Abstract
Background
Human norovirus (NoV) causes more than 80% of nonbacterial gastroenteritis in Europe and the United States. NoV transmission via contaminated surfaces may be significant for the spread of viruses. Therefore, measures for prevention and control, such as surface disinfection, are necessary to interrupt the dissemination of human NoV. Murine norovirus (MNV) as a surrogate for human NoV was used to study the efficacy of active ingredients of chemical disinfectants for virus inactivation on inanimate surfaces.
Methods
The inactivating properties of different chemical biocides were tested in a quantitative carrier test with stainless steel discs without mechanical action. Vacuum-dried MNV was exposed to different concentrations of alcohols, peracetic acid (PAA) or glutaraldehyde (GDA) for 5 minutes exposure time. Detection of residual virus was determined by endpoint-titration on RAW 264.7 cells.
Results
PAA [1000 ppm], GDA [2500 ppm], ethanol [50% (v/v)] and 1-propanol [30% (v/v)] were able to inactivate MNV under clean conditions (0.03% BSA) on the carriers by ≥ 4 log10 within 5 minutes exposure time, whereas 2-propanol showed a reduced effectiveness even at 60% (v/v). Furthermore, there were no significant differences in virus reduction whatever interfering substances were used. When testing with ethanol, 1- and 2-propanol, results under clean conditions were nearly the same as in the presence of dirty conditions (0.3% BSA plus 0.3% erythrocytes).
Conclusion
Products based upon PAA, GDA, ethanol and 1-propanol should be used for NoV inactivation on inanimate surfaces. Our data provide valuable information for the development of strategies to control NoV transmission via surfaces.
Similar content being viewed by others
Background
Human noroviruses (NoVs) are members of the genus Norovirus and belong to the family Caliciviridae possessing a single-stranded genome without an envelope. NoV is responsible for more than 80% of nonbacterial gastroenteritis in Europe and the United States [1, 2]. The most common routes of norovirus transmission are ingestion of contaminated food or water, direct and indirect person-to-person contact and aerosolization of viral particles.
In young children, a long-term shedding for more than one month was observed in 22.6% of those with adequate follow-up [3]. This study in a children's hospital also revealed that 59% of all NoV infections were hospital-acquired. A recent publication confirmed the extensive contamination of environmental surfaces resulting in a prolonged norovirus outbreak [4]. These findings highlight the role of widespread environmental contamination in hospitals and other medical settings. Effective disinfection of surfaces and healthcare equipment is crucial for the prevention of virus transmission. Consequently, inactivation studies simulating practical conditions with different chemical biocides are important to develop surface disinfectants effective against human NoVs.
Due to the lack of a cell culture system for important nosocomial pathogens such as human NoVs, it is necessary to use a surrogate virus which can easily be cultivated in cell culture. In the past, the most suitable surrogate for human NoVs when studying the virucidal activity of chemical disinfectants in suspension and on carriers was the feline calicivirus (FCV) [5–7]. Within the family Caliciviridae, FCV belongs to the genus Vesivirus and it is known to be a respiratory pathogen. Therefore, survival and inactivation characteristics may differ from those of human NoVs, which are transmitted by the fecal-oral route.
Recently, propagation of the murine norovirus (MNV) in dendritic cells and macrophages was achieved [8]. Like human NoV, MNV is a member of the genus Norovirus and passes through the gastrointestinal tract. Because of this, MNV seems to be a more suitable surrogate for human NoV than FCV [9, 10].
This study presents the results of activities of different biocides against the MNV dried on stainless steel to simulate practical conditions. These data should facilitate our understanding of inactivation of human NoV by surface disinfectants.
Methods
Preparation of the test virus suspension
Test virus suspension was prepared by infecting RAW 264.7 cells (ATCC TIB-71) with MNV (Berlin 06/06/DE isolate S99, kindly provided by the Robert Koch-Institute in Berlin) by using a multiplicity of infection of 1. Following 2 hours of adsorption at 37°C, the inoculum was replaced by Dulbecco's modified Eagle's medium (DMEM, 4.5 g/l glucose, Lonza Group Ltd., Verviers, Belgium) with 3% fetal calf serum (FCS, low endotoxin, HyClone, PerbioScience, Bonn, Germany). The tissue culture flask was incubated at 37°C and 5% CO2 until 70–95% of the cells showed a cytopathic effect (after 1–2 days). The cells were frozen and thawed twice, followed by centrifugation at 1600 g for 10 minutes. The supernatant was aliquoted as test virus suspension and stored at -80°C.
Chemical biocides
Dilutions of peracetic acid (PAA), glutaraldehyde (GDA) and the different kinds of alcohol (Sigma-Aldrich, Seelze, Germany) were prepared immediately before the inactivation experiments with hard water (300 ppm CaCO3, pH 7.0) prepared according to EN 14476:2007-02 and used within 2 hours [11].
Preparation of virus inoculum
The virus inoculum was prepared by mixing the test virus suspension with the interfering substances according to EN 14476:2007-02 [11]:
-
a)
Nine volumes of test virus suspension were mixed with one volume of 0.3% w/v of bovine serum albumin (clean conditions)
-
b)
Nine volumes of test virus suspension were mixed with one volume of 3% w/v of bovine serum albumin plus 3% v/v washed sheep erythrocytes (dirty conditions).
Preparation of the carrier
For cleaning, stainless steel discs (20 mm diameter, GK Formblech GmbH, Berlin, Germany) were incubated in a 5% (v/v) Decon®90-solution (Decon Laboratories Ltd., Hove, England) for 1 h. Afterwards the discs were rinsed off twice with freshly destilled water for 10 sec, ensuring that the carriers did not dry to any extent, and were then placed in 70% ethanol (v/v) for 15 min. Finally, the carriers were dried by evaporation in sterile petri dishes in a biological safety cabinet.
Experimental procedure
50 μl of the virus inoculum were pipetted in the middle of each pre-treated carrier and dried in a desiccator with a vacuum of 700–800 mbar for about 30 minutes to reach a constant humidity in all experiments. After drying, the virus-contaminated discs were transferred into 25 ml plastic vial holders (Sarstedt AG & Co. KG, Nümbrecht, Germany), which were previously filled with 0.5 g of glass beads (0.25–0.50 mm diameter, Carl Roth GmbH, Karlsruhe, Germany) to increase virus recovery by mechanical abrasion. 100 μl of the test biocides were then pipetted on the dried virus inoculum and incubated for 5 minutes. Control carriers received 100 μl of hard water instead of the chemical substance. In order to stop the activity of the test substance, 900 μl of culture medium were added immediately at the end of the exposure time. The vials were vortexed directly for 1 min to recover the residual virus before the eluate was diluted to determine viral infectivity. Each test was carried out with 3 replicates (carriers) per concentration of the substance and virus control respectively, with a minimum of two independent experiments.
Determination of infectivity
Infectivity was determined by endpoint dilution titration onto RAW 264.7 cells. At the end of the exposure time, aliquots of the mixture were taken and diluted immediately. 100 μl of each dilution were placed in eight wells of a sterile 96-well microtiter plate with the permissive RAW 264.7 cells. Plates were incubated for 4 to 5 days and infectivity was analysed by virus-induced cytopathic effect. Virus titres were determined by the method of Spearman and Kärber. Titre reduction was calculated as the difference between the virus titre of the water control (5 min) and the titre of the biocides after 5 min of exposure times and is presented as log10 reduction.
The criterion used for virucidal activity of the biocide was a four log10 reduction in this study (inactivation of 99.99%).
Results
Inactivation of MNV by peracetic acid and glutaraldehyde
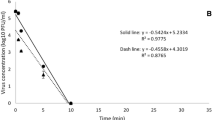
Different concentrations of PAA and GDA were evaluated in the carrier test against MNV under clean conditions with a contact time of 5 minutes. Concentrations of 50, 200 and 500 ppm were not able to reduce virus titres sufficiently, whereas PAA in a concentration of 1000 ppm reduced MNV titre by 4.05 log10. Testing GDA the concentration had to be increased up to 2500 ppm to achieve a 4 log10 reduction in virus titre (Figure 1a and 1b). Lower concentrations were not effective against the test virus on the carriers.
Effectiveness of peracetic acid and glutaraldehyde against murine norovirus. a) 100 μl of peracetic acid [50, 200, 500, 1000, 1500 ppm] or b) 100 μl of glutaraldehyde [125, 500, 1000, 2000, 2500 ppm] were pipetted on the dried virus inoculums (clean conditions) and incubated for 5 minutes. Results represent the mean log10 reduction with standard deviation of three replicates per concentration of the substance of two independent experiments.
Inactivation of MNV by different alcohols
Ethanol, 1-propanol and 2-propanol were tested for their ability to inactivate MNV on stainless steel discs. These different alcohols varied in their capability to inactivate MNV under clean conditions. Whereas the highest used concentration of 60% (v/v) 2-propanol only reduced the viral titre by 3.02 log10 within 5 minutes of exposure time (Figure 2a), 50–55% (v/v) ethanol was able to reduce the infectivity of MNV by 4.09 to 6.18 log10 (Figure 2b). The most effective alcohol in the carrier test was 1-propanol. 30% (v/v) 1-propanol was sufficient to inactivate 99.99% of MNV after a contact time of 5 minutes. A concentration of 40% (v/v) 1-propanol decreased virus titre on the carrier by an average of 6.04 log10 (Figure 2c).
Effectiveness of different concentrations of 2-propanol, ethanol and 1-propanol against murine norovirus. a) 100 μl of 2-propanol [20, 30, 40, 50, 60% (v/v)], b) 100 μl of ethanol [40, 45, 50, 55, 60% (v/v)] or c) 100 μl of 1-propanol [10, 20, 30, 40, 50, 60% (v/v)] were pipetted on the dried virus inoculums (clean conditions) and incubated for 5 minutes. Results represent the mean log10 reduction with standard deviation of three replicates per concentration of the substance of two independent experiments.
Influence of soil load on MNV inactivation by different alcohols
To compare the influence of soil load, we analyzed the three alcohols for their inactivation properties against MNV under clean and dirty conditions. Reduction factors achieved with the corresponding alcohols in concentrations of 40% (v/v) and 60% (v/v) under clean conditions were comparable to those measured under dirty conditions (Figure 3).
Comparison of the inactivation properties of different alcohols under clean and dirty conditions. 100 μl of ethanol [40, 60% (v/v)], 1-propanol [40, 60% (v/v)] or 2-propanol [40, 60% (v/v)] were pipetted on the dried virus inoculums and incubated for 5 minutes. Results represent the mean log10 reduction with standard deviation of two replicates per concentration of the substance of two independent experiments.
Discussion
Norovirus infections spread rapidly particularly in senior residences, hospitals, hotels, schools and even cruise ships. Apparently, contaminated surfaces may play an important role in the spread of human NoV [12–15]. In a rehabilitation centre, the protracted course of norovirus-induced gastroenteritis was also connected to an environmental contamination [16]. Therefore, measures for the prevention and control of NoV transmission should include surface disinfection with products highly effective against this viral pathogen.
Many surface cleaners in the household are based on 0.5% hypochlorite. It was shown by reverse transcriptase polymerase chain reaction (RT-PCR) for human NoVs that the cleaning of fecally contaminated surfaces with a cloth soaked in detergents or treatment with 0.5% hypochlorite alone failed to eliminate human NoVs. The virus could still be detected on up to 28% of the surfaces by RT-PCR. Only wiping the surface with a cloth soaked in detergents followed by applying a combined hypochlorite/detergent treatment was successful to achieve a sufficient efficacy against human NoVs [17].
Since there is only limited knowledge about human NoV inactivation on surfaces, it was the aim of this study to evaluate the virucidal properties of some often-used chemical ingredients of surface disinfectants in a standardized test procedure. The importance of carrier-based methods to test efficacy of disinfectants against vertebrate viruses has been stressed [18].
Meanwhile, carrier test methods including a ring trial launched under the initiative of the Organisation for Economic Cooperation and Development have been published [19–21]. In our study the protocol used followed the draft of the European Committee for Standardization TC 216 with 0.03% BSA and 0.3% BSA plus 0.3% sheep erythrocytes as interfering substances without mechanical action [22].
The absence of a cell culture assay for human NoV makes it necessary to introduce a reliable surrogate virus when studying the environmental persistence and efficacy of chemical disinfectants. Because FCV can be propagated in cell culture, it has been extensively studied in inactivation studies in the past [5, 23]. In contrast to FCV, MNV is transmitted by the fecal-oral route, thus making this virus a promising surrogate for human NoVs. Consequently, stability and inactivation by alcohol-based hand rubs and commercial products used in veterinary medicine for surface disinfection have been successfully tested with MNV in suspension and carrier tests [24–26].
In this study, MNV showed a remarkable stability while drying. The difference in the virus titre before and after drying on the carrier did not exceed 0.35 log10 (data not shown in table). In contrast, the poliovirus titre decreased by about 3 log10 during the drying process [27].
In general, non-enveloped viruses can persist for approximately up to two months on inanimate surfaces [28]. For example, reovirus was able to survive on polyvinyl chloride carriers for a period of 30 days dried in organic matrix [29].
MNV can persist in various environmental conditions. After 40 days at -20°C and 4°C only a < 2 log10 reduction was observed [30]. This study also revealed that MNV survived better in a stool suspension than on the surface of gauze or diaper material.
Recently, the stability of MNV mixed with artificial feces on stainless steel coupons was studied [25]. In this study, only a minimal loss of infectivity was measured at pH 2, whereas FCV was rather unstable at pH values lower than 3.
In this study we show that PAA and GDA were able to reduce the titre of MNV under clean conditions about more than 4 log10 within 5 minutes of exposure time. When testing PAA a concentration of 0.1% (1000 ppm) and when testing GDA a concentration of 0.25% (2500 ppm) was needed to inactivate 99.99% of the viruses. These results were confirmed by the ability of two commercial products based upon GDA and PAA to inactivate MNV [26].
In tests with other viruses simulating practical conditions, 2.0% GDA was able to inactivate HIV, rotavirus and HAV on carriers within one minute [31–33]. Additionally, reovirus embedded in an artificial test soil was inactivated by GDA within one minute, whereas a concentration of 0.1% took 12 minutes to inactivate this virus completely [29].
Generally, besides PAA and GDA, chlorine-based products are often recommended to inactivate non-enveloped viruses on contaminated inanimate surfaces. Testing nonporous and porous surfaces, 20 to 200 ppm of hypochlorous acid solution reduced the titres of human NoV and MNV by 3 log10 within a contact time of 10 minutes [34].
In further experiments we analysed the virucidal properties of ethanol, 1-propanol and 2-propanol against MNV in the quantitative carrier test under clean conditions with concentrations from 10 to 60% (v/v). Since results from the suspension test with the MNV where ethanol in a concentration of 60% (v/v) to 80% (v/v) was more effective than 1-propanol followed by 2-propanol (unpublished), we expected ethanol to be the most effective virucidal agent, followed by 1- and 2-propanol. Surprisingly, 1-propanol was the most effective alcohol to inactivate MNV on the carrier under clean conditions. Interestingly, a concentration of only 30% (v/v) 1-propanol was sufficient to inactivate 99.99% of the virus. In comparison, the two other alcohols were completely ineffective at this concentration. Ethanol achieved a similar effectiveness only at 50–55% (v/v), while 2-propanol at a concentration of 60% (v/v) did not reach a 4 log10 reduction. These results are in contrast to data with FCV. Here, 2-propanol inactivating 99% within one minute at 40 to 60% was found to be more effective than ethanol (6).
Approaching practical conditions, the soil load was increased from 0.03% BSA (clean conditions) to 0.3% BSA plus 0.3% sheep erythrocytes (dirty conditions). Previous results of suspension tests or carrier assays had shown that a higher organic load resulted in a decreased effectiveness of the biocide [35–37]. Interestingly, our results could not confirm these findings. There was no difference in log10 reduction detectable between clean and dirty conditions. All three alcohols tested showed similar reduction factors at 40% (v/v) and 60% (v/v) with 0.03% BSA as well as with 0.3% BSA plus 0.3% erythrocytes. At the tested concentration of 40% (v/v) 1-propanol was highly active against MNV after an exposure time of 5 minutes by reaching a reduction of 6 log10, whereas ethanol and 2-propanol could not inactivate the virus sufficiently. The effectiveness of ethanol and 2-propanol could be enhanced by increasing the concentration of alcohol. At an alcohol concentration of 60% (v/v) ethanol reached an efficacy comparable to 1-propanol, whereas 2-propanol was not capable of reducing the virus titre by more than 3 log10 at the same concentration.
In summary, our data indicate that 1-propanol was the most effective alcohol to inactivate MNV on stainless steel discs within 5 minutes of contact time. Independent of the amount of soil load, even a concentration of 30% (v/v) 1-propanol had the capability to decrease the virus titre by ≥ 4 log10 within 5 minutes.
In further studies, it would be interesting to compare the inactivating properties of these biocides against MNV on other carriers often used in hospitals and other medical settings.
Conclusion
To interrupt the transmission of human NoV it is necessary to use highly effective surface disinfectants tested under practical conditions. Our results show that MNV as a surrogate for human NoV is inactivated under clean conditions by PAA and GDA as well as by the two alcohols ethanol and 1-propanol on stainless steel discs after 5 minutes of exposure time, whereas 2-propanol only achieved insufficient effectiveness. The effective concentrations of the chemical biocides examined provide an informative basis when evaluating these substances as possible active ingredients of surface disinfectants with short contact times.
References
Lopman BA, Reacher MH, van Duijnhoven Y, Hanon F-X, Brown D, Koopmans M: Viral Gastroenteritis Outbreaks in Europe, 1995–2000. Emerg Infect Dis. 2003, 9: 90-96.
Fankhauser RL, Noel JS, Monroe SS, Ando T, Glass RI: Molecular epidemiology of "Norwalk-like viruses" in outbreaks of gastroenteritis in the United States. J Infect Dis. 1998, 178: 1571-1578. 10.1086/314525.
Beersma MFC, Schutten M, Vennema H, Hartwig NG, Mes THM, Osterhaus ADME, van Doornum GJJ, Koopmans M: Norovirus in a Dutch tertiary care hospital (2002–2007): frequent nosocomial transmission and dominance of GIIb strains in young children. J Hosp Infect. 2009, 71: 199-205. 10.1016/j.jhin.2008.11.018.
Wu HM, Fornek M, Schwab KJ, Chapin AR, Gibson K, Schwab E, Spencer C, Henning K: A norovirus outbreak at a long-term-care facility: the role of environmental surface contamination. Infect Control Hosp Epidemiol. 2005, 26: 802-810. 10.1086/502497.
Doultree JC, Druce JD, Birch CJ, Bowden DS, Marshall JA: Inactivation of feline calicivirus, a Norwalk virus surrogate. J Hosp Infect. 1999, 41: 51-57. 10.1016/S0195-6701(99)90037-3.
Malik YS, Maherchandani S, Goyal SM: Comparative efficacy of ethanol and isopropanol against feline calicivirus, a norovirus surrogate. Am J Infect Control. 2006, 34: 31-35. 10.1016/j.ajic.2005.05.012.
Mattison K, Karthikeyan K, Abebe M, Malik N, Sattar SA, Farber JM, Bidawid S: Survival of calicivirus in foods and on surfaces: experiments with feline calicivirus as a surrogate for norovirus. J Food Prot. 2007, 70: 500-503.
Wobus CE, Karst SM, Thackray LB, et al: Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLOS Biol. 2004, 2: e432-10.1371/journal.pbio.0020432.
Steinmann J, Becker B, Bischoff B, Paulmann D, Steinmann J, Steinmann E: Das murine Norovirus – ein neues Surrogatvirus für die humanen Noroviren?. HygMed. 2008, 33: 136-140.
Wobus CE, Thackray LB, Virgin HW: Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol. 2006, 80: 5104-5112. 10.1128/JVI.02346-05.
EN 14476:2007-02: Chemical disinfectants and antiseptics – Virucidal quantitative suspension test for chemical disinfectants and antiseptics used in human medicine – Test method and requirements (phase 2, step 1). 2007
Cheesbrough JS, Green J, Gallimore CI, Wright PA, Brown DW: Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol Infect. 2000, 125: 93-98. 10.1017/S095026889900432X.
Evans MR, Meldrum R, Lane W, et al: An outbreak of viral gastroenteritis following environmental contamination at a concert hall. Epidemiol Infect. 2002, 129: 355-360. 10.1017/S0950268802007446.
Green J, Wright PA, Gallimore I, Mitchell O, Morgan-Capner P, Brown DW: The role of environmental contamination with small round structured viruses in a hospital outbreak investigated by reverse-transcriptase polymerase chain reaction assay. J Hosp Infect. 1998, 39: 39-45. 10.1016/S0195-6701(98)90241-9.
Wu HM, Fornek M, Schwab KJ, Chapin AR, Gibson K, Schwab E, Spencer C, Henning K: A norovirus outbreak at a long-term facility: the role of environmental surface contamination. Infect Control Hosp Epidemiol. 2005, 26: 802-810. 10.1086/502497.
Kuusi M, Nuorti JP, Maunula L, Minh Tran NN, Ratia M, Karlsson J, von Bonsdorff CH: A prolonged outbreak of Norwalk-like calicivirus (NLV) gastroenteritis in a rehabilitation centre due to environmental contamination. Epidemiol Infect. 2002, 129: 133-138. 10.1017/S0950268802007276.
Barker J, Vipond IB, Bloomfield SF: Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via environmental surfaces. J Hosp Infect. 2004, 58: 42-49. 10.1016/j.jhin.2004.04.021.
Ijaz MK, Rubino J: Should test methods for disinfectants use vertebrate viruses dried on carriers to advance virucidal claims?. Infect Control Hosp Epidemiol. 2008, 29: 192-194. 10.1086/526441.
Organisation for Economic Cooperation and Development: OECD efficacy workshop on certain antimicrobial biocides. 2002, Arlington, Virginia, USA, workshop summary report. Arlington, VA: Organisation for Economic Cooperation and Development
Sattar SA, Springthorpe S, Adegbunrin O, Zafer AA, Busa M: A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. J Virol Meth. 2003, 112: 3-12. 10.1016/S0166-0934(03)00192-7.
Sattar SA, Springthorpe VS: Carrier tests to assess microbicidal activities of chemical disinfectants for use on medical devices and environmental surfaces. J AOAC Int. 2005, 88: 182-201.
WI 00216037: Chemical disinfectants and antiseptics. Quantitative non-porous surface test for the evaluation of virucidal activity of chemical disinfectants used in humane medicine. Test method and requirements without mechanical action (phase 2/step 2). CEN TC 216. 2008
Duizer E, Bijkerk P, Rockx B, de Groot A, Twisk F, Koopmans M: Inactivation of caliciviruses. Appl Environ Microbiol. 2004, 70: 4538-4543. 10.1128/AEM.70.8.4538-4543.2004.
Belliot G, Lavaux A, Souihel D, Agnello D, Pothier P: Use of murine norovirus as a surrogate to evaluate resistance of human norovirus to disinfectants. Appl Environ Microbiol. 2008, 74: 3315-3318. 10.1128/AEM.02148-07.
Cannon JL, Papafragkou E, Park GW, Osborne J, Jaykus L-A, Vinjé J: Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J Food Protect. 2006, 11: 2761-2765.
Poschetto LF, Ike A, Papp T, Mohn U, Böhm R, Marschang RE: Comparison to the sensitivities of noroviruses and feline calicivirus to chemical disinfection under field-like conditions. Appl Environ Microbiol. 2007, 73: 5494-5500. 10.1128/AEM.00482-07.
Peters J, Bräuniger S, Fischer I: Zur Prüfung der viruziden Wirksamkeit von Flächendesinfektionsmitteln. HygMed. 1995, 19: 20-28.
Kramer A, Schwebke I, Kampf G: How long do nosocomial pathogens persist on inanimate surfaces?. BMC Infectious Diseases. 2006, 6: 130-10.1186/1471-2334-6-130.
Howie R, Alfa MJ, Coombs K: Survival of enveloped and non-enveloped viruses on surfaces compared with other micro-organisms and impact of suboptimal disinfectant exposure. J Hosp Infect. 2008, 69: 368-376. 10.1016/j.jhin.2008.04.024.
Lee JE, Zoh KD, Ko GP: Inactivation and UV disinfection of murine norovirus with TiO2 under various environmental conditions. Appl Environ Microbiol. 2008, 74: 2111-2117. 10.1128/AEM.02442-07.
Hanson PJ, Gor D, Jeffries DJ, Collins JV: Chemical inactivation of HIV on surfaces. Br Med J. 1989, 298: 862-864. 10.1136/bmj.298.6677.862.
Lloyd-Evans N, Springthorpe VS, Sattar SA: Chemical disinfection of human rotavirus-contaminated inanimate surfaces. J Hyg (Lond). 1986, 97 (1): 163-173.
Mbithi JN, Springthorpe VS, Sattar SA: Chemical disinfection of hepatitis A virus on environmental surfaces. Appl Environ Microbiol. 1990, 56: 3601-3604.
Park GW, Boston DM, Kase JA, Sampson MN, Sobsey MD: Evaluation of liquid- and fog-based application of Sterilox hypochlorous acid solution for surface inactivation of human norovirus. Appl Environ Microbiol. 2007, 73: 4463-4468. 10.1128/AEM.02839-06.
Ferrier A, Garin D, Crance JM: Rapid inactivation of vaccinia virus in suspension and dried on surfaces. J Hosp Infect. 2004, 57: 73-79. 10.1016/j.jhin.2004.01.012.
van Bueren J, Larkin DP, Simpson RA: Inactivation of human immunodeficiency virus type 1 by alcohols. J Hosp Infect. 1994, 28: 137-148. 10.1016/0195-6701(94)90140-6.
Weber DJ, Barbee SL, Sobsey MD, Rutala WA: The effect of blood on the viral activity of Sodium Hypochlorite, a Phenolic, and a Quaternary Ammonium Compound. Infect Control Hosp Epidemiol. 1999, 20: 821-827. 10.1086/501591.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/9/107/prepub
Acknowledgements
We thank E. Schreier (Robert Koch-Institute, Berlin, Germany) for providing the Murine Norovirus (Berlin 06/06/DE isolate S99).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Dr. P. Goroncy-Bermes is employed by Schülke & Mayr, Norderstedt, Germany
Authors' contributions
All authors contributed to the conception and analysis of data. TM carried out the experiments. All authors are involved in drafting the manuscript. All authors have read and approved the final version of the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Magulski, T., Paulmann, D., Bischoff, B. et al. Inactivation of murine norovirus by chemical biocides on stainless steel. BMC Infect Dis 9, 107 (2009). https://doi.org/10.1186/1471-2334-9-107
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2334-9-107