodgins S, Mould GL, West SA, Schoenberg PL, Murray RM: Error-related processing dysfunction in children aged 9 to 12 years presenting putative antecedents of schizophrenia. Biol Psychiatry. 2010, 67 (3): 238-245. 10.1016/j.biopsych.2009.07.030.
Kelleher I, Harley M, Murtagh A, Cannon M: Are screening instruments valid for psychotic-like experiences ? A validation study of screening questions for psychotic-like experiences using in-depth clinical interview. Schizophr Bull. 2011, 37 (2): 362-369. 10.1093/schbul/sbp057.
Kaufman J, Birmaher B, Brent D, Rao U, Ryan N: The Schedule for Affective Disorders and Schizophrenia for School-aged Children: Present and Lifetime Version. 1996, University of Pittsburgh: Western Psychiatric Institute and Clinic
Berg P, Scherg M: Dipole models of eye movements and blinks. Electroencephalogr Clin Neurophysiol. 1991, 79 (1): 36-44. 10.1016/0013-4694(91)90154-V.
Ille N, Berg P, Scherg M: Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. J Clin Neurophysiol. 2002, 19 (2): 113-124. 10.1097/00004691-200203000-00002.
Michie PT, Budd TW, Todd J, Rock D, Wichmann H, Box J: Duration and frequency mismatch negativity in schizophrenia. Clin Neurophysiol. 2000, 111 (6): 1054-1065. 10.1016/S1388-2457(00)00275-3.
Baker K, Baldeweg T, Sivagnanasundaram S, Scambler P, Skuse D: COMT Val108/158 Met modifies mismatch negativity and cognitive function in 22q11 deletion syndrome. Biol Psychiatry. 2005, 58 (1): 23-31. 10.1016/j.biopsych.2005.03.020.
Umbricht D, Krljes S: Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005, 76 (1): 1-23. 10.1016/j.schres.2004.12.002.
Todd J, Michie PT, Schall U, Karayanidis F, Yabe H: Deviant matters: duration, frequency, and intensity deviants reveal different patterns of mismatch negativity reduction in early and late schizophrenia. Biol Psychiatry. 2008, 63: 58-64. 10.1016/j.biopsych.2007.02.016.
Belger A, Yucel GH, Donkers FCL: In search of psychosis biomarkers in high-risk populations: is the mismatch negativity the one we've been waiting for?. Biol Psychiatry. 2012, 71: 94-95. 10.1016/j.biopsych.2011.11.009.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-244X/13/45/prepub
Acknowledgements
This work was supported by an Essel Foundation NARSAD Independent Investigator Award. MC was supported by a Health Research Board (Ireland) Clinician Scientist Award. CR is supported by the Irish Research Council for the Humanities and Social Sciences (IRCHSS). DT and PSM were supported by SPUR-ON Summer studentships funded by Science Foundation Ireland (SFI). The research leading to these results has also received funding from the European Community’s Seventh Framework Programme under grant agreement No. HEALTH-F2-2010-241909 (Project EU-GEI). EU-GEI is the acronym of the project ‘European network of National Schizophrenia Networks studying Gene-Environment Interactions’. We wish to thank NUI Maynooth and the Clinical research Centre, Beaumont Hospital for the use of their facilities. We acknowledge the technical assistance provided by Derek Walsh (NUI Maynooth) during the data acquisition. We also acknowledge Dr. Kristin Laurens for her contributions to the task design. This data has been presented in poster form at Neuroscience Ireland 2011 at NUI Maynooth and also at the Annual Research Day at the Royal College of Surgeons in Ireland.
Financial disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JM, CR and IK recruited the participant sample. JM set up and recorded the EEG, analysed the data and wrote the first and final drafts of the manuscript. CR, DT and PSM helped carry out EEG set up and recording. IK conducted clinical interviews with the participants and their parents. RR aided the writing process and the data analysis. MC also helped to write and structure the manuscript. All authors read and approved the final manuscript.
Mary Cannon and Richard AP Roche contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Murphy, J.R., Rawdon, C., Kelleher, I. et al. Reduced duration mismatch negativity in adolescents with psychotic symptoms: further evidence for mismatch negativity as a possible biomarker for vulnerability to psychosis. BMC Psychiatry 13, 45 (2013). https://doi.org/10.1186/1471-244X-13-45
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-244X-13-45