Abstract
Background
We have previously reported that altered culture conditions (a broth media with shaking) could induce a strain of Helicobacter pylori to assume a long spiral morphology resembling that described for Helicobacter heilmannii. The present study was initiated to determine if other strains of H. pylori could be induced to assume that morphology and if doing so would alter the expression of immunodominant proteins.
Results
The six strains used in this study were American Type Culture Collection 43504, 43579, 49503, 51652, and 51653, and Sydney Strain I. Each strain was grown on solid media and in broth culture using conditions previously shown to induce the long spiral morphology in strain 43504. DNA from each was subjected to urease gene fingerprint analysis. Results of the molecular analysis showed identical fingerprint patterns for each strain independent of culture source, indicating that only a single strain was present in each culture. Expression of immunodominant proteins was assessed by SDS polyacrylamide gel electrophoresis and Western blotting with hyperimmune rabbit anti H. pylori sera or serum from an H. pylori infected patient. Analysis of protein profiles revealed some variation between strains but no significant differences associated with morphologic alterations.
Conclusions
These results indicate that growth of H. pylori in a long spiral form does not affect expression of immunodominant proteins, thus in vivo growth in the long spiral form (not documented to date) would not be distinguishable by serology.
Similar content being viewed by others
Background
Curved and spiral-shaped bacteria have been described in the gastric mucosa of humans for over a century, but it was not until the early 1980s that Helicobacter pylori was successfully cultured and determined to cause chronic gastritis and peptic ulcer disease [1–3]. It is now well established that this organism is a human pathogen, and evidence gathered to date suggests that chronic H. pylori infection can also predispose an individual to gastric cancer [4].
H. pylori is a gram negative bacterium typically appearing as a curved rod or short spiral. It is believed to be one of the more common pathogenic infections of man, with prevalence rates reaching 30–60% in developed countries, depending on age and socioeconomic status [4–7]. A related organism, Helicobacter heilmannii, has also been linked to gastritis in humans [8, 9]. Infection with H. heilmannii is thought to occur at a significantly lower frequency and to induce a more mild gastritis than infection with H. pylori[10, 11]. Current estimates place the frequency of infection with H. heilmannii between 0.2 and 1.7% [6, 12, 13].
Standard tests for detection of H. pylori infection include detection of urease activity, serologic detection of antibodies to the organism, histologic observation of the organism in gastric sections, and culture of the bacteria from gastric biopsies. Both H. pylori and H. heilmannii possess urease activity [14, 15] and antibody-based assays may not be able to distinguish between these organisms due to cross-reactivity to common immunoreactive proteins. As there is no routine culture method currently available for H. heilmannii, differentiation of these two organisms is dependent on morphology alone. H. heilmannii has been described as a corkscrew shape, typically appearing as a long spiral with greater than four turns, as compared to H. pylori's typical curved rod-like or short spiral (up to three turns) morphology [8, 10, 11].
We have previously reported that growth in a broth media with shaking can induce H. pylori to assume a long spiral morphology more closely resembling that described for H. heilmannii than for typical H. pylori. However, our initial report was based on only one American Type Culture Collection (ATCC) stock strain of H. pylori (strain 43504) [16]. The present study was initiated to determine if other strains of H. pylori could be induced to display the altered morphology, and to evaluate the expression of immunodominant proteins of bacteria in the altered state for each of these strains.
We obtained five stock strains of H. pylori (43504, 43579, 49503, 51652, and 51653) from ATCC, and Sydney Strain I (SS1) from the University of Delaware. Each strain was grown on both blood agar plates and in broth using our modified technique. Cultures grown in broth media with shaking showed organisms displaying a long spiral morphology with greater than 5 turns, while curved rods and short spiral organisms were seen when each of the six strains tested were grown on solid media. SDS polyacrylamide gel electrophoresis analysis of protein profiles and Western blot evaluation of immunoreactivity for each strain revealed no significant differences related to culture method. Urease gene fingerprint analysis, using DNA prepared from both plate and broth cultures for each strain indicated that only a single H. pylori strain was present in each culture. Our results suggest that in vivo growth of H. pylori in a long spiral (5 to 20 turns) or corkscrew form (which has not been documented to date) would be unlikely to affect currently available serologic tests used to screen patients for infection with the organism. Furthermore, our results indicate that reliance on morphology alone to distinguish between H. pylori and H. heilmannii in a clinical setting may need to be reevaluated.
Results
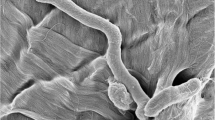
Examination of wet preparations and Gram-stained smears from cultures grown using our modified technique demonstrated that growth in broth with shaking resulted in Gram negative organisms displaying a long spiral morphology for each of the six H. pylori strains tested. Spirals containing greater than 5 (in some cases as many as 20) turns were observed in each instance (Figure 1A), consistent with our previous report. Blood agar-grown cultures for each of the six strains showed curved rod-like organisms and short spirals, morphology more typical for H. pylori (Figure 1B).
Atypical and classic H. pylori morphology. Gram-stained slides were prepared from a broth culture of H. pylori ATCC 43504 (A), or a plate culture of H. pylori ATCC 43579 (B). Slides were examined and photographed with a 63× objective. Atypical long spiral forms were seen in broth culture, while organisms displaying classic H. pylori morphology were seen in cultures grown on solid media.
Protein profiles of both plate and broth cultures of H. pylori ATCC strains 43504, 43579, 49503, 51652, 51653, and strain SS1 generated by denaturing polyacrylamide gel electrophoresis did not reveal any apparent differences in expression pattern. Although some slight differences were noted between the strains tested, the expression profiles for the blood agar- and broth-grown forms of each strain appeared to be identical (Figure 2A, 2B). Similarly, Western Blot using polyclonal rabbit anti-H. pylori sera or selected human sera revealed no significant differences in immunoreactivity between plate-grown (Figure 3A) and broth-grown (Figure 3B) H. pylori when the same strain was examined. Although some variation between strains was observed, there did not appear to be any significant difference in production of immunoreactive proteins that could be attributed to the altered morphology.
SDS-PAGE of plate and broth cultures of H pylori strains. Cultures of H. pylori ATCC strains 43504, 43579, 49503 (A), and ATCC strains 51652, 51653, and strain SS1 (B), were harvested from both plate (P) and broth (B) cultures and electrophoresed in 11% SDS polyacrylamide gels as described. No differences in protein expression due to the variations in morphology were observed. Apparent molecular weights (kDa) are indicated.
Immunoblots probed with rabbit anti- H. pylori or patient sera. Western blot membranes of H. pylori ATCC strains 43504, 43579, 49503 (A), ATCC 51652, 51653, and strain SS1 (B) plate (P) and broth (B) grown forms prepared as described in Methods were reacted with sera from a rabbit immunized with H. pylori ATCC 43504. Western blot membranes of H. pylori ATCC strains 43504, 43579, 49503 (C), ATCC 51652, 51653, and strain SS1 (D) plate (P) and broth (B) grown forms prepared as described in Methods were reacted with sera from a patient infected with H. pylori. No apparent differences in the expression of immunoreative proteins as due to the observed morphologic shift were noted. Apparent molecular weights (kDa) are indicated.
Molecular analysis of the urease A/B region of the H. pylori genome revealed no differences in fingerprint pattern between blood agar- and broth grown cultures for each strain, indicating that only the particular organism of interest was present in each culture. PCR on all DNA examined resulted in the expected 2.4-kb product (Figure 4A), and five distinct restriction patterns were observed among the six strains tested (Figure 4B). Urease gene fingerprints for ATCC strains 43579 and 49503 appeared to be identical.
Molecular analysis of the H. pylori urease A/B region. (A) Amplification of the ureA / ureB region of six different strains of H. pylori was performed using primer pair HpR1 / HpR2 as described. Molecular size standards (kb) and the expected 2.4-kb product are indicated. Lanes 1 to 13 are polymerase chain reactions with DNA from H. pylori strains grown on solid media (lanes 1, 3, 5, 7, 9, and 11), DNA from H. pylori strains grown in broth (lanes 2, 4, 6, 8, 10, and 12), and a water negative control (lane 13). Lanes 1 and 2 are ATCC strain 43504; lanes 3 and 4 are ATCC strain 43579; lanes 5 and 6 are ATCC strain 49503; lanes 7 and 8 are ATCC strain 51652; lanes 9 and 10 are ATCC strain 51653, and lanes 11 and 12 are SS1. (B) Restriction patterns resulting from Hae III digestion of the 2.4 kb PCR product obtained from the amplification of H. pylori genomic DNA with primers HpR1 and HpR2 are shown. Molecular size standards (kb) are indicated. Lanes 1 to 13 are digests from DNA from H. pylori strains grown on solid media (lanes 1, 3, 5, 7, 9, and 11), DNA from H. pylori strains grown in broth (lanes 2, 4, 6, 8, 10, and 12), and a water negative control (lane 13). Lanes 1 and 2 are ATCC strain 43504; lanes 3 and 4 are ATCC strain 43579; lanes 5 and 6 are ATCC strain 49503; lanes 7 and 8 are ATCC strain 51652; lanes 9 and 10 are ATCC strain 51653, and lanes 11 and 12 are SS1.
Discussion
Members of the genus Helicobacter can be found in a variety of forms including rods, curved rods, short spirals, long spirals or corkscrews, and non-culturable coccoid forms. Many of the species are differentiated by their morphology, which is particularly true for H. pylori and the more recently discovered H. heilmannii. Both of these organisms have been shown to be a cause of gastric disease in humans, and they are identified in clinical material based on their morphologic appearance on histologic section or Gram stain. We previously reported that growth of a single H. pylori strain (strain ATCC 43504) in a liquid media with shaking yielded bacteria that more closely resembled the morphology reported for H. heilmannii than that known to be typical for H. pylori[8, 10, 11, 16]. The present study was performed in order to extend these early observations to determine if multiple strains of this organism have the ability to form long spiral or corkscrew shapes under certain culture conditions, and to determine if significant changes in immunologically relevant proteins are seen with this morphology shift. Our results demonstrate that H. pylori ATCC 43504 and five additional H. pylori strains (ATCC strains 43579, 49503, 51652, and 51653, and Sydney Strain I) reversibly undergo the expected morphologic change using our modified broth culture technique. We further demonstrate that the alteration of the bacterial morphology does not appear to change the expression pattern of the immunodominant proteins. In addition we demonstrate, using PCR amplification and restriction digest typing methods, that a single strain of H. pylori is present in each culture and is responsible for the morphologic shift observed.
While growth of H. pylori in a long spiral form may occur only under the conditions reported in this study, it seems likely that the morphologic changes would also occur under specific conditions in the environment of the gastric epithelium. This conclusion is supported by the observation that H. pylori may appear corkscrew shaped in biopsy specimens [12]. As these morphologically variant organisms are indistinguishable from H. heilmannii under the microscope, a number of the reported cases of H. heilmannii infection may actually be due to H. pylori. A report describing review of antral biopsy smears where H. heilmannii was previously observed found that a number of these diagnoses could not be confirmed as the organisms present appeared more similar to H. pylori[17]. Other studies have found that H. pylori and H. heilmannii are rarely co-localized, although mixed infections have been reported [13, 15, 17–22]. These observations, taken in the context of our recent results, indicate that while it may be possible to harbor both organisms, it may also be possible that the differences observed are due to a single bacterial strain found in specific microenvironments within the gastric mucosa.
The gold standard for diagnosis of H. pylori infection is considered to be culture or histologic assessment of the organism's presence. Despite reported limitations in the value of serology as more than a screening tool, it is significantly less expensive and more convenient than other more invasive methods [4, 5, 23]. While the conditions which may allow H. pylori to assume a long spiral form in vivo are not known at the present time, our results indicate that under these conditions expression of major immunoreactive proteins would remain unchanged, thus current serologic methods would be unaffected by this shift in bacterial morphology. The failure to detect alterations in protein expression was surprising given the dramatic differences in morphology. Certainly the differences observed could be expected to influence the motility of the organism and possibly its ability to associate with or adhere to gastric tissues. Furthermore, although there were no detected differences in the qualitative expression of proteins, there may well be a difference in quantitative expression of some proteins that may have pathophysiologic significance.
Conclusions
In this report we provide evidence that multiple strains of H. pylori can undergo a morphologic shift under appropriate culture conditions, implying that it is a trait common to this Helicobacter species. It is conceivable that under the right conditions, this organism may also be able to undergo this change in vivo. Our work demonstrates that while such an alteration in vivo could affect the ability to distinguish gastric Helicobacters on stained sections, the ability to diagnose H. pylori infection serologically would remain unaffected by the shift. We are currently expanding our studies in an attempt to determine which bacterial proteins undergo changes in expression pattern allowing the organism to alter its morphology. Future efforts in this area will focus on attempts to isolate and identify long spiral organisms from clinical specimens, and to determine whether culture conditions could be used to support the growth of these organisms in either the long spiral or conventional morphology.
Methods
Culture
Lyophilized H. pylori stocks (ATCC 43504, 43579, 49503, 51652, and 51653, Rockville, MD) were each reconstituted with 0.5 ml Brucella broth (Difco, Detroit, MI) with 0.1% cyclodextrin (BBCD) and transferred to 25 ml of BBCD in a 25 cm2 tissue culture flask. Similarly, 1.0 ml of a frozen 15% glycerol stock containing Sydney Strain I (University of Delaware) was thawed and added to 25 ml BBCD in a 25-cm2 tissue culture flask. Flasks were incubated at 37°C on a rocking platform (28 cycles per minute) under microaerophilic conditions using the BBL Campy Pack Plus System (Becton Dickinson, Cockeysville, MD). After three days, 5 ml of each culture was transferred to 100 ml BBCD in a 150 ml filter unit receiver flask (Nalgene, Rochester, NY) and incubated at 37°C with rocking as described above in the GasPak CO2 Jar System (Becton Dickinson, Cockeysville, MD). After four days, 6 drops of broth culture were used to inoculate 4 plates of Trypticase Soy Agar with 5% sheep blood (Becton Dickinson, Cockeysville, MD) for each strain. All plates were incubated inverted in a GasPak CO2 Jar System. Each remaining broth culture was harvested by centrifugation at 10,000 g for 10 minutes at 4°C. Pellets were resuspended in phosphate buffered saline (PBS) with 5 mM MgCl2 and washed a total of five times. After four days, the plate cultures were harvested by scraping the colonies from the agar, suspending them in PBS with 5 mM MgCl2, and washing as described above. The protein concentration of each suspension was determined using a Bradford microassay (BioRad, Richmond, CA).
Microscopy
Wet preparations and Gram-stained (Becton Dickinson, Cockeysville, MD) smears were prepared for all cultures at times of transfer and harvest. These were examined using a Zeiss Axioplan Photomicroscope with 63× and 100× objectives under brightfield, phase contrast, and differential interference contrast settings.
Protein analysis
Harvested bacteria from broth and plate cultures were diluted to a concentration of 0.5 mg/ml in sample buffer containing 0.1 M dithiothreitol as the reducing agent. The samples were electrophoresed on 11% SDS polyacrylamide mini gels using a Hoeffer Mighty Small II electrophoresis unit (Pharmacia Biotech, Piscataway, NJ). Separated antigens were transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, NH) using a Nova blot semi-dry transfer system (Pharmacia Biotech, Piscataway, NJ). Membranes were blocked overnight at 4°C with 0.5% bovine serum albumin in PBS, dried, and stored desiccated until use.
Western blot
Membranes were incubated with polyclonal rabbit anti H. pylori [diluted 1:1000 in blotting buffer (PBS with 1% nonfat dry milk)] or serum from a patient with H. pylori (diluted 1:500 in blotting buffer) for 1 hour at room temperature. Membranes were washed three times with PBS and then incubated with a 1:1000 dilution of biotinylated goat anti rabbit IgG (H + L) or a 1:1000 dilution of biotinylated goat anti human IgG (H + L) (Kirkegaard and Perry Labs, Gaithersburg, MD). Membranes were washed with PBS as described above and incubated with a 1:1000 dilution of peroxidase conjugated streptavidin (Kirkegaard and Perry Labs, Gaithersburg, MD) for 1 hour at room temperature. Membranes were washed again as described and incubated with 4-chloro-1-naphthol substrate (7.8 mM 4-chloro-1-naphthol diluted 1:2 with 1:1500 30% H2O2 in citrate phosphate buffer, pH 4.0). Membranes were washed with distilled water, air dried, and evaluated for banding patterns.
DNA isolation
DNA was isolated from harvested cultures of H. pylori from either plate or broth medium. Briefly, 7.5 volumes of 6 M guanidine HCl/0.1 M sodium acetate, pH 5.5 was added to 100 μl aliquots of harvested bacteria. Preparations were mixed by inversion and placed on an orbital shaker (Lab-Line, Melrose Park, IL) at 150 rpm for 60 minutes at room temperature. Samples were centrifuged at 18,000 × g for 30 minutes at 4°C. Each supernatant was extracted twice with phenol:chloroform:isoamyl alcohol (25:24:1) and once with chloroform:isoamyl alcohol (24:1), followed by addition of 0.1 volume 3 M sodium acetate and 2.5 volumes 95% ethyl alcohol to precipitate the DNA. Samples were placed at -20°C for a minimum of 60 minutes, followed by centrifugation as described above. Pellets were washed with 70% ethyl alcohol, dried, and resuspended in sterile Tris-EDTA (TE) buffer.
PCR and urease gene fingerprinting analysis
A 2.4 kilobase (kb) product encompassing the H. pylori urease A and urease B (ureA and ureB) structural genes was amplified as described by Foxall [24] using primers HpR1 (5' AGGAGAATGAGATGA 3') and HpR2 (5' ACTTTATTGGCTGGT 3'). Oligonucleotides were purchased from Integrated DNA Technologies (Coralville, IA). Each reaction contained 100 nanograms (ng) H. pylori genomic DNA in a 50 μl reaction volume (2.0 ng/μl) unless otherwise noted. Amplification was carried out in a Perkin Elmer 9600 thermocycler (Foster City, CA). Reaction parameters were as follows: denaturation for 5 minutes at 95°C, followed by 34 cycles of 94°C for 1 minute, 53°C for 1 minute, and 72°C for 3 minutes. This was followed by 1 cycle of 94°C for 1 minute, 53°C for 1 minute, and 72°C for 5 minutes. Following amplification, 20 μl samples of each reaction were mixed with 5 μl gel loading solution (Sigma Biochemical, St. Louis, MO) and analyzed on 0.7% Seakem GTG agarose gels (FMC Bioproducts, Rockland, ME) to verify the amplification of the 2.4 kb product. Amplified products were ethanol precipitated and resuspended in sterile Tris-EDTA buffer. Each DNA sample was incubated with 20 U of restriction enzyme Hae III (Promega, Madison, WI) in the appropriate buffer overnight at 37°C. Individual restriction patterns for each strain were analyzed by electrophoresis of each digested sample on 4.0% Nusieve 3:1 agarose (FMC Bioproducts, Rockland, ME) gels.
References
Lee A, O'Rourke J: Gastric bacteria other than Helicobacter pylori. Gastroenterol Clin North Am. 1993, 22: 21-42.
Marshall B: Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983, i: 1273-1275.
Warren JR: Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983, i: 1273-10.1016/S0140-6736(83)92719-8.
Czinn SJ: Serodiagnosis of Helicobacter pylori in pediatric patients. J Pediatr Gastroenterol Nutr. 1999, 28: 132-134. 10.1097/00005176-199902000-00006.
Fennerty MB:Helicobacter pylori.Arch Intern Med. 1994, 154: 721-727. 10.1001/archinte.154.7.721.
Mention K, Michaud L, Guimber D, De Lasalle E, Vincent P, Turck D, Gottran F: Characteristics and Prevalence of Helicobacter heilmannii infection in Children Undergoing Upper Gastrointestinal Endoscopy. J Pediatr Gastroenterol Nutr. 1999, 29: 533-539. 10.1097/00005176-199911000-00012.
Talley NJ, Noack KB: The worldwide prevalence of Helicobacter pylori: Asymptomatic infection and clinical states associated with infection in adults. In: Helicobacter pylori Biology and Clinical Practice. Edited by: Goodwin CS, Worsley BW. 1993, Boca Raton, CRC Press, 63-84.
Morris A, Ali MR, Thomsen L, Hollis B: Tightly spiral shaped bacteria in the human stomach: another cause of active chronic gastritis?. Gut. 1990, 31: 139-143.
Solnick JV, O'Rourke J, Lee A, Paster B, Dewhirst F, Tompkins L: An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J Infect Dis. 1993, 168: 379-385.
Heilmann K, Borchard F: Gastritis due to Spiral Shaped Bacteria Other than Helicobacter pylori: Clinical, Histological, and Ultrastructural Findings. Gut. 1991, 32: 137-140.
Oliva MM, Lazenby A, Perman JA: Gastritis associated with Gastrospirillum hominis in children. Comparison with Helicobacter pylori and review of the literature. Mod Path. 1993, 6: 513-515.
Andersen LP: New Helicobacter species in humans. Dig Dis. 2001, 19 (2): 112-115. 10.1159/000050664.
Dieterich C, Wiesel P, Neiger R, Blum A, Corthesy-Theulaz I: Presence of Multiple " Helicobacter heilmannii " Strains in an Individual Suffering from Ulcers and in His Two Cats. J Clin Microbiol. 1998, 36 (5): 1366-1370.
Andersen LP, Norgaard A, Holck S, Blom J, Elsborg L: Characterization of a Culturable Gastrospirillum hominis (Helicobacter heilmannii) Strain isolated from Human Gastric Mucosa. J Clin Microbiol. 1999, 37 (4): 1069-1076.
Solnick JV, O'Rourke J, Lee A, Tompkins L: Molecular Analysis of Urease Genes from a Newly Identified Uncultured Species of Helicobacter. Infect Immun. 1994, 62 (5): 1631-1638.
Fawcett PT, Gibney KM, Vinette KMB: Helicobacter pylori can be induced to assume the morphology of Helicobacter heilmannii. J Clin Microbiol. 1999, 37: 1045-1048.
Debongnie JC, Donnay M, Mairese J: Gastrospirillum hominis ("Helicobacter heilmannii"): A Cause of Gastritis, Sometimes Transient, Better Diagnosed by Touch Cytology?. Am J Gastroenterol. 1995, 90 (3): 411-416.
Dent JC, McNulty CAM, Uff JC, Wilkinson SP, Gear MW: Spiral Organisms in the Gastric Antrum. Lancet. 1987, i: 96-10.1016/S0140-6736(87)92754-1.
Fischer R, Samisch W: "Gastrospirillum hominis ": another four cases. Lancet. 1990, 335: 59-10.1016/0140-6736(90)90195-B.
Kern SE, Yardley JH, Kafonek DR, Epstein JI, Edlow DW, Morrison S, Moore GW, Gebhardt FC, Diamond MP: Three Cases of Gastric Spirochetelike Organisms. Gastroenterology. 1989, 96: 266-268.
Mazzucchelli L, Wilder-Smith CH, Ruchti C, Meyer-Wyss B, Merki HS: Gastrospirillum hominis in Asymptomatic, Healthy Individuals. Dig Dis Sci. 1993, 38 (11): 2087-2089.
Queiroz DMM, Cabral MMDA, Nogueira AMMF, Barbosa AJA, Rocha GA, Mendes EN: Mixed gastric infection by Gastrospirillum hominis and Helicobacter pylori. Lancet. 1990, 336: 507-508. 10.1016/0140-6736(90)92057-O.
Vaira D, Vakil N: Blood, urine, stool, breath, money, and Helicobacter pylori. Gut. 2001, 48: 287-289. 10.1136/gut.48.3.287.
Foxall PA, Hu LT, Mobley HL: Use of Polymerase Chain Reaction-Amplified Urease Structural Genes for Differentiation of Isolates. J Clin Microbiol. 1992, 30: 739-741.
Acknowledgements
We thank Dr. Diane Herson at the University of Delaware for providing us with H. pylori Sydney Strain I and Megan Mills and Laura Vella for technical support and culture work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
PTF conceived of the study and was primarily responsible for study design and coordination. KMG participated in study design and performed the SDS-PAGE gels and immunoblots. KMBV participated in study design, performed the molecular analyses, and drafted the manuscript. RP participated in study design. All authors participated in review and preparation of the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Vinette, K.M., Gibney, K.M., Proujansky, R. et al. Growth of Helicobacter pylori in a long spiral form does not alter expression of immunodominant proteins. BMC Microbiol 2, 24 (2002). https://doi.org/10.1186/1471-2180-2-24
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2180-2-24