Abstract
Background
Miliary pulmonary nodules are commonly caused by various infections and cancers. We sought to identify the relative frequencies of various aetiologies and the clinical and radiographic predictors of miliary tuberculosis (TB) in patients with miliary pulmonary nodules.
Methods
We performed a retrospective cohort study of patients who presented with micronodules occupying more than two-thirds of the lung volume, based on computed tomography (CT) of the chest, between November 2001 and April 2007, in a tertiary referral hospital in South Korea.
Results
We analyzed 76 patients with miliary pulmonary nodules. Their median age was 52 years and 38 (50%) were males; 18 patients (24%) had a previous or current malignancy and five (7%) had a history of TB. The most common diagnoses of miliary nodules were miliary TB (41 patients, 54%) and miliary metastasis of malignancies (20 patients, 26%). Multivariate analysis revealed that age ≤30 years, HIV infection, corticosteroid use, bronchogenic spread of lesions, and ground-glass opacities occupying >25% of total lung volume increased the probability of miliary TB. However, a history of malignancy decreased the probability of miliary TB.
Conclusion
Miliary TB accounted for approximately half of all causes of miliary pulmonary nodules. Young age, an immune-compromised state, and several clinical and radiographic characteristics increased the probability of miliary TB.
Similar content being viewed by others
Background
Miliary pulmonary nodules are commonly caused by various infections and cancers. In fact, a heterogeneous groups of conditions comprising more than 80 entities may result in miliary nodules [1]. Among them, miliary tuberculosis (TB) is one of the most frequent aetiologies in areas with a high prevalence of TB [2]. However, approximately two-thirds of all miliary TB cases are acid-fast bacilli (AFB) smear-negative, and even transbronchial biopsy frequently fails to reveal AFB or caseating granulomas [3–5]. In such cases, the remaining options for the rapid diagnosis of miliary TB include invasive procedures such as open lung biopsy or empirical anti-TB treatment, depending on the probability of miliary TB based on clinical and radiographic findings. Nonetheless, no recent report has examined the role of clinical characteristics in the differential diagnosis of miliary pulmonary nodules. Moreover, the clinical role of radiographic findings in differentiating between miliary TB and other aetiologies of miliary pulmonary nodules has not been established, although differential diagnosis based on the distribution of miliary nodules in high-resolution computed tomography has been studied [2, 6–8].
We examined the relative frequencies of various aetiologies of miliary pulmonary nodules and sought to identify clinical and radiographic predictors of miliary TB in a tertiary referral hospital in South Korea, where the incidence of active TB was reported to be about 96/100,000 persons in 2005 [9].
Methods
Study subjects
We included all adult patients (≥18 years of age) who presented with miliary nodules, based on computed tomography (CT) of the chest, between November 2001 and April 2007. Patients were selected from Seoul National University Hospital, a tertiary referral hospital in South Korea. Miliary nodules were defined as micronodules (<7 mm in diameter [10]), regardless of solid or non-solid nature, occupying more than two-thirds of the lung volume on a conventional chest CT or high-resolution chest CT. We excluded patients whose physician had not fully evaluated the cause of the miliary nodules, patients who defaulted before obtaining a final diagnosis, and patients with concurrent discrete macronodules (>7 mm in diameter). However, patients with concurrent consolidations were included.
The study protocol was approved by the institutional review board of Seoul National University Hospital.
Study design
We performed a retrospective cohort study to examine the relative frequencies of aetiologies of miliary pulmonary nodules and to assess clinical and radiographic predictors of miliary TB. Enrolled patients were classified into two groups, miliary TB and miliary nodules due to other causes, according to the final diagnosis of their miliary pulmonary nodules. To reveal predictors of miliary TB, clinical and radiographic characteristics were analyzed between the groups.
Review of clinical findings and laboratory tests
We retrospectively reviewed the demographic and clinical characteristics, including age, gender, body mass index (BMI), and symptoms, of the selected patients. In addition, we checked for the presence of previous TB, malignancy, diabetes mellitus, immunosuppressive drug therapy, solid organ transplantation, connective tissue disease, chronic kidney disease, chronic liver disease, HIV infection, and alcoholism. We also reviewed the results of laboratory tests, including complete blood cell counts, blood chemistry, and C-reactive protein levels. The results of histopathological examinations and microbiological studies, including acid-fast staining, mycobacterial cultures, and fungal cultures, were also reviewed.
Radiographic evaluations
Chest CT scans performed at the time of diagnosis were reviewed by two board-certified radiologists, by consensus. They were blinded to patient clinical data and had not participated in the case selection.
Characteristics and distribution of miliary nodules
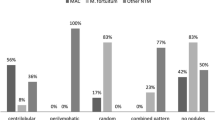
Miliary nodules were assessed for size, margin, profusion, and dominant distribution. Considering previous reports that miliary nodules from cancer usually consist of micronodules of variable size or relatively large micronodules (4–7 mm in diameter) [1, 11], we described the size of the miliary nodules as 'homogeneous' when the sizes of all micronodules were confined to 0–4 mm. The profusion of nodules was estimated by counting all nodules in two contiguous squares of 4 cm2 on three levels: just above the aortic arch, the right upper lobar bronchus, and the lower portion of the left atrium [7]. The dominant distribution of miliary nodules within secondary pulmonary lobules was described as centrilobular, perilymphatic, or random [1, 2, 6, 11].
Definition of bronchogenic spread and pre-existing tuberculosis (TB) sequelae
Poorly defined centrilobular nodules, branching linear lesions, tree-in-bud appearance, and bronchial wall thickening were classified as indicating the bronchogenic spread of lesions [7]. Patients with fibrotic bands, small calcified nodules, or bronchiectasis observable in the upper lobes were regarded as having pre-existing TB sequelae [12, 13]. Mediastinal lymph nodes >1 cm in short-axis diameter were considered positive signs of lymphadenopathy. Additionally, we assessed the extent of accompanying ground-glass opacities, consolidations, and pleural effusions.
Diagnostic criteria
Miliary TB was confirmed if any of the following was met: (1) positive staining for AFB, the growth of Mycobacterium tuberculosis, or positive PCR for M. tuberculosis DNA in sputum, bronchial lavage fluid, pleural fluid, or tissue; (2) the presence of caseating granulomas in lung tissue and clinical and radiographic improvement with anti-TB medications; or (3) definite clinical and radiographic improvement with empirical anti-TB medications. Miliary metastasis of malignancies was identified when any of the following was met: (1) cytological/histological examination of miliary nodules showing malignant cells; or (2) concurrent changes in the size and number of miliary nodules relative to the size of measurable primary malignant lesions. For example, concurrent decreases in the sizes of both primary lesions and miliary nodules in a patient receiving anti-cancer chemotherapy would be such a case. Pneumoconiosis was diagnosed based on occupational history and compatible clinical and radiographic findings. Disseminated fungal diseases were defined using bacteriological or pathological analyses of miliary nodules. Hypersensitivity pneumonitis was diagnosed by environmental or occupational history and improvement with environmental control or corticosteroid treatment.
Statistical analyses
To identify factors for differentiating between TB-associated miliary nodules and miliary nodules due to other causes, we performed univariate analyses using the χ2 test or Fisher's exact test for categorical variables, and using t tests for continuous variables. To determine predictors of miliary TB, we performed binary logistic regression analysis with significant (P < 0.05) variables from the univariate analysis. Co-linearity was eliminated by stepwise selection (entry, p = 0.05; removal, p = 0.1). Because some predictors were likely to be found only within the miliary TB group, we used the penalized likelihood ratio test to determine significance. For the same reason, odds ratios and 95% confidence intervals were estimated using the penalized maximum likelihood and profile penalized likelihood confidence intervals, respectively. Statistical significance was determined at P < 0.05. All analyses were performed using SPSS (version 12.0, SPSS Inc., Chicago, IL, USA) or SAS (version 8.2, SAS Institute Inc., Cary, NC, USA).
Results
Demographic and clinical characteristics of patients with miliary pulmonary nodules
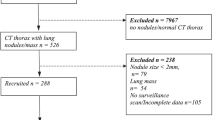
A total of 182 patients diagnosed with and treated for miliary pulmonary nodules during the study period were initially screened for inclusion in this study. Of these, 102 patients were excluded because of concurrent discrete macronodules (>7 mm in diameter), mass lesions suggestive of primary lung cancer, or cavitations suggestive of pulmonary TB. Indeed, the diagnoses of the 102 patients excluded from further analysis were metastatic cancer (65 patients), miliary TB (29 patients), and pneumoconiosis (8 patients). An additional four patients were excluded because they defaulted before obtaining a final diagnosis. Finally, data for 76 patients were included and analyzed. Their median age was 52 years and 38 (50%) of them were males; 18 patients (24%) had a previous or current malignancy and five (7%) had a history of TB (Table 1).
Aetiologies of miliary pulmonary nodules
Among the 76 patients, miliary TB accounted for 54% of the miliary pulmonary nodules (41 patients). Of these, 31 patients (76%) were diagnosed by positive staining for AFB, the growth of Mycobacterium tuberculosis, or positive PCR for M. tuberculosis DNA in sputum, bronchial lavage fluid, pleural fluid, cerebrospinal fluid, or tissue. In five patients (12%), diagnoses were made by the presence of caseating granulomas in lung tissue and clinical and radiographic improvement with anti-TB medications. The other five patients (12%) were diagnosed based on definite clinical and radiographic improvement with empirical anti-TB medications. Miliary metastasis of malignancies accounted for 26% of the miliary pulmonary nodules (20 patients). Of these, direct histological confirmation of miliary pulmonary nodules via lung biopsy was achieved in one patient. Three patients were diagnosed with disseminated candidiasis, all with an underlying hematologic malignancy (Table 2).
Clinical and radiological findings associated with miliary TB
With univariate analysis, we found that age ≤30 years, HIV infection, corticosteroid use, connective tissue disease, low BMI, body temperature >37.7°C, homogeneous nodule size, high profusion, bronchogenic spread of lesions, and ground-glass opacities occupying >25% of total lung volume were associated with miliary TB. However, a history of solid organ malignancy decreased the probability of miliary TB (Tables 3, 4). Subsequent multivariate analysis supported the association between miliary TB and age ≤30 years, HIV infection, corticosteroid use, bronchogenic spread of lesions, and ground-glass opacities occupying >25% of total lung volume, as well as the association between miliary nodules of non-miliary TB and a history of malignancy (Table 5).
Discussion
Although the prevalence of active pulmonary TB in South Korea decreased from 5.1% in 1965 to 0.33% in 2005 [14], this study showed that miliary TB still accounted for approximately half of all cases of miliary pulmonary nodules in the patients examined. The proportion of miliary TB among miliary pulmonary nodules in the present study was higher than that in a previous study by Lee et al. (22.5%) in a South Korean population [2]. This discrepancy is probably the result of differences in study design. In the previous study, patients without biopsy-proven diagnoses were not included [2], which might have excluded a considerable portion of miliary TB patients who may have been identified by bacteriological studies.
Our study indicated that age ≤30 years, HIV infection, and corticosteroid use were strong independent predictors of miliary TB (Table 5). Although miliary TB is relatively rare among young adults without HIV infection in developed nations [15], about one-quarter of the non-HIV-infected patients with miliary TB in this study were ≤30 years of age. This difference may be attributable to the relatively high incidence of TB in South Korea, where the annual incidence of active TB has been reported to be about 96/100,000 persons per year [9].
Other risk factors for miliary TB, including diabetes mellitus [3–5], a history of prior TB [4, 5], chronic kidney disease [3–5], and hematologic malignancies [16], failed to show an association in our study population (Table 3). These differences may also be attributable to study design. All of the subjects in the present study had miliary pulmonary nodules, and clinical characteristics were subsequently compared between miliary TB patients and patients with other miliary pulmonary nodules. However, previous studies compared clinical characteristics between miliary TB patients and healthy controls without TB [3–5]. Certain clinical features are more common in miliary TB patients than in the general population, but these factors may have less significance in the clinical setting of miliary pulmonary nodules of various aetiologies.
A history of hematologic or solid malignancy (OR, 0.1; 95% CI, 0.0–0.7) was a strong negative predictor of miliary TB in our study (Table 5). Although hematologic and solid organ malignancy is a known risk factor for the reactivation of TB [16–22], the presence of a history of malignancy decreased the probability of miliary TB in our study. The miliary nodules found in 13 of 18 patients (72%) with underlying malignancies represented metastases of those underlying malignancies.
Among radiographic findings, this study revealed that the bronchogenic spread of lesions and ground-glass opacities occupying >25% of total lung volume were associated with miliary TB. This confirmed a previous observation that these parameters are frequently found in miliary TB [7, 23]. However, a random distribution of miliary nodules on high-resolution computed tomography scans, which is a well-known characteristic of miliary TB [1, 2, 6–8, 23], was not identified as a predictor in this study. This may reflect the fact that all malignant miliary nodules showed a random distribution in our study. Although a random distribution can be helpful in differentiating between miliary TB and diffuse panbronchiolitis [2, 11] or sarcoidosis [1, 2, 6, 7, 11], its usefulness in diagnosis may be limited in clinical situations where the majority of miliary nodules are caused by miliary TB or malignant metastasis.
To correctly appreciate these results, the limitations of this study should be considered. First, this study was performed in South Korea, where the annual incidence of active TB has been reported to be about 96/100,000 persons per year [9]. Any generalization of the results to patients in areas with higher or lower TB rates should be made with caution. Second, we did not include patients who had miliary nodules but no CT scan, and we excluded patients with discrete lung masses. Thus, the results should be applied cautiously and should not be applied to cases with miliary nodules of clinically apparent causes. For example, a case of miliary nodules with a concurrent spiculated mass highly suggestive of lung cancer would not be applicable to the results of this study. Third, the confidence intervals of the odds ratios for some variables were wide, as a result of the small numbers of patients in some categories. Future studies prospectively recruiting a larger number of patients should be performed to confirm the results of this study.
Conclusion
In conclusion, although the prevalence of TB has markedly decreased in South Korea, miliary TB still accounts for about half of all causes of miliary pulmonary nodules. To avoid delayed anti-TB treatment, miliary TB should be seriously considered in patients with the factors identified in this study: ≤30 years of age, HIV infection, corticosteroid use, absence of a history of malignancy, bronchogenic spread of lesions, or ground-glass opacities occupying >25% of total lung volume in chest CT scans.
References
Andreu J, Mauleon S, Pallisa E, Majo J, Martinez-Rodriguez M, Caceres J: Miliary lung disease revisited. Curr Probl Diagn Radiol. 2002, 31 (5): 189-197. 10.1016/S0363-0188(02)90002-2.
Lee KS, Kim TS, Han J, Hwang JH, Yoon JH, Kim Y, Yoo SY: Diffuse micronodular lung disease: HRCT and pathologic findings. J Comput Assist Tomogr. 1999, 23 (1): 99-106. 10.1097/00004728-199901000-00022.
Maartens G, Willcox PA, Benatar SR: Miliary tuberculosis: rapid diagnosis, hematologic abnormalities, and outcome in 109 treated adults. Am J Med. 1990, 89 (3): 291-296. 10.1016/0002-9343(90)90340-J.
Al-Jahdali H, Al-Zahrani K, Amene P, Memish Z, Al-Shimemeri A, Moamary M, Alduhaim A: Clinical aspects of miliary tuberculosis in Saudi adults. Int J Tuberc Lung Dis. 2000, 4 (3): 252-255.
Mert A, Bilir M, Tabak F, Ozaras R, Ozturk R, Senturk H, Aki H, Seyhan N, Karayel T, Aktuglu Y: Miliary tuberculosis: clinical manifestations, diagnosis and outcome in 38 adults. Respirology. 2001, 6 (3): 217-224. 10.1046/j.1440-1843.2001.00328.x.
Voloudaki AE, Tritou IN, Magkanas EG, Chalkiadakis GE, Siafakas NM, Gourtsoyiannis NC: HRCT in miliary lung disease. Acta Radiol. 1999, 40 (4): 451-456.
Hong SH, Im JG, Lee JS, Song JW, Lee HJ, Yeon KM: High resolution CT findings of miliary tuberculosis. J Comput Assist Tomogr. 1998, 22 (2): 220-224. 10.1097/00004728-199803000-00011.
McGuinness G, Naidich DP, Jagirdar J, Leitman B, McCauley DI: High resolution CT findings in miliary lung disease. J Comput Assist Tomogr. 1992, 16 (3): 384-390. 10.1097/00004728-199205000-00009.
World Health Organization: Global tuberculosis control: surveillance, planning, financing. WHO report 2007, Geneva. 2007
Austin JH, Muller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, Webb WR, Zerhouni EA: Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology. 1996, 200 (2): 327-331.
Raoof S, Amchentsev A, Vlahos I, Goud A, Naidich DP: Pictorial essay: multinodular disease: a high-resolution CT scan diagnostic algorithm. Chest. 2006, 129 (3): 805-815. 10.1378/chest.129.3.805.
Im JG, Itoh H, Shim YS, Lee JH, Ahn J, Han MC, Noma S: Pulmonary tuberculosis: CT findings – early active disease and sequential change with antituberculous therapy. Radiology. 1993, 186 (3): 653-660.
Moon WK, Im JG, Yeon KM, Han MC: Mediastinal tuberculous lymphadenitis: CT findings of active and inactive disease. AJR Am J Roentgenol. 1998, 170 (3): 715-718.
Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC: The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998, 2 (1): 27-36.
Hopewell PC: Tuberculosis and other mycobacterial diseases. Murray and Nadel's textbook of respiratory medicine. Edited by: Mason RJ, Broaddus VC, Murray JF, Nadel JA. 2005, Philadelphia: Elsevier Inc, 1013-1014. 4
Khan B, Ahmed P, Ullah K, Hussain CA, Hussain I, Raza S: Frequency of tuberculosis in haematological malignancies and stem cell transplant recipients. J Coll Physicians Surg Pak. 2005, 15 (1): 30-33.
Kaplan MH, Armstrong D, Rosen P: Tuberculosis complicating neoplastic disease. A review of 201 cases. Cancer. 1974, 33 (3): 850-858. 10.1002/1097-0142(197403)33:3<850::AID-CNCR2820330334>3.0.CO;2-H.
Feld R, Bodey GP, Groschel D: Mycobacteriosis in patients with malignant disease. Arch Intern Med. 1976, 136 (1): 67-70. 10.1001/archinte.136.1.67.
Ibrahim EM, Uwaydah A, al-Mulhim FA, Ibrahim AM, el-Hassan AY: Tuberculosis in patients with malignant disease. Indian J Cancer. 1989, 26 (2): 53-57.
De La Rosa GR, Jacobson KL, Rolston KV, Raad II, Kontoyiannis DP, Safdar A: Mycobacterium tuberculosis at a comprehensive cancer centre: active disease in patients with underlying malignancy during 1990–2000. Clin Microbiol Infect. 2004, 10 (8): 749-752. 10.1111/j.1469-0691.2004.00954.x.
Kamboj M, Sepkowitz KA: The risk of tuberculosis in patients with cancer. Clin Infect Dis. 2006, 42 (11): 1592-1595. 10.1086/503917.
Kim HR, Hwang SS, Ro YK, Jeon CH, Ha DY, Park SJ, Lee CH, Lee SM, Yoo CG, Kim YW, et al: Solid-organ malignancy as a risk factor for tuberculosis. Respirology. 2008, 13 (3): 413-419. 10.1111/j.1440-1843.2008.01282.x.
Oh YW, Kim YH, Lee NJ, Kim JH, Chung KB, Suh WH, Yoo SW: High-resolution CT appearance of miliary tuberculosis. J Comput Assist Tomogr. 1994, 18 (6): 862-866. 10.1097/00004728-199411000-00003.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2334/8/160/prepub
Acknowledgements
The authors appreciate the statistical analysis and advice provided by the Medical Research Collaborating Center, Seoul National University College of Medicine/Seoul National University Hospital.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SMJ collected and analysed the data, drafted and edited the manuscript. HJL designed and advised the radiographic analysis. EAP and HYL reviewed the computed tomography of enrolled patients. SML, SCY, CGY, YWK, SKH, YSS participated in design and contributed to editing the manuscript. JJY conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Jin, SM., Lee, H.J., Park, EA. et al. Frequency and predictors of miliary tuberculosis in patients with miliary pulmonary nodules in South Korea: A retrospective cohort study. BMC Infect Dis 8, 160 (2008). https://doi.org/10.1186/1471-2334-8-160
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2334-8-160