Abstract
Background
Although adolescent idiopathic scoliosis affects approximately 3% of adolescents, the genetic contributions have proven difficult to identify. Work in model organisms, including zebrafish, chickens, and mice, has implicated the lysyl oxidase family of enzymes in the development of scoliosis. We hypothesized that common polymorphisms in the five human lysyl oxidase genes (LOX, LOXL1, LOXL2, LOXL3, and LOXL4) may be associated with the phenotype of adolescent idiopathic scoliosis.
Methods
This was a case-control genetic association study. A total of 112 coding and tag SNPs in LOX, LOXL1, LOXL2, LOXL3, and LOXL4 were genotyped in a discovery cohort of 138 cases and 411 controls. Genotypes were tested for association with adolescent idiopathic scoliosis by logistic regression with a two degree of freedom genotypic model and gender as a covariate. Fourteen SNPs with p < 0.1 in the discovery phase were genotyped in an independent replication cohort of 400 cases and 506 controls.
Results
No evidence for significant association was found between coding or tag SNPs in LOX, LOXL1, LOXL2, LOXL3, and LOXL4 and the phenotype of adolescent idiopathic scoliosis.
Conclusions
Despite suggestive evidence in model organisms, common variants and known coding SNPs in the five human lysyl oxidase genes do not confer increased genotypic risk for adolescent idiopathic scoliosis. The above methodology does not address rare variants or individually private mutations in these genes, and future research may focus on this area.
Similar content being viewed by others
Background
Adolescent idiopathic scoliosis (AIS) affects 2-3% of the pediatric population [1] and often requires bracing or surgical treatment. AIS is currently recognized as a multifactorial disease with multiple influences, both environmental and genetic [2, 3]. Multiple attempts have been made to identify the genetic etiologies of AIS with only limited success, despite evidence for genetic contributions.
Multiple studies have made a strong case for heritability of the incidence of AIS. A meta-analysis of 68 sets of twins found concordance in 73% of monozygous twins and 36% of dizygous twins [4]. A more recent study relying on self report survey methodology confirmed the increased concordance in monozygotic twin pairs (6/44) relative to dizygotic pairs (0/91) [5]. The search for underlying genes has only uncovered a few viable candidates, namely SNTG1 [6] and CHD7 [7]. Additionally, a recent genome-wide agnostic approach put forward a promising finding in CHL1, but the authors were unable to replicate the association in all independent populations [8].
The human lysyl oxidases are a family of copper-dependent enzymes involved in the modeling of connective tissue. These enzymes oxidize the side chain of peptidyl lysine converting specific lysine residues to residues of α-aminoadipic-d-semialdehyde, allowing crosslinking of collagen and elastin. The lysyl oxidase enzymes employ a copper ion (Cu2+) as one of the essential cofactors [9]. This family of enzymes has been proposed to play a role in a range of human diseases including exfoliation glaucoma [10], myocardial fibrosis [11], intracranial aneurysms [12] and cancer metastases [13].
Research in model organisms has linked lysyl oxidase activity with scoliotic phenotypes. Zebrafish researchers have shown that disrupting lysyl oxidase activity in embryonic fish results in notochord distortion leading to defects of the axial skeleton [14]. Work in a line of chickens susceptible to scoliosis implicated the lysyl oxidases as a causative feature [15, 16], but this has not yet been definitively shown. Recent work has investigated the murine lysyl oxidases with bone development [17–19], but a scoliotic phenotype has not been specifically studied in a murine model.
Specific investigations of human lysyl oxidases and scoliosis have been limited to hypotheses generated by findings of increased copper content in hair and plasma samples from scoliotic patients [20–22]. To our knowledge, the relationship between adolescent idiopathic scoliosis and variants in the lysyl oxidase family of genes has not been investigated. This study was designed to test for association between common polymorphisms in the five human lysyl oxidase genes with the phenotype of adolescent idiopathic scoliosis.
Methods
Sample Size
Power calculations were performed prior to initiation of the study with the Genetic Power Calculator [23]. As expected, the genotypic relative risk detectable with our estimated discovery cohort sample size of 180 cases and 360 controls varied with allele frequency over the range of 0.05 to 0.35. Our anticipated detectable difference in the discovery cohort ranged from an odds ratio of 1.7 to 2.0. Parameters included the 2 degree of freedom genotypic test with alpha = 0.1 and 80% power; assumptions included genotyping the causative SNP, a dominant model without additive or multiplicative effects, and a population prevalence of 3%.
Populations
Discovery Cohort
Patients with AIS collected at Washington University School of Medicine, St. Louis Children's Hospital, and Shriner's Hospital for Children in St. Louis, MO were genotyped as cases in the discovery cohort. Inclusion criteria for this cohort were spinal curvature >10 degrees on radiograph and Caucasian ancestry. Exclusion criteria were known or suspected associated diagnoses such as Marfan syndrome or neuromuscular disease. Patients provided written informed consent as part of an IRB protocol approved by Washington University School of Medicine and Shriner's Hospital for Children. DNA was isolated from either lymphocytes or saliva via standard procedures. An aliquot of each sample was then whole genome amplified with the QIAGEN REPLI-g kit per the manufacturer's directions (Valencia, CA). Amplification was confirmed by agarose gel electrophoresis (data not shown).
The population control patients for the discovery cohort were recruited at an ambulatory outpatient clinical laboratory in Kansas City, MO as previously described [24]. Patients provided written informed consent as part of an IRB protocol approved by Saint Luke's Hospital of Kansas City. Scoliosis status was not assessed clinically or radiographically. Ancestry and gender information was obtained by self-report. Only participants identified as Caucasian were included in this study, and controls were frequency matched for gender. DNA was previously extracted from lymphocytes by standard procedures and whole genome amplified via QIAGEN REPLI-g per the manufacturer's directions (Valencia, CA).
Replication Cohort
A portion of the replication cohort cases (n = 130) were recruited at the University of Iowa. Inclusion criteria were Cobb angle of at least 10 degrees with pedicle rotation by radiograph and Caucasian ancestry. Exclusion criteria were evidence of neuromuscular or congenital scoliosis, or other recognizable syndromes involving scoliosis. The remainder of the cases in the replication cohort (n = 270) were collected at Texas Scottish Rite Hospital in Dallas, TX. Inclusion criteria were scoliosis of at least 15 degrees by radiograph and Caucasian ancestry. Exclusion criteria were neuromuscular, congenital, or syndromic scoliosis, or family history of the same. In both populations, genomic DNA was isolated from whole blood or saliva by standard procedures and resuspended in water. All patients provided written informed consent as part of IRB protocols approved by the University of Iowa and University of Texas Southwestern Medical Center and Texas Scottish Rite Hospital.
Population control patients were obtained from patients recruited in a clinical laboratory in Kansas City, MO, as described above, without overlap of individuals between cohorts. Women recruited by the Perinatal Research Center in Nashville, TN also served as controls. These participants provided written informed consent as part of a protocol approved by TriStar Nashville IRB, Nashville, TN and Western IRB, Seattle, WA. Scoliosis status was not assessed clinically or radiographically. Inclusion criterion was Caucasian ancestry, obtained by self report. The gender ratio of controls was again frequency matched to that of the cases in the replication cohort.
A summary of the populations is given in Table 1. The ages of the controls are more advanced than those of cases, indicating that they were beyond the age of developing the AIS phenotype. We maintained the assumption of 3% prevalence of AIS in the control populations, as we would not expect AIS to increase the chances of inclusion in the control populations.
SNP Selection
All SNPs from the lysyl oxidase genes denoted as coding in dbSNP build 129 [25] were candidates for genotyping regardless of the minor allele frequency. HapMap data [26] from the CEU population was analyzed into the Haploview (version 2) program [27] for 100 kb upstream and downstream of the five genes studied, LOX, LOXL1, LOXL2, LOXL3 and LOXL4. The Tagger algorithm [28] selected tag SNPs with the following parameters: pairwise tagging only; r2 threshold 0.8; MAF cutoff 0.1; Design score 1. The lists of coding SNPs were marked as included SNPs in the Tagger algorithm and no SNPs were excluded a priori. This resulting list was submitted to the Vanderbilt DNA Resources Core for genotyping on the Sequenom MassARRAY system (San Diego, CA). After assay design with proprietary Sequenom software, those tag SNPs which failed probe design or did not pool with other markers were added to the excluded SNPs list, and iterations performed until all regions were tagged. SNPs that associated with AIS after controlling for gender with p < 0.1 prior to multiple test correction were carried forward to replication genotyping.
Genotyping
The Vanderbilt DNA Resources Core performed the genotyping with the use of the Sequenom MassARRAY system (San Diego, CA). This technology is based on a single-base primer extension reaction coupled with mass spectrometry. Quality-control procedures included examination of marker and sample genotyping efficiency, allele frequency calculations, accuracy of known HapMap samples, and testing of Hardy-Weinberg equilibrium.
Analysis
Genetic analyses including Hardy Weinberg equilibrium testing, allele frequency, logistic regression, and allele association were performed with gPLINK v2.050 [29, 30]. SNPs with total genotyping efficiency of <85% were excluded from further analysis. Individual samples with genotyping efficiency of <70% were also excluded. Allele frequencies were calculated in both cases and controls, and were analyzed for differences between cohorts. Genotype results were tested for deviation from Hardy-Weinberg equilibrium in control samples.
Genotypes in both cohorts were analyzed by multivariate logistic regression with gender as a covariate and case/control status as the outcome. The analyses did not assume a prespecified genetic model and was conducted with a genotypic model requiring two degrees of freedom. In the discovery cohort, the SNPs with p-values <0.1 for the overall model prior to multiple test correction were carried forward for genotyping in the replication cohort. In the replication cohort, stratified analyses were conducted to ensure that the origin of the samples or gender of the subject did not act as a confounder or effect modifier. As a secondary analysis, the results of an allele association test requiring only 1 degree of freedom was performed.
Results
For initial SNP selection, the Tagger algorithm initially identified a total of 132 candidate coding and tag SNPs. After reiterations to accommodate the Sequenom Genotyping platform, final selection included 112 SNPs. These were genotyped in 138 case and 411 control samples (Additional File 1). Of these, 9 SNPs and 15 samples (8 cases and 7 controls) failed genotyping and were excluded from further analyses. A total of 16 SNPs in LOXL1, LOXL2, and LOXL4 (Table 2) showed a difference by genotype with an uncorrected adjusted p < 0.1. These SNPs were carried forward for genotyping in the replication population.
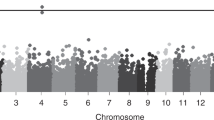
Of the 16 SNPs carried forward to the replication samples, rs751160 (LOXL4) and rs12442211 (LOXL1) were not successfully genotyped. In the cohort of 400 cases and 506 controls, 17 samples (7 cases and 10 controls) failed genotyping. A total of 14 SNPs were analyzed within the replication cohort using the same two degree of freedom genotypic model with multivariate logistic regression, adjusting for gender. The minor allele frequencies were consistent between populations (Figure 1). No SNPs showed a difference by genotype between cases and controls with p < 0.004 (Table 3). Because the cases in the replication cohort were obtained from two different recruiting centers, the analysis was stratified by recruiting center to assess for confounding. No SNP attained significance in a single cohort that was masked by combining the analysis. In addition, the analysis was stratified by gender to determine if gender was acting as an effect modifier. No significant associations or evidence for effect modification were detected.
A secondary analysis of traditional allele association was performed for the SNPs genotyped in the replication cohort. The results indicate that no SNPs were significantly correlated after accounting for multiple testing.
Retrospective power calculations with performed with measured allele frequencies. Our discovery cohort of 138 cases and 411 controls had allele frequencies ranging from 0.06 - 0.49. We had 80% power to detect minimum odds ratios in the range of 1.7 - 2.1 using a 2 degree of freedom genotypic test with alpha = 0.1 (cutoff for inclusion in replication set). Assumptions included genotyping of the causative SNP, a dominant model without additive or multiplicative effects, and a population prevalence of 3%. In the replication cohort of 400 cases and 506 controls, the measured allele frequencies ranged from 0.13 - 0.46. This allowed for 80% power to detect minimum odds ratios in the range of 1.5 - 1.6 with the same parameters. The detection limit increased to 2.6 - 3.6 with a Bonferroni correction and alpha = 0.00357. Note the lowest minor allele frequency measured in the discovery cohort was 0.06, with minimum detectable odds ratio of 2.0. No SNPs carried forward in the replication set had a measured allele frequency of less than 0.13, indicating that rare polymorphisms were not fully assessed in this study.
Additional polymorphisms are added to public databases such as dbSNP with each build. To assess for the extent of coverage, the successfully genotyped SNPs were tested as proxy markers for all SNPs with allele frequency > 0.1 within 100 kb of each lysyl oxidase gene included in the 1000 Genomes pilot data release. We achieved an average r2 of 0.76 over 131 SNPs in LOX, 0.68 over 404 SNPs in LOXL1, 0.60 over 741 SNPs in LOXL2, 0.36 over 224 SNPs in LOXL3, and 0.64 over 344 SNPs in LOXL4.
Discussion
Although work in model organisms suggests a role for lysyl oxidases in scoliosis, common variants in the five human lysyl oxidase genes did not show significant association with the adolescent idiopathic phenotype.
These negative results do not provide support for the underlying hypothesis that lysyl oxidases are involved in the development of adolescent idiopathic scoliosis. The mechanisms by which lysyl oxidase enzymes putatively influence scoliosis in experimental models show effect modification, specifically with copper exposure. Prior work demonstrated that some mutations in a zebrafish model did not overtly cause the abnormal phenotype, but instead allowed the expression of the phenotype at previously subclinical levels of copper deprivation [14]. Likewise, the incidence and severity of scoliosis in the genetically predisposed chickens were sensitive to dietary copper [15, 16]. Measures of copper intake or homeostasis were not available in this population.
The inherent limitations of this study must be acknowledged. Although we studied a large combined cohort of patients with AIS, this study was not powered to detect small risks. We recognize that in addition to sample size, the minor allele frequency of a particular polymorphism and the linkage disequilibrium between the putative causative variant and the genotyped SNP are the primary driving forces behind the detectable genotype relative risk. In addition, the analysis was performed without predefining a specific model such as additive or multiplicative. We selected a genotypic model which uses two degrees of freedom, resulting in lower power because it allows for the genotypes to have a relationship other than additive or multiplicative. The use of an unscreened control population with an assumed AIS prevalence of 3% also decreased the effective power of this study, and had the potential to lead to misclassification bias.
Only tag SNPs with a minor allele frequency above 0.10 and previously described coding SNPs were included in this analysis. As a result, only variants which are relatively common in the population were evaluated. The post hoc evaluation of coverage indicates that additional common polymorphisms in these genes were not well captured in this study. Since newly reported variants were not all sufficiently tagged, this study does not address their potential to have association with AIS. Additionally, a phenotype with complex inheritance such as adolescent idiopathic scoliosis may be due relatively rare, but more penetrant, variants and these were not examined in this study.
Conclusions
Common polymorphisms in the lysyl oxidase family of genes were not found to associate with the phenotype of adolescent idiopathic scoliosis. These results suggest future research in a number of different directions. The lysyl oxidase genes could be examined in other idiopathic scoliotic phenotypes. Adolescent idiopathic scoliosis has onset with puberty, but the lysyl oxidases may impact spinal development during earlier windows resulting in congenital scoliosis or juvenile scoliosis. Alternatively, adolescent idiopathic scoliosis may be the appropriate phenotype, but the impact is mediated through individually rare mutations that have a large impact on the overall phenotype in a subset of patients. Further study of rare variants would necessitate sequencing individuals with adolescent idiopathic scoliosis for variants in at least exonic regions, a timely and more costly approach.
References
Rogala EJ, Drummond DS, Gurr J: Scoliosis: incidence and natural history. A prospective epidemiological study. J Bone Joint Surg Am. 1978, 60: 173-176.
Miller NH: Genetics of familial idiopathic scoliosis. Clin Orthop Relat Res. 2007, 462: 6-10.
Wang WJ, Yeung HY, Chu WC, Tang NL, Lee KM, Qiu Y, Burwell RG, Cheng JC: Top theories for the etiopathogenesis of adolescent idiopathic scoliosis. J Pediatr Orthop. 2011, 31: S14-27.
Kesling KL, Reinker KA: Scoliosis in twins. A meta-analysis of the literature and report of six cases. Spine (Phila Pa 1976). 1997, 22: 2009-2014. 10.1097/00007632-199709010-00014. discussion 2015
Andersen MO, Thomsen K, Kyvik KO: Adolescent idiopathic scoliosis in twins: a population-based survey. Spine (Phila Pa 1976). 2007, 32: 927-930. 10.1097/01.brs.0000259865.08984.00.
Bashiardes S, Veile R, Allen M, Wise CA, Dobbs M, Morcuende JA, Szappanos L, Herring JA, Bowcock AM, Lovett M: SNTG1, the gene encoding gamma1-syntrophin: a candidate gene for idiopathic scoliosis. Hum Genet. 2004, 115: 81-89. 10.1007/s00439-004-1121-y.
Gao X, Gordon D, Zhang D, Browne R, Helms C, Gillum J, Weber S, Devroy S, Swaney S, Dobbs M, Morcuende J, Sheffield V, Lovett M, Bowcock A, Herring J, Wise C: CHD7 gene polymorphisms are associated with susceptibility to idiopathic scoliosis. Am J Hum Genet. 2007, 80: 957-965. 10.1086/513571.
Sharma S, Gao X, Londono D, Devroy SE, Mauldin KN, Frankel JT, Brandon JM, Zhang D, Li QZ, Dobbs MB, Gurnett CA, Grant SF, Hakonarson H, Dormans JP, Herring JA, Gordon D, Wise CA: Genome-wide association studies of adolescent idiopathic scoliosis suggest candidate susceptibility genes. Hum Mol Genet. 2011, 20: 1456-1466. 10.1093/hmg/ddq571.
Lucero HA, Kagan HM: Lysyl oxidase: an oxidative enzyme and effector of cell function. Cell Mol Life Sci. 2006, 63: 2304-2316. 10.1007/s00018-006-6149-9.
Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K: Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 2007, 317: 1397-1400. 10.1126/science.1146554.
Lopez B, Querejeta R, Gonzalez A, Beaumont J, Larman M, Diez J: Impact of treatment on myocardial lysyl oxidase expression and collagen cross-linking in patients with heart failure. Hypertension. 2009, 53: 236-242.
Akagawa H, Narita A, Yamada H, Tajima A, Krischek B, Kasuya H, Hori T, Kubota M, Saeki N, Hata A, Mizutani T, Inoue I: Systematic screening of lysyl oxidase-like (LOXL) family genes demonstrates that LOXL2 is a susceptibility gene to intracranial aneurysms. Hum Genet. 2007, 121: 377-387. 10.1007/s00439-007-0333-3.
Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ: Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009, 15: 35-44. 10.1016/j.ccr.2008.11.012.
Gansner JM, Mendelsohn BA, Hultman KA, Johnson SL, Gitlin JD: Essential role of lysyl oxidases in notochord development. Dev Biol. 2007, 307: 202-213. 10.1016/j.ydbio.2007.04.029.
Opsahl W, Abbott U, Kenney C, Rucker R: Scoliosis in chickens: responsiveness of severity and incidence to dietary copper. Science. 1984, 225: 440-442. 10.1126/science.6740317.
Greve C, Trachtenberg E, Opsahl W, Abbott U, Rucker R: Diet as an external factor in the expression of scoliosis in a line of susceptible chickens. J Nutr. 1987, 117: 189-193.
Iftikhar M, Hurtado P, Bais MV, Wigner N, Stephens DN, Gerstenfeld LC, Trackman PC: Lysyl oxidase-like-2 (LOXL2) is a major isoform in chondrocytes and is critically required for differentiation. J Biol Chem. 2011, 286: 909-918. 10.1074/jbc.M110.155622.
Ito H, Akiyama H, Iguchi H, Iyama K, Miyamoto M, Ohsawa K, Nakamura T: Molecular cloning and biological activity of a novel lysyl oxidase-related gene expressed in cartilage. J Biol Chem. 2001, 276: 24023-24029. 10.1074/jbc.M100861200.
Pischon N, Maki JM, Weisshaupt P, Heng N, Palamakumbura AH, N'Guessan P, Ding A, Radlanski R, Renz H, Bronckers TA, Myllyharju J, Kielbassa AM, Kleber BM, Bernimoulin JP, Trackman PC: Lysyl oxidase (lox) gene deficiency affects osteoblastic phenotype. Calcif Tissue Int. 2009, 85: 119-126. 10.1007/s00223-009-9252-8.
Dastych M, Cienciala J, Krbec M: Changes of selenium, copper, and zinc content in hair and serum of patients with idiopathic scoliosis. J Orthop Res. 2008, 26: 1279-1282. 10.1002/jor.20629.
Pratt WB, Phippen WG: Elevated hair copper level in idiopathic scoliosis: preliminary observations. Spine (Phila Pa 1976). 1980, 5: 230-233. 10.1097/00007632-198005000-00005.
Pratt WB, Schader JB, Phippen WG: Elevation of hair copper in idiopathic scoliosis. A follow-up report. Spine (Phila Pa 1976). 1984, 9: 540-10.1097/00007632-198407000-00027.
Purcell S, Cherny SS, Sham PC: Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003, 19: 149-150. 10.1093/bioinformatics/19.1.149.
Morgan TM, Krumholz HM, Lifton RP, Spertus JA: Nonvalidation of reported genetic risk factors for acute coronary syndrome in a large-scale replication study. JAMA. 2007, 297: 1551-1561. 10.1001/jama.297.14.1551.
Database of Single Nucleotide Polymorphisms (dbSNP). [http://www.ncbi.nlm.nih.gov/projects/SNP/]
Thorisson GA, Smith AV, Krishnan L, Stein LD: The International HapMap Project Web site. Genome Res. 2005, 15: 1592-1593. 10.1101/gr.4413105.
Barrett JC, Fry B, Maller J, Daly MJ: Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005, 21: 263-265. 10.1093/bioinformatics/bth457.
de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D: Efficiency and power in genetic association studies. Nat Genet. 2005, 37: 1217-1223. 10.1038/ng1669.
Purcell S: PLINK. [http://pngu.mgh.harvard.edu/~purcell/plink/]
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC: PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007, 81: 559-575. 10.1086/519795.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2350/12/92/prepub
Acknowledgements
TLM: Supported by Vanderbilt Environmental Health Science Scholars (VEHSS) grant K12 ES015855 from the National Institute of Environmental Health Sciences, NIH, and by Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, NIH
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
TLM conceived of the study, selected SNPs for genotyping, performed primary analysis, and drafted the manuscript. CAG, MBD, CAW, JAM, TMM and RM collected biospecimens from participants and characterized the phenotypes. LMM participated in study design, data acquisition, and analysis. All authors read and approved the final manuscript.
Electronic supplementary material
12881_2011_828_MOESM1_ESM.XLSX
Additional file 1: Genotyped SNPs in the discovery phase with resulting p-values and odds ratios Excel spreadsheet of all SNPs (N = 112) submitted for genotyping in the discovery phase (138 case and 411 control samples). The indicated p-value resulted from a logistic regression genotypic model controlling for gender. The odds ratios for each SNP are given in reference to the homozygous major allele genotype. (XLSX 21 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
McGregor, T.L., Gurnett, C.A., Dobbs, M.B. et al. Common polymorphisms in human lysyl oxidase genes are not associated with the adolescent idiopathic scoliosis phenotype. BMC Med Genet 12, 92 (2011). https://doi.org/10.1186/1471-2350-12-92
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2350-12-92