Abstract
Background
A series of Rps (resistance to P ytophthora s ojae) genes have been protecting soybean from the root and stem rot disease caused by the Oomycete pathogen, Phytophthora sojae. Five Rps genes were mapped to the Rps1 locus located near the 28 cM map position on molecular linkage group N of the composite genetic soybean map. Among these five genes, Rps1-k was introgressed from the cultivar, Kingwa. Rps1-k has been providing stable and broad-spectrum Phytophthora resistance in the major soybean-producing regions of the United States. Rps1-k has been mapped and isolated. More than one functional Rps1-k gene was identified from the Rps1-k locus. The clustering feature at the Rps1-k locus might have facilitated the expansion of Rps1-k gene numbers and the generation of new recognition specificities. The Rps1-k region was sequenced to understand the possible evolutionary steps that shaped the generation of Phytophthora resistance genes in soybean.
Results
Here the analyses of sequences of three overlapping BAC clones containing the 184,111 bp Rps1-k region are reported. A shotgun sequencing strategy was applied in sequencing the BAC contig. Sequence analysis predicted a few full-length genes including two Rps1-k genes, Rps1-k-1 and Rps1-k-2. Previously reported Rps1-k-3 from this genomic region [1] was evolved through intramolecular recombination between Rps1-k-1 and Rps1-k-2 in Escherichia coli. The majority of the predicted genes are truncated and therefore most likely they are nonfunctional. A member of a highly abundant retroelement, SIRE1, was identified from the Rps1-k region. The Rps1-k region is primarily composed of repetitive sequences. Sixteen simple repeat and 63 tandem repeat sequences were identified from the locus.
Conclusion
These data indicate that the Rps1 locus is located in a gene-poor region. The abundance of repetitive sequences in the Rps1-k region suggested that the location of this locus is in or near a heterochromatic region. Poor recombination frequencies combined with presence of two functional Rps genes at this locus has been providing stable Phytophthora resistance in soybean.
Similar content being viewed by others
Background
Many plant disease resistance (R) genes from different plant species have been isolated and characterized; but are grouped into a limited number of classes [2, 3]. R loci are usually organized in clusters, and genes within one cluster are mostly derived from a common ancestor [4]. The clustering feature can facilitate the expansion of R gene number and the generation of new R gene specificities through recombination and positive selection [5]. Long contiguous sequences containing several R genes or resistance gene analogues (RGA) have been determined [6–8]. These sequences provided insights into the mechanisms of R gene evolution and generation of novel recognition specificity. Insertions of retroelements in genomic regions containing R genes or RGAs have been documented in these studies. Retroelements are suggested to create variability among paralogous R gene members [9].
Soybean (Glycine max L. Merr.) is a legume crop of great economic and agricultural importance across the world. Its estimated genome size is 1,115 Mb, of which approximately 40–60% is composed of repetitive sequence [10–12]. Repetitive DNA sequences have been shown to be the major determinant of plant genome sizes [13]. There are two main types of repetitive sequences, tandem repeat DNA sequences and dispersed DNA sequences such as retroelements [13]. Several tandem repeats, SB92, STR120 and STRR102 have been reported in soybean [14–16]. It has been suggested that soybean has experienced at least two rounds of genome-wide duplications [17–19]. Despite the availability of genomics resources such as densely saturated genetic maps, BAC and YAC libraries, large EST collections, BAC end sequences, a soybean genome database (SoyGD) browser, and the legume information system (LIS) [20], our knowledge of soybean genome structure is still largely limited [21–27].
Root and stem rot disease caused by Pytophthora sojae is one of the most destructive soybean diseases in the United States [28]. Use of Phytophthora resistance conferred by single dominant Rps genes has been providing reasonable protection of soybean against this pathogen. Five Rps genes including Rps1-k were mapped to the Rps1 locus located near the 28 cM map position on molecular linkage group N of the composite genetic soybean map [29, 30]. Among these five genes, Rps1-k was introgressed from the cultivar, Kingwa. Rps1-k confers resistance to most races of P. sojae, and has been widely used for the past two decades [31]. By applying a positional cloning approach two classes of functional coiled coil-nucleotide binding-leucine rich repeat (CC-NB-LRR)-type resistance genes were isolated from the soybean Rps1-k locus [1]. A large cluster of highly polymorphic paralogous Rps1-k sequences is located at the adjacent Rps1-k region [32]. The Rps1-k locus was mapped to two overlapping BAC clones encompassing 184 kb, located at one end of an approximately 600 kb contiguous DNA spanned by several overlapping BAC clones [32]. CC-NB-LRR-type genes of the 184 kb Rps1-k region were evaluated and two classes of highly similar genes were shown to confer race-specific Phytophthora resistance [1]. To gain insights into the soybean genome organization and evolution of Rsp1-k genes, BAC clones encompassing the Rps1 locus were sequenced and analyzed.
Results
Sequence of three BAC clones spanning the Rps1-k locus
Rps1-k was previously mapped to a region flanked by two markers CG1 and 18R [30, 32]. To understand the composition of the Rps1-k region, three overlapping BAC clones, GS_18J19, GS_43D16 and GS_99I16 that may encompass the Rps1 locus were chosen for sequencing [32]. Phytophthora resistance genes were previously identified from these three BAC clones through positional cloning [1].
A total of 4,093 reads (829, 1,189 and 2,065 reads for GS_18J19, GS_43D16 and GS_99I16, respectively) were generated from these BAC clones. GS_18J19, GS_43D16 and GS_99I16 were sequenced to a 14-, 12- and 9-fold redundancies, respectively. A single contig of 38,498 bp was obtained for GS_18J19 after the initial assembly (GenBank accession EU450800). Three and five contigs were obtained from assembling of sequences derived from GS_43D16 and GS_99I16, respectively. The resulting contigs of GS_43D16 and GS_99I16 were ordered into individual scaffolds manually, in which the order and orientation of the contigs were inferred by mate pairs (sequences obtained from both ends of a ~20 kb shotgun clone) [33]. The clones that span the gaps between two adjacent contigs were identified based on mate pairs and were used to obtain sequences of the gap regions. Gaps were filled out by applying the primer walking approach. Primers were designed based on the sequences of contig ends from which walking were initiated. To guarantee the high sequence quality, less sequenced regions were further sequenced by using suitable primers. After initial assembly and gap filling, 70,829 and 164,411 bp sequences were obtained from GS_43D16 and GS_99I16, respectively (GenBank accession EU450800). The assembled GS_18J19 sequences represent one end of the GS_43D16.
Directional sequencing of GS_43D16
Earlier, partial sequencing of the three BAC clones had allowed us to identify candidate genes underlying Rps1-k. The functional identities of Rps1-k genes were confirmed through stable transformation in soybean [1]. Two classes of Rps1-k genes were identified. The three Class I Rps1-k genes were identical in their ORF sequences. The Class I gene, Rps1-k-3, showed a recombination breakpoint at the 3' untranslated region originating from sequence exchange between members of both classes of genes [1].
Rps1-k-1, Rps1-k-2 and Rps1-k-3 were isolated from GS_43D16 [1]. The existence of abundant repetitive sequences made it difficult to assemble the sequences of the BAC clones. To avoid any misassembly, GS_43D16 containing Rps1-k-1, Rps1-k-2 and Rps1-k-3 was subjected to directional sequencing using the EZ::TN <NotI/KAN-3> transposon of the EZ::TN in-Frame Linker Insertion Kit (Epicentre, Madison, WI). Two hundred and twenty-four EZ::TN <NotI/KAN-3> transposon insertion GS_43D16 clones were randomly selected for further analysis. This approach allowed us to map physically the individual sequence reads onto the GS_43D16 sequence as follows. Transposon insertion sites of individual transposition events were utilized to select EZ::TN <NotI/KAN-3> transposon containing GS_43D16 clones. Each clone was digested with NotI and hybridized to GS_43D16 end-specific probes in Southern analyses (Figure 1). There are three NotI sites in GS_43D16; one in the insert soybean genomic DNA and two in the pBeloBAC11 vector flanking the cloning HindIII site. Therefore, NotI digestion of GS_43D16 resulted in three NotI fragments (Figure 1); (I) a large DNA fragment of ~55 kb, (II) a small DNA fragment of ~15 kb, and (III) the pBeloBAC11 vector sequence. There are two NotI sites flanking the kanamycin resistance gene in the EZ::TN <NotI/KAN-3> transposon. Therefore, if there is a single transposon insertion in the GS_43D16 clone, then five fragments including the ~1.2 kb transposon, should be generated following NotI digestion (Figure 1).
Physical mapping of the locations of individual EZ::TN < Not I/KAN-3> transposon insertions in a soybean bacterial artificial chromosome. Individual GS_43D16 clones containing the EZ::TN <NotI/KAN-3> transposon were digested with NotI. Three NotI fragments, I, II and III released from NotI digestion of GS_43D16 are shown in the last lane. Note that fragment III is comprised of the pBeloBAC11 vector sequence. The top panel shows the gel of NotI digested DNA of GS_43D16 clones carrying the transposon in the NotI Fragment II. The middle panel shows the Southern hybridization data of the gel shown in the top panel. The 245 bp probe for Southern analysis was obtained by PCR of the GS_43D16 end that overlaps with GS_18J19, but not GS_99I16. The lower panel shows the distribution of clones carrying the transposon at various regions of the NotI Fragment II. One dot represented one clone containing the transposon at that particular location of the NotI Fragment II.
Of the analyzed 224 random transposon-inserted GS_43D16 clones, 162 were shown to contain the transposon in the large fragment; 40 of them in the small fragment; and 22 in the pBeloBAC11 vector. Clones containing transposon insertions in the vector pBeloBAC11 were not considered for further study. Approximate physical locations of transposon insertions in individual NotI genomic DNA fragments were determined by Southern analyses as shown in Figure 1. Based on the physical location of transposon insertions, 114 GS_43D16 clones containing transposon insertions in either the 15 or 55 kb NotI fragment were selected for sequencing by using transposon end-specific primers. Only about 50% percent of the clones produced sequences that were readable. Pairwise sequence comparison between the assembled GS_43D16 sequence and sequences obtained from individual transposon inserted GS_43D16 clones revealed the transposon insertion sites in GS_43D16.
Among the randomly picked 224 transposon-inserted clones, the number of transposon insertions was proportional to the size of NotI fragments. However, there were no insertions in two regions, one of about 5 kb in the ~15 kb fragment and the other one is about 10 kb in the ~55 kb fragment. Whether this was due to bias in transposon insertion or due to sampling variance is yet to be determined.
The quality of 78,313 bp assembled GS_43D16 sequence was verified through restriction mapping as follows. Clones carrying transposon insertions at various regions were selected and double digested with KpnI and NotI. The predicted KpnI – NotI restriction maps based on the assembled GS_43D16 sequence is shown in Figure 2A. Eight fragments are expected from Kpn1 and NotI double digestion of GS_43D16. Only five fragments were resolved in the gel analyses, because some of the fragments are of similar sizes. For example, there are two 17 kb fragments termed Fragment I. Following digestion of clones carrying single transposons with both enzymes released two additional fragments and the 1.2 kb transposon. Depending upon the position of the transposon in a given KpnI or KpnI-NotI fragment two fragments of variable sizes were produced (Table 1). Comparison of observed fragment sizes with that of expected fragment sizes showed that there is general agreement between the observed and expected fragment sizes. SalI-NotI map (Figure 2C) based on the assembled sequence was also verified by digesting GS_43D16 with SalI and NotI. Eight fragments were expected from the double digestion. Two fragments, 7.9 kb and 7.11 kb were not resolved and termed Fragment IV (Figure 2C and 2D). Smallest fragment (0.6 kb) is not shown in Fig 2D. Taking these data together, it was concluded that the generated GS_43D16 sequence represents the physical distance of the soybean DNA present in that clone and no large fragments were remained to be sequenced.
Verification of the restriction maps of GS_43D16. A, KpnI and NotI map of the assembled GS_43D16 sequence. B, KpnI and NotI double digestion of selected GS_43D16 clones carrying the EZ::TN <NotI/KAN-3> transposon insertions. Eight fragments were expected from KpnI and NotI digestion of GS_43D16 carrying no transposons (A). Only five fragments were observed, because some of the fragments had similar mobilities in the gel. Some of these fragments were resolved after transposon insertion. A close relationship was observed between the restriction fragment sizes determined by gel electrophoresis and that by sequence data and location of transposon insertions (Table 1). m1, λHindIII ladders, m2, 1 kb DNA ladder (New England Biolabs Inc., Beverly, MA). C, SalI-NotI map of the assembled GS_43D16 sequence. D, SalI and NotI digestion of GS_43D16. Eight fragments were expected from the double digestion of GS_43D16 (Figure 2C). Six fragments were resolved from the digestion of the clone (43 in 2D). 7.9 kb and 7.11 kb fragments were not resolved (Fragment IV, twice the intensity of either Fragment III or Fragment V) and 0.6 kb SalI-NotI fragment is not included in 2D.
Genes underlying the Rps1-k
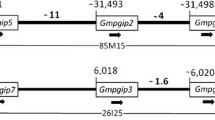
GS_18J19 overlaps with one end of GS_43D16. GS_99I16 comprised 51,109 bp sequences of GS_43D16 (Figure 3). There were 99.99%, 99.85% and 99.96% identities between the overlapping sequences of GS_18J19 and GS_43D16, GS_43D16 and GS_99I16, and GS_18J19 and GS_99I16, respectively. These results indicate high quality of the assembled sequences. High identity of GS_43D16 sequence with the overlapping regions of GS_18J19 and GS_99I16 suggested that there was no rearrangement in GS_43D16, from which Rps1-k-1, Rps1-k-2 and Rps1-k-3 were previously isolated [1].
Molecular characterization of the Rps1 -k region. A) The overlapping three BAC clones containing two CC-NB-LRR genes (green box) of the Rps1-k region are depicted. Sequences of individual BACs were utilized to show their overlapping regions. The composite Rps1-k region, shown at the top of the figure, is based on the sequences of these three BACs. GS_99I16 does not carry the 5'-end of Rps1-k-1, which is shown with a truncated green box. B) Arrangement of predicted genes and retrotransposons in the Rps1-k region. The green colored boxes represent full-length genes (Rps1-k-1 and Rps1-k-2); the red colored boxes represent partial genes; the blue colored boxes represent retroelements; white boxes represent introns in the predicted genes. Boxes above the ruler represent genes that have coding sequence on the forward strand, whereas the boxes under the ruler indicate the genes that are on the reverse strand. Detailed annotation data are presented in Table 2.
The gene content of an 184,111 bp contig sequence (GenBank Accession Number EU450800) carrying the Rps1-k locus derived from the GS_43D16 and GS_99I16 sequences was determined. Genes were predicted with GeneScan and GeneMark.hmm ES-3.0 programs [34]. To get a better gene prediction, genes predicted by GeneScan and GeneMark.hmm, and/or sequences having similarities to soybean ESTs were further analyzed by different NCBI Blast programs and sequence alignment programs. Putative annotations of the predicted genes were accomplished by BlastP searches. The gene content in the Rsp1-k region appears to be poor. Only a few full-length genes were predicted. These include two coiled coil-nucleotide binding-leucine rich repeat (CC-NB-LRR)-type Rps1-k genes and retrotransposons (Figure 3, Table 2).
Most of the identified genes are truncated. Genes were considered truncated when their predicted reading frames are partial. For example, the predicted cysteine proteinase shares an 88% identity with the first 126 amino acids of a soybean cysteine proteinase protein (BAA06030) followed by a premature stop codon. BlastN search against the soybean EST database was performed to support our gene prediction. ESTs showing high similarities but no complete identities to all predicted genes were identified (Table 2).
The Rps1-k region is composed of repetitive sequences
The major portion of the contiguous 184,111 bp sequence of the Rps1-k region is comprised of repetitive sequences including simple repeat sequences, tandem repeats and retroelements. The simple repeat and tandem repeat sequences were identified using Sputnik and tandem repeats finder. Sixteen simple repeat sequences were identified (Table 3). Sixty-three tandem repeats were revealed with copy numbers ranging from 1.8 to 72 and unit length varying from 7 to 310 bp (Table 4). The consensus motif length of the tandem repeat containing 72 copies is 24 bp. Sequence data from individual reads confirmed that they are tandem repeats in head-to-tail orientation. This sequence was used to query the soybean GSS (genomic survey sequence) database and a number of sequences with high identities were revealed. The one (CL868124) showing highest identity to the consensus 24 bp tandem sequence came from the project on characterization of the heterochromatic, gene-poor centric regions of soybean chromosomes.
Another abundant tandem repeat contains the consensus AATCAAG sequence. There are 12.3 copies of this repeat sequence between positions 163,795 and 163,880 and 11.3 copies between 178,234 and 178,312. Several soybean tandem repeat sequences, SB92, STR120 and STR102, have been identified [14–16]. Seven copies of a tandem repeat sequence with 102 bp unit length were also found in the Rps1-k locus, but it shares no similarity with STR120 or STR102.
The ~20 kb intergenic sequence between Rps1-k-1 and Rps1-k-2 is primarily made up of repetitive sequences. Four simple repeat sequences were localized in this interval. Notably, a 220 bp sequence was found at two locations, one between positions 24,318 and 24,537 and the other one between positions 29,963 and 30,182. This sequence encodes part of a protein sharing high similarity to the receptor-like protein kinase, Xa21 (BAD27933).
A copia/Ty1-like retroelement, SIRE1–8, was identified from the assembled 184,111 bp sequence of the Rps1-k region [35]. The 9.5 kb sequence encoding the SIRE1–8 element was used to query the soybean EST database. Two ESTs (CB063565 and CO983516) showed 99% identity to part of the gag-pol encoding sequence, one EST showed 92% identity to the LTRs and one EST exhibited similarities to the envelope-like sequence.
The CC-NB-LRR-type gene, Rps1-k-3 evolved through intramolecular recombination in Escherchia coli
Previously it was reported that a CC-NB-LRR gene, Rps1-k-3 was evolved from recombination between Rps1-k-1 and Rps1-k-2 [1]. The gene was comprised of 5' end from Rps1-k-1 and 3'-untranslated region from Rps1-k-2. This gene was isolated form GS_43D16 but not from either GS_18J19 or GS_99I16. This observation suggested that these two BAC clones did not overlap [1]. Therefore, it was concluded that the two Class I CC-NB-LRR genes isolated from GS_18J19 and GS_99I16 were unique.
Following sequencing, Rps1-k-3 was not identified from GS_43D16. Physical mapping of GS_43D16 (Figure 2) and high identities between overlapping sequences of three independent BAC clones (Figure 3) suggested strongly that the complete sequence of GS_43D16 was obtained and this clone did not go through any rearrangement. Rps1-k-3 was identified from a binary clone, p43-10 cloned in the pTF101.1 vector [1]. The clone was isolated from a library of binary clones prepared from GS_43D16 DNA partially digested with BamHI. Since this gene was not found in GS_43D16, the gene must have evolved through intramolecular recombination in Escherichia coli. Comparison of the insert sequence of p43-10 with GS_43D16 sequence revealed that the insert of the binary clone carries sequences identical to a BamHI fragment that contains an internal BamHI site (Figure 4). However, p43-10 insert DNA does not contain a segment of the GS_43D16 including the internal BamHI site. This gene presumably originated from recombination in E. coli. By looking at the recombinant breakpoint in Rps1-k-3, it was hypothesized that two identical 174 bp sequences (21,980 through 22,154 and 46,473 through 46,647 of the Rps1-k region shown in Figure 3B) of the 3'-end of both Rps1-k-1 and Rps1-k-2 were involved in the RecA-independent recombination process as shown in Figure 4.
Generation of the Rps1 -k-3 through intramolecular recombination. Locations of Rps1-k-1 and Rps1-k-2 on the GS_43D16 sequence are shown. Partial BamHI digested GS_43D16 DNA was cloned into the binary vector pTF101.1 and a library of binary clones was obtained. The library was screened for LRR sequences. Binary clone p43-10 contained the Rps1-k-3 gene, which is not present in GS_43D16 (Figure 3). This gene was presumably originated from intramolecular recombination in E. coli. Three BamHI (B) sites involved in generation of the Rps1-k-3 [1] are shown on the map. Solid line shows the region cloned in p43-10 and broken line indicates the region not found in p43-10. Presumably this region was lost during the recombination process in E.coli. The possible recombination process involved in the evolution of Rps1-k-3 is shown at lower part of the figure. The two identical 174 bp sequences of Rps1-k-1 (red line) and Rps1-k-2 (black line) involved in the recombination process are shown within the blue open circle (21,980 through 22,154 of Rps1-k-1 and 46,473 through 46,647 of Rps1-k-2 in the Rps1-k region shown in Figure 3B). The proposed model for the recombination event in E. coli is based on the article by Weisberg and Adhya [65].
The major recombination pathway in E. coli requires RecA [36]. Therefore, recA- strains, such as DH10B used in our experiment, are considered for recombinant gene cloning experiments to avoid any recombination events. Unfortunately, RecA-independent intraplasmid recombination does occur in these recA- strains. The frequency of recombination is however much lower as compared to that observed in recA+ strains [37, 38]. A recent study suggested that RecA-independent recombination is suppressed by single-strand DNA exonuclease (ssExos) activity. In absence ssExos, the extent of RecA-independent recombination in recA- strains is comparable to that in recA+ strains [39].
Discussion
Genomes of higher plants vary significantly in their size and complexity. Repetitive DNA sequences have been shown to be the major determinant of genome sizes in higher plants [13]. The prevalence of transposable elements and retroelements can promote unequal crossing-over leading to transposon-mediated rearrangements and gene duplications [40]. It has been hypothesized that transposable elements play a major role in the expansion and diversification of transmembrane receptor kinase-type disease resistance Xa21 gene family [9]. The abundance of retroelements has been observed in several genomic regions containing R genes or RGA loci, such as barley powdery mildew resistance gene, Mla, and Citrus virus resistance gene, Ctv [7, 8]. The variability among 14 rice Xa21 gene members has been considered to be generated mainly from the rearrangements mediated by transposon-like elements [9]. Rps1-k genes are arranged closely. About 38 copies of Rps1-k-like sequences were predicted to exist in the soybean genome. Most of the copies are clustered in the Rps1-k region [32]. A copia-like retroelement, Tgmr, has previously been reported from the Rps1-k region [41]. It is possible that retrotransposons facilitated the amplification of the Rps1-k gene family.
In many plant species such as Arabidopsis thaliana and Medicago tuncatula, chromosome arms are differentiated into euchromatic and heterochromatic regions [42–44]. Recently, Lin et al. [14] showed that in soybean heterochromatic regions are also delimited from euchromatin. Studies in Arabidopsis, Medicago and Lycopersicon esculentum have shown that the euchromatin has a high gene density, whereas pericentromeric heterochromatin is largely comprised of repetitive sequences [44–46]. The Rps1-k region is composed of mostly tandem repeat sequences and retroelements (Figure 3; Table 2). The gene content is very similar to that of a soybean BAC clone identified from the pericentromeric heterochromatin [14]. FISH mapping showed that SIRE1 and other retroelements are sequestered to the heterochromatic and/or pericentromeric regions [14]. The tandem repeat sequences and retroelements including SIRE1 are commonly abundant in heterochromatic and/or pericentromeric regions of the soybean genome. Therefore, the Rps1-k region could be located in heterochromatic region which may be pericentromeric.
The possible microcollinearities of the Rps1-k locus with genomic sequences of plant species such as Arabidopsis, Medicago and Lotus japonicus were investigated. An R protein-like sequence of Medicago genomic clone, MTH2-138E10, showed 65% identities to Rps1-k-2. However, no synteny was observed between the Rps1-k region and MTH2-138E10 sequence. A limited synteny of the Rps1-k locus was observed with the Lotus genome. Two copies of a Lotus Rps1-k homolog located five kb apart showed 54%–58% identity with the Rps1-k-2 protein. These two genes are located in two overlapping Lotus BAC clones, LjT02F05 and LjT20J15. Apart from the Lotus Rps1-k homolog, no nucleic acid sequences of these two Lotus BAC clones showed similarity to sequences of the Rps1-k region. In order to identify susceptible haplotype (rps1), the Rps1-k contig sequence was compared with the available BAC end sequences from the SoyGD database that contains sequences of the cultivar Forrest carrying the susceptible rps1 gene [27]. No sequence from LG N of the Forrest haplotype was identified that showed similarity to the Rps1-k contig reported here (Figure 3B).
It has been reported that plant disease resistance gene loci exhibit extensive loss of synteny. R gene-like sequences frequently lack syntenic map locations between the cereal species rice, barley, and foxtail millet [47]. An effort to clone the rice homolog of the barley Rpg1 gene was unsuccessful; because, although the DNA markers flanking Rpg1 were syntenic between rice and barley, the region containing the gene is absent in the syntenic rice genome [48]. These observations imply that R gene loci evolve faster than the rest of the genomes. This is further supported by comparative sequence analysis conducted in crucifers and grasses [49]. R genes may be located in less stable regions of the genome such as telomeric or pericentromeric regions where synteny is poorly conserved [50]. The tomato Tm-2 gene resides in a heterochromatic region near the centromere of chromosome 9 [51]. The Rpg1 gene is located near the telomere of the short arm of barley chromosome 1 [48]. The tomato Mi-1 gene is located at the border region between euchromatin and heterochromatin [52]. The lack of microsynteny of the Rps1-k region with the currently available genome sequences and abundance of repeat sequences including retroelements suggested that Rps1-k is located in a heterochromatic region which could be pericentromeric.
The Rps1-k-1 and Rps1-k-2 genes are about 20 kb apart (Figure 3). Most frequently R genes are arranged in clusters, and genes within one cluster are mostly derived from a common ancestral gene [4]. This clustering feature is considered to facilitate the expansion of R gene numbers and race-specificities through recombination and positive selection [5]. Study of multiple, genetically linked R gene families has provided insight into the molecular mechanisms of R gene evolution and the generation of novel recognition specificity. Seven family members of Xa21 were identified within a 230-kb region [9]. Similarly, seven members of the tomato I2 gene family reside in a 90 kb region [53]. The tomato Cf-2 locus contains two nearly identical resistance genes in a 17 kb fragment [54]. Both tomato Cf4 and Cf9 loci comprised of four additional tandemly duplicated paralogous copies within a 36-kb region [55]. Paralogous R gene sequences have also been reported to map more distantly. For example, members of the lettuce Dm3 family span at least 3.5 Mb with at least 120 kb distance between two gene members [56]. Although in these examples members are evolved from one progenitor gene [4], three distinct CC-NB-LRR gene families were identified in the Mla locus within a 240-kb region [7, 57]. The potato R1 locus also contains three fast evolving CC-NB-LRR genes that undergo frequent sequence exchanges among members of individual groups [58].
Plants have to generate novel resistance specificities to combat the quickly evolved pathogens. This clustering feature can facilitate the expansion of R gene numbers and the generation of new R gene specificities through recombination and positive selection [5]. Paralogous R gene sequences were most likely evolved through unequal recombination. The maize Rp1 locus, carrying nine paralogues, is probably the best example of unequal recombination for evolution of tandem paralogous R gene sequences [59]. An unequal crossing over between Arabidopsis RPP8 and its paralog, RPHA8 most likely resulted in rpp8 [60]. An unequal crossing over event was detected at the Rps1-k region leading to tandem duplication [32].
Conclusion
Genomes of higher plants vary significantly in their size and complexity because of the existence of a large amount of repetitive sequences. It was observed that the Rps1-k region is composed of mostly tandem repeat sequences and retroelements. Several disease resistance genes have been found in the less stable regions of the genome such as telomeric or pericentromeric regions where synteny is poorly conserved. The lack of microsynteny of the Rps1-k region with the currently available genome sequences and abundance of repeat sequences in the locus suggest that Rps1-k is located in a heterochromatic region that could be pericentromeric.
Methods
BAC DNA sequencing
The details of sequencing strategies of the three BACs, GS_18J19, GS_43D16 and GS_99I16 were described previously [1]. The sequence reads generated were assembled using the Phred/Phrap/Consed package [61, 62].
Directional sequencing of GS_43D16
The EZ::TN <NotI/KAN-3> transposon insertion BAC clones were generated using the EZ::TN in-Frame Linker insertion kit (Epicentre, Madison, WI). The transposon insertion sites were mapped by NotI digestion. Southern hybridization was carried out to physically map the position of transposon insertion in each clone. Both ends of GS_43D16 were used as probes. The 5'-end sequence of GS_43D16 was amplified with primers: (i) GS_43D16 end1F: CTGTAAATTATAAACACATGCCAT and (ii) GS_43D16-end1R: GCTGAATTTCAGTGTAGTGGCGTTTAC. The 3'-end sequence of GS_43D16 was amplified with primers: (i) GS_43D16 end2F: CCCATCCTCATTAATACTTCACACCAC and (ii) GS_43D16 end2R: GTAGTGGAAGTCTATAGTTGTATACCTCTC. BAC DNA was prepared using the alkaline lysis minipreparation procedure. The clones were sequenced in a 96-well plate using either NotI/KAN-3 FP-2 or NotI/KAN-3 RP-2 primer provided in the EZ::TN in-Frame Linker insertion kit (Epicentre, Madison, WI). Sequencing was conducted at the Iowa State University DNA Facility.
Gene prediction and sequence analysis
Two gene prediction software packages were used in analyzing the BAC sequences: GENSCAN and GeneMark.hmm ES-3.0 (E – eukaryotic; S – self-training; 3.0 – the version) [34]. The Arabidopsis-based scoring matrix was applied when using GENSCAN. Arabidopsis, maize, rice and Medicago were used as model species when GeneMark.hmm was applied. To more accurately predict gene content in the Rps1-k region, the predicted genes were further analyzed using different BLAST programs of the NCBI Basic Local Alignment Search Tool (Blast) server [63]: (i) discontiguous Mega Blast program with entrez query limited to Arabidopsis, lotus, Medicago and soybean; (ii) Blastn against the soybean EST database; (iii) BlastX and (iv) BlastP. Soybean EST distribution on the BAC sequence was evaluated using the BlastN program. The simple repeat sequences and tandem repeat sequences were identified using Sputnik and tandem repeats finder program [64], respectively.
References
Gao H, Narayanan NN, Ellison L, Bhattacharyya MK: Two classes of highly similar coiled coil-nucleotide binding-leucine rich repeat genes isolated from the Rps1-k locus encode Phytophthora resistance in soybean. Mol Plant Microbe Interact. 2005, 18: 1035-1045. 10.1094/MPMI-18-1035.
Hammond-Kosack KE, Parker JE: Deciphering plant-pathogen communication: fresh perspectives for molecular resistance breeding. Curr Opin Biotechnol. 2003, 14: 177-193. 10.1016/S0958-1669(03)00035-1.
Martin GB, Bogdanove AJ, Sessa G: Understanding the functions of plant disease resistance proteins. Annu Rev Plant Biol. 2003, 54: 23-61. 10.1146/annurev.arplant.54.031902.135035.
Richly E, Kurth J, Leister D: Mode of amplification and reorganization of resistance genes during recent Arabidopsis thaliana evolution. Mol Biol Evol. 2002, 19: 76-84.
Michelmore RW, Meyers BC: Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process. Genome Res. 1998, 8: 1113-1130.
Graham MA, Marek LF, Shoemaker RC: Organization, expression and evolution of a disease resistance gene cluster in soybean. Genetics. 2002, 162: 1961-1977.
Wei F, Wing RA, Wise RP: Genome dynamics and evolution of the Mla (powdery mildew) resistance locus in barley. Plant Cell. 2002, 14: 1903-1917. 10.1105/tpc.002238.
Yang ZN, Ye XR, Molina J, Roose ML, Mirkov TE: Sequence analysis of a 282-kilobase region surrounding the citrus Tristeza virus resistance gene (Ctv) locus in Poncirus trifoliata L. Raf. Plant Physiol. 2003, 131: 482-492. 10.1104/pp.011262.
Song WY, Pi LY, Wang GL, Gardner J, Holsten T, Ronald PC: Evolution of the rice Xa21 disease resistance gene family. Plant Cell. 1997, 9: 1279-1287. 10.1105/tpc.9.8.1279.
Arumuganathan K, Earle ED: Nuclear DNA content of some important plant species. Plant Mol Biol Rep. 1991, 9: 208-218. 10.1007/BF02672069.
Goldberg RB: DNA sequence organization in the soybean plant. Biochem Genet. 1978, 16: 45-68. 10.1007/BF00484384.
Gurley WB, Hepburn AG, Key JL: Sequence organization of the soybean genome. Biochim Biophys Acta. 1979, 561: 167-183.
Kubis S, Schmidt T, Heslop-Harrison JS: Repetitive DNA Elements as a Major Component of Plant Genomes. Annals of Botany. 1998, 82: 45-55. 10.1006/anbo.1998.0779.
Lin JY, Jacobus BH, SanMiguel P, Walling JG, Yuan Y, Shoemaker RC, Young ND, Jackson SA: Pericentromeric regions of soybean (Glycine max L. Merr.) chromosomes consist of retroelements and tandemly repeated DNA and are structurally and evolutionarily labile. Genetics. 2005, 170: 1221-1230. 10.1534/genetics.105.041616.
Morgante M, Jurman I, Shi L, Zhu T, Keim P, Rafalski JA: The STR120 satellite DNA of soybean: organization, evolution and chromosomal specificity. Chromosome Res. 1997, 5: 363-373. 10.1023/A:1018492208247.
Vahedian MA, Shi L, Zhu T, Okimoto R, Danna K, Keim P: Genomic organization and evolution of the soybean SB92 satellite sequence. Plant Mol Biol. 1995, 29: 857 -8862. 10.1007/BF00041174.
Blanc G, Wolfe KH: Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell. 2004, 16: 1667-1678. 10.1105/tpc.021345.
Schlueter JA, Dixon P, Granger C, Grant D, Clark L, Doyle JJ, Shoemaker RC: Mining EST databases to resolve evolutionary events in major crop species. Genome. 2004, 47: 868-876. 10.1139/g04-047.
Shoemaker RC, Polzin K, Labate J, Specht J, Brummer EC, Olson T, Young N, Concibido V, Wilcox J, Tamulonis JP, Kochert G, Boerma HR: Genome duplication in soybean (Glycine subgenus soja). Genetics. 1996, 144: 329-338.
(LIS) LIS: [http://www.comparative-legumes.org/]
Cregan PB, Jarvik T, Bush AL, Shoemaker RC, Lark KG, Kahler AL, Kaya N, VanToai TT, Lohnes DG, Chung J, Specht JE: An integrated genetic linkage map of the soybean genome. Crop Sci. 1999, 39: 1464-1490.
Danesh D, Penuela S, Mudge J, Denny RL, Nordstrom H, Martinez JP, Young ND: A bacterial artificial chromosome library for soybean and identification of clones near a major cyst nematode resistance gene. Theor Appl Genet. 1998, 96: 196-202. 10.1007/s001220050727.
Marek LF, Shoemaker RC: BAC contig development by fingerprint analysis in soybean. Genome. 1997, 40: 420-427.
Meksem K, Zobrist K, Ruben E, Hyten D, Quanzhou T, Zhang HB, Lightfoot D: Two large-insert soybean genomic libraries constructed in a binary vector: applications in chromosome walking and genome wide physical mapping. Theor Appl Genet. 2001, 101: 747-755. 10.1007/s001220051540.
Salimath SS, Bhattacharyya MK: Generation of a soybean BAC library, and identification of DNA sequences tightly linked to the Rps1-k disease resistance gene. Theor Appl Genet. 1999, 98: 712-720. 10.1007/s001220051125.
Santra DK, Sandhu D, Tai T, Bhattacharyya MK: Construction and characterization of a soybean yeast artificial chromosome library and identification of clones for the Rps6 region. Funct Integr Genomics. 2003, 3: 153-159. 10.1007/s10142-003-0092-8.
Shultz JL, Kurunam D, Shopinski K, Iqbal MJ, Kazi S, Zobrist K, Bashir R, Yaegashi S, Lavu N, Afzal AJ, Yesudas CR, Kassem MA, Wu C, Zhang HB, Town CD, Meksem K, Lightfoot DA: The Soybean Genome Database (SoyGD): a browser for display of duplicated, polyploid, regions and sequence tagged sites on the integrated physical and genetic maps of Glycine max. Nucleic Acids Res. 2006, 34: D758-65. 10.1093/nar/gkj050.
Wrather JA, Stienstra WC, Koenning SR: Soybean disease loss estimates for the United States from 1996 to 1998. Can J Plant Pathol. 2001, 23: 122-131.
Song QJ, Marek LF, Shoemaker RC, Lark KG, Concibido VC, Delannay X, Specht JE, Cregan PB: A new integrated genetic linkage map of the soybean. Theor Appl Genet. 2004, 109: 122-128. 10.1007/s00122-004-1602-3.
Kasuga T, Salimath SS, Shi J, Gijzen M, Buzzell RI, Bhattacharyya MK: High resolution genetic and physical mapping of molecular markers linked to the Phytophthora resistance gene Rps1-k in soybean. Mol Plant-Microbe Interact. 1997, 10: 1035-1044. 10.1094/MPMI.1997.10.9.1035.
Schmitthenner AF, Hobe M, Bhat RG: Phytophthora sojae races in Ohio over a 10-year interval. Plant Dis. 1994, 78: 269-276.
Bhattacharyya MK, Narayanan NN, Gao H, Santra DK, Salimath SS, Kasuga T, Liu Y, Espinosa B, Ellison L, Marek L, Shoemaker R, Gijzen M, Buzzell RI: Identification of a large cluster of coiled coil-nucleotide binding site-leucine rich repeat-type genes from the Rps1 region containing Phytophthora resistance genes in soybean. Theor Appl Genet. 2005, 111: 75-86. 10.1007/s00122-005-1993-9.
Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigo R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X: The sequence of the human genome. Science. 2001, 291: 1304-1351. 10.1126/science.1058040.
Lomsadze A, Ter-Hovhannisyan V, Chernoff YO, Borodovsky M: Gene identification in novel eukaryotic genomes by self-training algorithm. Nucleic Acids Res. 2005, 33: 6494-6506. 10.1093/nar/gki937.
Laten HM, Havecker ER, Farmer LM, Voytas DF: SIRE1, an endogenous retrovirus family from Glycine max, is highly homogeneous and evolutionarily young. Mol Biol Evol. 2003, 20: 1222-1230. 10.1093/molbev/msg142.
Clark AJ, Margulies AD: Isolation and Characterization of Recombination-Deficient Mutants of Escherichia Coli K12. Proc Natl Acad Sci U S A. 1965, 53: 451-459. 10.1073/pnas.53.2.451.
Bi X, Liu LF: recA-independent and recA-dependent intramolecular plasmid recombination. Differential homology requirement and distance effect. J Mol Biol. 1994, 235: 414-423. 10.1006/jmbi.1994.1002.
Sosio M, Bossi E, Donadio S: Assembly of large genomic segments in artificial chromosomes by homologous recombination in Escherichia coli. Nucleic Acids Res. 2001, 29: E37-10.1093/nar/29.7.e37.
Dutra BE, Sutera VA, Lovett ST: RecA-independent recombination is efficient but limited by exonucleases. Proc Natl Acad Sci U S A. 2007, 104: 216-221. 10.1073/pnas.0608293104.
Fedoroff N: Transposons and genome evolution in plants. Proc Natl Acad Sci USA. 2000, 97: 7002-7007. 10.1073/pnas.97.13.7002.
Bhattacharyya MK, Gonzales RA, Kraft M, Buzzell RI: A copia-like retrotransposon Tgmr closely linked to the Rps1-k allele that confers race-specific resistance of soybean to Phytophthora sojae. Plant Mol Biol. 1997, 34: 255-264. 10.1023/A:1005851623493.
Fransz P, Armstrong S, Alonso-Blanco C, Fischer TC, Torres-Ruiz RA, Jones G: Cytogenetics for the model system Arabidopsis thaliana. Plant J. 1998, 13: 867-876. 10.1046/j.1365-313X.1998.00086.x.
Fransz PF, Armstrong S, de Jong JH, Parnell LD, van Drunen C, Dean C, Zabel P, Bisseling T, Jones GH: Integrated cytogenetic map of chromosome arm 4S of A. thaliana: structural organization of heterochromatic knob and centromere region. Cell. 2000, 100: 367-376. 10.1016/S0092-8674(00)80672-8.
Kulikova O, Gualtieri G, Geurts R, Kim DJ, Cook D, Huguet T, de Jong JH, Fransz PF, Bisseling T: Integration of the FISH pachytene and genetic maps of Medicago truncatula. Plant J. 2001, 27: 49-58. 10.1046/j.1365-313x.2001.01057.x.
Initative AG: Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000, 408: 796-815. 10.1038/35048692.
Wang Y, Tang X, Cheng Z, Mueller L, Giovannoni J, Tanksley SD: Euchromatin and pericentromeric heterochromatin: comparative composition in the tomato genome. Genetics. 2006, 172: 2529-2540. 10.1534/genetics.106.055772.
Leister D, Kurth J, Laurie DA, Yano M, Sasaki T, Devos K, Graner A, Schulze-Lefert P: Rapid reorganization of resistance gene homologues in cereal genomes. Proc Natl Acady Sci U S A. 1998, 95 (1): 370-375. 10.1073/pnas.95.1.370.
Han F, Kilian A, Chen JP, Kudrna D, Steffenson B, Yamamoto K, Matsumoto T, Sasaki T, Kleinhofs A: Sequence analysis of a rice BAC covering the syntenous barley Rpg1 region. Genome. 1999, 42: 1071-1076. 10.1139/gen-42-6-1071.
Gale MD, Devos KM: Plant comparative genetics after 10 years. Science. 1998, 282: 656-659. 10.1126/science.282.5389.656.
Michelmore R: Genomic approaches to plant disease resistance. Curr Opin Plant Biol. 2000, 3: 125-131. 10.1016/S1369-5266(99)00050-3.
Motoyoshi F, Ohmori T, Murata M: Molecular characterization of heterochromatic regions around the Tm-2 locus in chromosome 9 of tomato. Symp Soc Exp Biol. 1996, 50: 65-70.
Zhong XB, Bodeau J, Fransz PF, Williamson VM, van Kammen A, de Jong JH, Zabel P: FISH to meiotic pachytene chromosomes of tomato locates the root-knot nematode resistance gene Mi-1 and the acid phosphatase gene Aps-1 near the junction of euchromatin and pericentromeric heterochromatin of chromosome arms 6S and 6L, respectively. Theor ApplGenet. 1999, 98: 365-370. 10.1007/s001220051081.
Simons G, Groenendijk J, Wijbrandi J, Reijans M, Groenen J, Diergaarde P, Van der Lee T, Bleeker M, Onstenk J, de Both M, Haring M, Mes J, Cornelissen B, Zabeau M, Vos P: Dissection of the Fusarium I2 gene cluster in tomato reveals six homologs and one active gene copy. Plant Cell. 1998, 10: 1055-1068. 10.1105/tpc.10.6.1055.
Dixon MS, Jones DA, Keddie JS, Thomas CM, Harrison K, Jones JD: The tomato Cf-2 disease resistance locus comprises two functional genes encoding leucine-rich repeat proteins. Cell. 1996, 84: 451-459. 10.1016/S0092-8674(00)81290-8.
Parniske M, Hammond-Kosack KE, Golstein C, Thomas CM, Jones DA, Harrison K, Wulff BB, Jones JD: Novel disease resistance specificities result from sequence exchange between tandemly repeated genes at the Cf-4/9 locus of tomato. Cell. 1997, 91: 821-832. 10.1016/S0092-8674(00)80470-5.
Meyers BC, Chin DB, Shen KA, Sivaramakrishnan S, Lavelle DO, Zhang Z, Michelmore RW: The major resistance gene cluster in lettuce is highly duplicated and spans several megabases. Plant Cell. 1998, 10: 1817-1832. 10.1105/tpc.10.11.1817.
Wei F, Gobelman-Werner K, Morroll SM, Kurth J, Mao L, Wing R, Leister D, Schulze-Lefert P, Wise RP: The Mla (powdery mildew) resistance cluster is associated with three NBS-LRR gene families and suppressed recombination within a 240-kb DNA interval on chromosome 5S (1HS) of barley. Genetics. 1999, 153: 1929-1948.
Kuang H, Wei F, Marano MR, Wirtz U, Wang X, Liu J, Shum WP, Zaborsky J, Tallon LJ, Rensink W, Lobst S, Zhang P, Tornqvist CE, Tek A, Bamberg J, Helgeson J, Fry W, You F, Luo MC, Jiang J, Robin Buell C, Baker B: The R1 resistance gene cluster contains three groups of independently evolving, type I R1 homologues and shows substantial structural variation among haplotypes of Solanum demissum. Plant J. 2005, 44: 37-51.
Hulbert SH, Webb CA, Smith SM, Sun Q: Resistance gene complexes: evolution and utilization. Annu Rev Phytopathol. 2001, 39: 285-312. 10.1146/annurev.phyto.39.1.285.
McDowell JM, Dhandaydham M, Long TA, Aarts MG, Goff S, Holub EB, Dangl JL: Intragenic recombination and diversifying selection contribute to the evolution of downy mildew resistance at the RPP8 locus of Arabidopsis. Plant Cell. 1998, 10: 1861-1874. 10.1105/tpc.10.11.1861.
Ewing B, Hillier L, Wendl MC, Green P: Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998, 8: 175-185.
Gordon D, Abajian C, Green P: Consed: a graphical tool for sequence finishing. Genome Res. 1998, 8: 195-202.
NCBI Basic Local Alignment Search Tool (Blast): [http://www.ncbi.nlm.nih.gov/BLAST/]
Weisberg RA, Adhya S: Illegitimate recombination in bacteria and bacteriophage. Annu Rev Genet. 1977, 11: 451-473. 10.1146/annurev.ge.11.120177.002315.
Acknowledgements
We are grateful to Drs. Phil Becraft, Adam Bogdanove, Randy C. Shoemaker and Steven Whitham for invaluable discussion and James Baskett for critically reading this manuscript. This research has been supported by USDA-NRI Grant No. 2001-35301-10577 and a grant from Iowa Soybean Association and ISU Agronomy Department Endowment Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
HG carried out all the studies presented in the paper. MKB conceived the study and participated in the experiment design and coordination, and helped with drafting and finalizing the manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Gao, H., Bhattacharyya, M.K. The soybean-Phytophthora resistance locus Rps1-k encompasses coiled coil-nucleotide binding-leucine rich repeat-like genes and repetitive sequences. BMC Plant Biol 8, 29 (2008). https://doi.org/10.1186/1471-2229-8-29
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2229-8-29