Abstract
Background
Enterobacter aerogenes and Klebsiella pneumoniae are common isolates in clinical microbiology and important as producers of extended spectrum β-lactamases (ESBL). The discrimination between both species, which is routinely based on biochemical characteristics, is generally accepted to be straightforward. Here we report that genotypically unrelated strains of E. aerogenes can be misidentified as K. pneumoniae by routine laboratories using standard biochemical identification and using identification automates.
Results
Ten clinical isolates, identified as K. pneumoniae or K. terrigena with the routinely used biochemical tests and with API-20E, were identified as E. aerogenes by tDNA-PCR – an identification that was confirmed by 16S rRNA gene sequencing for five of these isolates. Misidentification also occurred when using the automated identification systems Vitek 2 and Phoenix, and was due to delayed positivity for ornithine decarboxylase and motility. Subculture and prolonged incubation resulted in positive results for ornithine decarboxylase and for motility. It could be shown by RAPD-analysis that the E. aerogenes strains belonged to different genotypes.
Conclusions
Clinical E. aerogenes isolates can be easily misidentified as Klebsiella due to delayed positivity for ornithine decarboxylase and motility. The phenomenon may be widespread, since it was shown to occur among genotypically unrelated strains from different hospitals and different isolation dates. A useful clue for correct identification is the presence of an inducible β-lactamase, which is highly unusual for K. pneumoniae. In several instances, the use of genotypic techniques like tDNA-PCR may circumvent problems of phenotypic identification.
Similar content being viewed by others
Background
Enterobacteriaceae with β-lactam resistance due to the production of Extended-Spectrum β-Lactamases (ESBL) were discovered in the eighties and since that time became epidemic and endemic in hospitals worldwide [1]. Since two decades, 20 to 40 ESBL-producing strains are isolated monthly in our hospital. Amongst the clinical isolates from our hospital, two new TEM-β-lactamase genes were described [2]. In Belgium, as well as in other countries, a shift occurred from Klebsiella pneumoniae isolates as the predominant ESBL-producers [3] to predominance of Enterobacter aerogenes. It is also known that most of the E. aerogenes isolates in the Belgian hospitals belong to one of two predominant Belgian clones (BEI and BEII) [4], a situation which is comparable to that in other countries [5]. During the last years however, we found that several isolates that were identified as K. pneumoniae or K. terrigena by conventional biochemical testing were in fact E. aerogenes as could be shown by the use of genotypic methods, i.e. tDNA-PCR, validated by 16S rRNA gene sequencing, and by extensive phenotypic testing (including subculture and prolonged incubation).
Results
For ten clinical isolates, i.e. seven ESBL-producing clinical isolates collected during 2001 and three more recently collected isolates (Table 1), the API20E codes 5205773 (isolates GA1, GA2, GA3, MN2, MN3 and VGM), 5205753 (isolates DHJ1 and DHJ3) or 5204673 (isolates RA and DBH) were obtained. The first code resulted in a weak identification as either E. aerogenes, K. pneumoniae or Raoultella (Klebsiella) planticola, the second code did not yield any identification, and the third code resulted in a weak but acceptable identification as K. terrigena. The isolates with the codes 5205773 and 5205753 were identified as K. pneumoniae by additional biochemical testing due to negative reactions for motility (tested in semi-solid agar) and ornithine decarboxylase. However, these isolates all possessed an inducible cefalosporinase, as detected on the antibiogram using a disk approximation test, a finding which strongly contradicts an identification as K. pneumoniae or Klebsiella sp.
In fact, the first hint that these strains, phenotypically identified as K. pneumoniae, were actually E. aerogenes, came from tDNA-PCR based identification. Using this method, all isolates were identified as E. aerogenes, and this by comparison of the obtained fingerprint – composed of amplified intergenic tRNA spacers of 101, 106, 111, 115, 121, 189, 190, 198 or 289, and 391 bp in length – with a library containing fingerprints of more than 3000 strains belonging to hundreds of species, available at http://allserv.ugent.be/~mvaneech/All_C.txt.
Confirmation of this genotypic identification was obtained by 16S rRNA gene sequencing for five isolates (Table 1). Analysis yielded a similarity of between 99.8% and 100% to E. aerogenes Genbank entries.
The presence of a genuine K. pneumoniae isolate in patient DHJ (Table 1) further complicated the identification.
This observation lead us to carry out additional phenotypic testing. Using the hanging drop method for testing motility, a few motile cells were observed, and upon retesting in semi-solid medium, weak migration could be observed. Like most biochemical tests in the routine laboratory, ornithine decarboxylase is read after overnight or 24 hours of incubation, but when the incubation period was prolonged to up to 2–5 days, all isolates tested positive.
Because at present automated systems are frequently used for routine identification, a selection of six strains, containing four isolates of the study and two controls, i.e. phenotypically correctly identified E. aerogenes (LBV268) and K. pneumoniae (BG) isolates, were tested in two different systems, i.e. Vitek 2 (bioMérieux, Marcy l'Etoile, France) and Phoenix (BD Biosciences, Sparks, Md.). Both automated systems yielded the same results as the API20E, i.e. that the E. aerogenes isolates, aberrant due to a slow reaction for motility and ornithine decarboxylase, were misidentified as K. pneumoniae (Table 1). This misidentification by the automated systems is not unexpected, since they are based on biochemical testing only and a reading time of 24 hours or less. The control strains were correctly identified.
Disk diffusion antibiotic susceptibility testing, carried out according the NCCLS guidelines, revealed basically the same resistotype for all isolates, characterized by resistance to ceftazidime and susceptibility to ceftriaxone. Additional resistance to aztreonam was observed for some isolates, reflecting the most dominant resistance patterns for the E. aerogenes isolates in our hospital. All isolates were also found to carry high-level resistance to cefoxitin, which is highly unusual for Klebsiella spp. Furthermore, the disk-approximation test with an amoxycillin-clavulanic acid disk close to β-lactam disks on Mueller-Hinton II agar, showed a combined pattern of synergy (broadening of the inhibition zone in the direction of clavulanic acid) and antagonism (flattening of the inhibition zone), which is suggestive for a combination of an ESBL and an inducible β-lactamase. Again, inducible β-lactamases are very rare in Klebsiella spp. but typical for Enterobacter spp. It should be noticed that this phenomenon will not be detected by automated MIC-determination systems like Vitek 2 and Phoenix. Using PCR and sequencing as described previously [2], the presence of TEM-5 could be shown in the isolates of patients GA and MN, and SHV-4 in the isolate of patient DHJ.
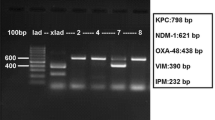
The genotypic relationship of the phenotypically aberrant isolates was investigated with AP-PCR. Isolates GA1, GA2, GA3, MN2, MN3 and DBH were corresponding to Belgian clone BEI (Figure 1, pattern A), isolates VGM, DHJ1 and DHJ3 were closely related to Belgian clone BEII (Figure 1, pattern B, differing from pattern C, characteristic of clone BEII, by a single extra band) while isolate RA was not related to any of the others (Figure 1, pattern D). This genetic diversity among the phenotypically aberrant strains makes it probable that strains with this kind of aberrant phenotype are not restricted to a single clone within E. aerogenes.
RAPD analysis of Enterobacter aerogenes and Klebsiella pneumoniae strains used in this study Lane M: DNA molecular weight marker (100 base pair ladder). The E. aerogenes RAPD-types are indicated as A, B, C and D and the K. pneumoniae types are indicated as K1 an K2. RAPD-type A corresponds to clone BEI, type B to clone BEII, type C to BEII-related clone and type D presents a strain unrelated to clones BEI and BEII. Negative image of ethidium bromide stained agarose electrophoresis
Discussion
K. pneumoniae and E. aerogenes are taxonomically closely related species [6] which share many characteristics. Our sequencing results (unpublished) and those of others [6] confirm that the genus Enterobacter is polyphyletic and that E. aerogenes should be placed within the genus Klebsiella. However, differentiation between E. aerogenes and K. pneumoniae is usually straightforward when based on testing for ornithine decarboxylase and motility, both positive for E. aerogenes. This is also reflected in the name "Klebsiella mobilis" [7], which is known as a valid synonym for E. aerogenes. Apparently, in some E. aerogenes isolates, the expression of these characteristics can be weak and/or delayed, and these are therefore scored negative when reading is done after the incubation periods that are routinely applied (overnight – 24 hours).
However, the combination of an inducible β-lactamase and/or high-level cefoxitin resistance, which are rare in Klebsiella spp., and an identification as Klebsiella sp. should warrant further investigation.
The phenomenon of E. aerogenes misidentified as K. pneumoniae or K. terrigena due to delayed or negative ornithine decarboxylase and motility was reported previously, and was also discovered because of unexpected imipenem resistance of the so-called K. pneumoniae isolates [8]. Also in this case, subculture and prolonged incubation restored the positivity for these characteristics.
The problem of misidentification of E. aerogenes as K. pneumoniae (or even K. terrigena) is probably not uncommon and probably also geographically widespread. This can be deduced from the following considerations: i) this phenomenon of misidentification of E. aerogenes was already reported in 1993 [8], ii) the phenomenon occurred in genotypically different organisms, iii) the isolates were found over an extended period of time – also recently, and finally iv) we received similar strains from other Belgian hospitals (unpublished data). It should be noted that misidentification also occurred when using the newer and automated systems like Vitek2 and Phoenix.
On the other hand, the problem seems to be largely unknown. In a recent study, Hansen and colleagues [9] carried out an interlaboratory comparison of the efficacy of 18 biochemical tests for the identification of 242 strains of different Klebsiella species and of Enterobacter aerogenes, but do not mention the problem of possible delayed activity, possibly also because the study started from validated strains of each species.
Conclusions
Identification in a routine clinical microbiology laboratory of the most commonly encountered Enterobacteriaceae is usually considered to be fast and straightforward, but apparently identification problems may occur due to diminished or delayed expression of some characteristics, even for well-established species like E. aerogenes and K. pneumoniae. Here we showed that E. aerogenes isolates exist for which ornithine decarboxylase and motility are negative or delayed positive, and that as such these isolates can be misidentified as K. pneumoniae. This phenomenon may be quite frequent and geographically widespread. Genotypic identification techniques like tDNA-PCR, which moreover are cheaper than phenotypic testing for many bacterial species, can be semi-automatized, are faster and mostly have a higher discriminatory power, which is also reflected in this study.
Methods
tDNA-PCR
tDNA-PCR was carried out using the outwardly directed tRNA-gene consensus primers T5A (5'AGTCCGGTGCTCTAACCAACTGAG) and T3B (5'AGGTCGCGGGTTCGAATCC), thus amplifying the intergenic tRNA-spacers, as described previously [10, 11]. Electropherograms were normalized using GeneScan Analysis software, version 2.1 (Applied Biosystems). Transformation of GeneScan tables (ABI310, McIntosh) to tables on IBM, separation into separate digital fingerprints, and comparison of the digital tDNA-PCR fingerprints with a library of tDNA-PCR-fingerprints obtained from a large collection of reference strains, was done using in house software described previously [10].
16S rRNA gene sequencing
For five of the phenotypically aberrant isolates, the complete 16S rRNA sequence was determined by amplification of the 16S rRNA-gene with the primers 5'-AGTTTGATCCTGGCTCAG and 5'-TACCTTGTTACGACTTCGTCCCA [12], and sequencing was performed as described previously [12]. Comparison of the obtained 16S rDNA-sequence with all known sequences in Genbank was carried out using the BLAST software (National Center for Biotechnology Information http://www.ncbi.nlm.nih.gov/BLAST/).
RAPD-analysis for strain typing
The genotypic relationship of the isolates was investigated using arbitrarily primed PCR with RAPD Ready-to-Go beads (Amersham Pharmacia, Uppsala, Sweden) and the ERIC II primer 5'-AAGTAAGTGACTGGGGTGAGCG [13]. Analysis of the fingerprints was obtained by visual interpretation on ethidium bromide stained electrophoresis gels.
References
Gniadkowski M: Evolution and epidemiology of extended-spectrum β-lactamases (ESBLs) and ESBL-producing microorganisms. Clin Microbiol Inf. 1991, 7: 597-608. 10.1046/j.1198-743x.2001.00330.x.
Claeys G, De Baere T, Vaneechoutte M, Verschraegen G: Caz-hi, an extended-spectrum TEM β-lactamase (TEM-61), is derived from Caz-lo (TEM-11) by in vivo-selection. Antimicrob Agents Chemother. 1998, 42: 3328-9.
Podschum R, Ulmann U: Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998, 11: 589-603.
De Gheldre Y, Struelens MJ, Glupczynski Y, De Mol P, Maes N, Nonhoff C, Chetoui H, Sion C, Ronveaux O, Vaneechoutte M: National epidemiologic surveys of Enterobacter aerogenes in Belgian hospitals from 1996 to 1998. J Clin Microbiol. 2001, 39: 889-96. 10.1128/JCM.39.3.889-896.2001.
Bosi C, Davin-Regli A, Bornet C, Mallea M, Pages J-M, Bollet C: Most Enterobacter aerogenes strains in France belong to a prevalent clone. J Clin Microbiol. 1999, 37: 2165-9.
Boye K, Hansen DS: Sequencing of the 16S rDNA of Klebsiella: taxonomic relations within the genus and to other Enterobacteriaceae. Int J Med Microbiol. 2003, 292: 495-503.
Bascomb S, Lapage SP, Willcox WR, Curtis MA: Numerical classification of the tribe Klebsielleae. J Gen Microbiol. 1971, 66: 279-95.
Ehrhardt AF, Sanders CC, Thomson KS, Watanakunakorn C, Trujilan-Martin : Emergence of resistance to imipenem in Enterobacter isolates masquerading as Klebsiella pneumoniae during therapy with imipenem/cilastatin. Clin Infect Dis. 1993, 17: 120-2.
Hansen DS, Aucken HM, Abiola T, Podschun R: Recommended test panel for differentiation of Klebsiella species on the basis of a trilateral interlaboratory evaluation of 18 biochemical tests. J Clin Microbiol. 42: 3665-9. 10.1128/JCM.42.8.3665-3669.2004.
Baele M, Baele P, Vaneechoutte M, Storms V, Butaye P, Devriese LA, Verschraegen G, Gillis M, Haesebrouck F: Application of tDNA-PCR for the identification of Enterococcus species. J Clin Microbiol. 2000, 38: 4201-7.
Welsh J, McClelland M: Genomic fingerprints produced by PCR with consensus tRNA gene primers. Nucl Acids Res. 1991, 19: 861-6.
Vaneechoutte M, Claeys G, Steyaert S, De Baere T, Peleman R, Verschraegen G: Moraxella canis isolation from an ulcerated metastatic lymph node. J Clin Microbiol. 2000, 38: 3870-1.
Grundmann HJ, Towner KJ, Dijkshoorn L, Gerner-Smidt P, Maher M, Seifert H, Vaneechoutte M: Multicenter study using standardized protocols and reagents for evaluation of reproducibility of PCR-based fingerprinting of Acinetobacter spp. J Clin Microbiol. 1997, 35: 3071-7.
Acknowledgements
We thank Leen Van Simaey, Catharine De Ganck, Inge Bocquaert and Rita De Beul for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
GC, GV and AM were responsible for sample collection and initial biochemical identification. PVD and GW carried out automated biochemical identification. GW in addition carried out extended biochemical characterization. TDB and MV carried out the molecular analysis. GC, GV, TDB and MV drafted the manuscript. All authors read and approved the final manuscript.
Geert Claeys, Thierry De Baere contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Claeys, G., De Baere, T., Wauters, G. et al. Extended-Spectrum β-lactamase (ESBL) producing Enterobacter aerogenes phenotypically misidentified as Klebsiella pneumoniae or K. terrigena. BMC Microbiol 4, 49 (2004). https://doi.org/10.1186/1471-2180-4-49
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2180-4-49