Abstract
Background
The genus Morus, known as mulberry, is a dioecious and cross-pollinating plant that is the sole food for the domesticated silkworm, Bombyx mori. Traditional methods using morphological traits for classification are largely unsuccessful in establishing the diversity and relationships among different mulberry species because of environmental influence on traits of interest. As a more robust alternative, PCR based marker assays including RAPD and ISSR were employed to study the genetic diversity and interrelationships among twelve domesticated and three wild mulberry species.
Results
RAPD analysis using 19 random primers generated 128 discrete markers ranging from 500–3000 bp in size. One-hundred-nineteen of these were polymorphic (92%), with an average of 6.26 markers per primer. Among these were a few putative species-specific amplification products which could be useful for germplasm classification and introgression studies. The ISSR analysis employed six anchored primers, 4 of which generated 93 polymorphic markers with an average of 23.25 markers per primer. Cluster analysis of RAPD and ISSR data using the WINBOOT package to calculate the Dice coefficient resulted into two clusters, one comprising polyploid wild species and the other with domesticated (mostly diploid) species.
Conclusion
These results suggest that RAPD and ISSR markers are useful for mulberry genetic diversity analysis and germplasm characterization, and that putative species-specific markers may be obtained which can be converted to SCARs after further studies.
Similar content being viewed by others
Background
Mulberry (genus Morus) is an economically important plant used for sericulture, as it is the sole food plant for the domesticated silkworm, Bombyx mori. The genus Morus, which is widely distributed in Asia, Europe, North and South America, and Africa, is cultivated extensively in East, Central and South Asia for silk production. A few species of mulberry are also valued for their edible fruit (M. alba, M. indica and M. laevigata), timber (M. laevigata and M. serrata). Whereas it has been widely believed that mulberry species originated on the low slopes of the Himalayas bordering China and India, the study of Hou suggests a multicentered origin [1]. Since the classification of the genus Morus is mainly based on morphological characteristics, considerable differences exist among systematists as to the number of species that exist in this genus [2–7]. So far, more than 150 species of mulberry have been cited in the Index Kewensis, but a majority of them have been treated either as synonyms or as varieties rather than species, and some have been transferred to allied genera. A study carried out by Koidzumi in 1917 [3] recognised 24 species and one variety under the genus Morus based on the style length in female flowers and the nature of the stigma in male flowers. In contrast, more than 60 years later by analyzing the electrophoretic patterns of seven enzymes and sap proteins in 131 varieties of three mulberry species, M. bombycis, M. alba, and M. latifolia Hirano categorised them into seven varietal groups, and affinities among them [8]. Because of environmental influence, phenotypic traits in many cases fail to serve as unambiguous markers for systematics and diversity analysis [9]. Moreover, most of the putative mulberry species are dioecious and can cross-pollinate among themselves to produce fertile hybrids, suggesting that they have relatively close genetic relationships. Such a high degree of cross-species reproductive success is not encountered often in nature, and has thus created considerable doubt with regard to the species status of mulberry.
Molecular markers successfully developed during the last two decades have largely overcome the problems that are associated with phenotype-based classification. Initially, isozymes [10–12] and Restriction Fragment Length Polymorphisms (RFLPs) [13–17] served as reliable markers for genetic analyses in plants. But PCR based techniques developed in recent years such as Random Amplified Polymorphic DNA (RAPDs) [18, 19], Inter Simple Sequence Repeats (ISSR) [20], Amplified Fragment Length Polymorphisms (AFLPs) [21], and Simple Sequence Repeats (SSRs) [22], also called microsatellites, provide DNA markers that are dispersed throughout plant genomes [23] and are easier to reproduce and analyse. High levels of polymorphism and their co-dominant nature have made SSRs ideal markers for studying genetic diversity in plants [24–26]. However, the time and cost of identifying SSR motifs and designing primers for regions flanking SSRs have restricted the widespread use of microsatellites in plants [27, 28]. ISSR markers, which show dominant inheritance, use SSR repeat-anchored primers and are being used as an alternate tool in diversity studies. ISSR markers are useful in detecting genetic polymorphisms among accessions by generating a large number of markers that target multiple microsatellite loci distributed across the genome. Further, they are simpler to use than the SSR technique as prior knowledge of the target sequences flanking the repeat regions is not required [20, 29–31].
So far only a few attempts have been made to characterise the genetic diversity in mulberry by using molecular markers. These include AFLP based marker analysis [32], RAPD and DAMD profiles [33], ISSR based analysis [34], and genetic polymorphism estimation in mulberry hybrids using RAPDs [35]. In the current study we report the use of RAPD and ISSR markers for assessing the genetic diversity and relationships among 12 cultivated and 3 wild mulberry species.
Results
RAPD analysis
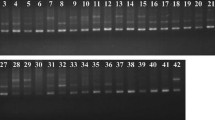
A total of 19 decamer oligonucleotide primers was used to investigate fifteen mulberry species. Each of the random primers produced distinct polymorphic banding patterns in all of the species examined. Typical results obtained with the primers OPY-13 and OPW-03 are shown in Fig. 1a and 1b, respectively. The size of the amplified products ranged from 500–3000 bp, with 3 – 9 bands per primer. A total of 119 RAPD polymorphic markers were generated by the 19 primers, at a rate of 6.26 markers per primer. The lowest number of polymorphic bands was obtained in a hexaploid, M. tiliaefolia (42), whereas a diploid species, M. sinensis, gave the maximum number of polymorphic bands (81) with all of the polymorphic primers. Triploid species M. laevigata and M. bombycis produced 57 and 73 polymorphic bands, respectively.
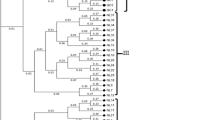
Distance matrix analysis of the RAPD data was calculated using WINDIST software. The values obtained for each pairwise comparison of RAPD fragments are shown in Table 3. Genetic distances among different mulberry species ranged from 0.220 (between M. rubra and M. bombycis) to 0.728 (between M. tiliaefolia and M. alba). The distance matrix based on RAPD data sets is graphically represented as a dendrogram using the UPGMA method shown in Fig. 3a.
RAPD analysis also revealed putative species-specific amplified products. One such band was observed in the species, M. sinensis, M. latifolia, and M. serrata, whereas two such bands were obtained in M. tiliaefolia and M. laevigata (Table 4).
ISSR analysis
Out of six ISSR primers tested, four gave distinct polymorphic products. Two primers, a 5' anchored (CA)7 and a 3' anchored (GT)8, did not yield any amplification products. The results obtained with the primer (TG)7 are shown in Fig. 2. The four ISSR primers produced 93 polymorphic markers at an average of 23.25 markers per primer. The highest number of markers was obtained with the primers (TG)7 and (CA)7Y (30), whereas the primer (TG)7Y resulted in the lowest number of markers (11). Among different species, M. rotundiloba showed the lowest number of polymorphic bands (19), whereas M. bombycis resulted in the highest number of polymorphic products (35). ISSR analysis, like RAPD analysis, did not show any correlation between the number of amplified fragments and the mulberry ploidy level. The genetic distance values based on ISSR analysis are presented in Table 5. The highest value of 0.909 was between M. rotundiloba and M. tiliaefolia, and the lowest value was 0.419, between M. rubra and M. bombycis. The distance matrix based on ISSR data sets was used to construct a dendrogram, which is shown in Fig. 3b.
Discussion
Mulberry is a perennial, heterogeneous outbreeding tree, the leaves of which are the exclusive food of the silk secreting insect, B. mori. Outbreeding in cultivated mulberry species is common and inter-species hybridization is often observed. Because of phenotypic plasticity, the occurrence of interspecific hybridization, mutation, and the absence of an unambiguous set of criteria for designating a true species, there is confusion in the systematic classification of mulberry. The present work evaluates the genetic diversity and relationships among fifteen mulberry species using RAPD and ISSR markers.
The wide variation in genetic distance among the different mulberry species revealed by both RAPD and ISSR techniques reflected a high level of polymorphism at the DNA level. Earlier studies by Sharma et al. using the AFLP technique [32] also showed a large genetic variation among different Morus genotypes. Such a high level of polymorphism, especially in cultivated mulberry, reflects the outcrossing nature of the species. Cluster analysis of RAPD and ISSR data using UPGMA revealed that the three wild species, namely, M. laevigata, M. serrata, and M. tiliaefolia, are genetically distant from the domesticated species studied here. The distinctness of the wild species as revealed by both RAPD and ISSR in the present study and that of Sharma et al. [32] can be attributed to their geographical isolation, which is in strong contrast to the outbreeding and high heterozygosity which have accompanied the long history of cultivation of domesticated mulberry species.
The ISSR profiles of mulberry generated by CA/TG repeat anchored primers showed that these repeats are abundant in the Morus genome. Vijayan and Chatterjee [34] also observed amplification of AC rich repeat based ISSR primers. However, this type of repeat is less abundant in plant genomes compared to other kinds of repeats [36], and the abundance of other classes of microsatellite repeats in the mulberry genome awaits to be demonstrated. The RAPD analysis revealed a close relationship between M. cathayana and M. rotundiloba, whereas these two species were found to be distinct in ISSR analysis. Such variation between RAPD and ISSR may be due to the fact that the PCR amplified profiles in the two marker assays originated from different repetitive and non-repetitive regions of the genomes, and the possibility that many co-migrating bands may be non-homologous, producing a background noise that could influence the results [37, 38]. Similar to the RAPD analysis, the ISSR results showed no correlation to ploidy status or to the number of amplified products. For example, in the triploid species, M. laevigata and M. bombycis, 23 and 35 polymorphic products were observed, respectively, whereas the tetraploid species M. serrata revealed 20 ISSR products, and in the hexaploid, M. tiliaefolia, 25 products were amplified. These results are consistent with the earlier studies of RAPD patterns in Chrysanthemum by Wolf & Rijn [39], which show that the ploidy level of a plant does not appear to influence the number of fragments amplified per primer.
Classification of Morus based on phenotypic variations or isozyme patterns should be reconsidered in the context of molecular analyses by RAPD and ISSR as well as that of AFLP [32]. Hirano [8] studied 131 varieties of cultivated mulberry morphologically classified as belonging to three species, viz., M. bombycis, M. alba, and M. latifolia. Examining isozymes and several sap proteins, he found no significant difference among these three species, prompting him to conclude that all three species are the same. However, our study as well as the AFLP study unambiguously place M. alba as a separate species distinct from the other two, which are likely to be independent species. Thus, RAPD and ISSR based molecular markers were able to distinguish differences between the species which were indistinguishable by isozyme based markers. Similarly, Koidzumi [3, 4] considered that M. lhou, M. multicaulis, and M. latifolia are similar and belong to a single species. However, their wide separation in the present study contradicts this observation. Additional phylogenetic studies using chloroplast or mitochondrial gene sequences or appropriate nuclear gene sequences can help to evaluate the systematic positions of these species.
Conclusions
The present study reveals that PCR based fingerprinting techniques, RAPD and ISSR, are informative for estimating the extent of genetic diversity as well as to determine the pattern of genetic relationships between different species of Morus, with polymorphism levels sufficient to establish informative fingerprints with relatively fewer primer sets. The information obtained from the present study could be of practical use for mapping the mulberry genome as well as for classical breeding. The genetic similarity of wild mulberry with other cultivated species is low as indicated by the separation of these two groups in both RAPD and ISSR analyses. The informative primers identified in our studies will be useful in genetic analysis of mulberry accessions in germplasm holdings. The putative species-specific bands can be used as probes to ascertain whether they are in low or high copy numbers in the mulberry genome, and such specific bands may be used for genotype characterization and grouping germplasm accessions. Further, putative species-specific RAPD markers could be converted to sequence characterized amplification regions (SCARs) after sequencing and designing primer pairs to develop robust species specific markers. The study also provides a basis for mulberry breeders to make informed choices on selection of parental material based on genetic diversity to help overcome some of the problems usually associated with a tree crop improvement program.
Methods
Plant materials
The wild and cultivated mulberry species were obtained from the Central Sericultural Germplasm Research Center, Hosur, India. The species used in the present study along with their key morphological characters and country of origin are listed in Table 1.
DNA extraction
DNA was extracted from fresh young leaves of five individuals of a species using a Nucleon Phytopure System (Amersham Pharmacia Biotech, UK) according to the manufacturer's instruction and pooled. The genomic DNA was quantified on 0.8% agarose gels and diluted to uniform concentration (10 ng/μl) for RAPD and ISSR analysis.
RAPD amplification
PCR reactions were performed according to the protocol of Williams et al. [18]. Briefly, PCR amplifications were carried out in an MJ Research Thermal Cycler PTC-200, in a reaction volume of 20 μl containing 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2 mM MgCl2, 0.2 μM primer, 0.1 mM each of dATP, dTTP, dCTP and dGTP, 0.5 U of Taq DNA polymerase (Gibco BRL, USA) and 20 ng of template DNA. The 19 random primers used in the current study were obtained from Operon Technologies Inc. (Alameda, CA, USA); these included OPW-1-4, OPY-1-11, OPY-13-14, and OPY-16-17. Amplification reactions were carried out using the following cycle profile: Initial denaturation at 93°C for 2 min followed by 45 cycles at 93°C for 1 min, 35°C for 1 min, 72°C for 2 min and a final 7 min extension at 72°C. PCR products were electrophoresed on a 1.5% agarose gel according to Sambrook et al. [40] in 1X TBE buffer (89 mM Tris-borate, 2 mM EDTA, pH 8.0) and stained with ethidium bromide. The gel image was recorded using a Gel Documentation System (UVP, UK).
ISSR amplification
The ISSR primers were synthesized on an Applied Biosystem DNA synthesizer based on core repeats [20], anchored either at the 5' or 3' end (Table 2). Amplification reactions were carried out in 20 μl containing 10 mM Tris-HCl (pH 8.4), 50 mM KCl, 0.1% Triton X-100, 2 mM MgCl2, 10 μM of each primer, 0.1 mM each of dCTP, dGTP, dTTP and 0.075 mM of cold dATP with 2 μCi of α-32P dATP, (6000 Ci/mmol from BRIT, JONAKI, Hyderabad, India), 1 U of Taq DNA polymerase (Gibco BRL, USA) and 20 ng of template DNA. PCR reactions were performed using an MJ Research Thermal Cycler PTC-200 with following amplification conditions: Initial denaturation of 2 min at 94°C, followed by 30 cycles of 94°C for 30s, annealing at 52°C for 45s, extension at 72°C for 2 min and a final extension at 72°C for 7 min. PCR reactions were terminated by adding 13.2 μl of stop solution (95% formamide, 20 mM EDTA, 0.5% bromophenol blue and xylene cyanol). 4 μl of sample DNA was denatured at 75°C for 2 min, chilled on ice and then run on a non-denaturing sequencing gel containing 6% polyacrylamide, 3 M urea and 1X TBE at 900 V for 20 hr. The gels were dried and exposed for 2–10 hr on X-ray film (Kodak-Biomax) for autoradiography.
Data analysis
DNA banding patterns generated by RAPD and ISSR were scored for the presence (1) or for absence (0) of each amplified band. All RAPD and ISSR assays were repeated twice and only the reproducible bands were scored. For considering a marker as polymorphic, the absence of an amplified product in at least one species was used as a criterion. For genetic distance analysis, WINDIST software of the WIN BOOT package was used with the NTSYS format. Cluster analysis was based on similarity matrices using the unweighted pair group method analysis (UPGMA) program in the WIN BOOT software package [41]. The Dice coefficient was used for dendrogram construction with a sample number of 100.
References
Hou YJ: Mulberry breeding. Sericulture Department, Zhejiang Agriculture University, Hangzhou, China. 1994, 4-
Linnaeus C: Species Plantarum. 1753, Stockholm, 2: 986-
Koidzumi G: Taxonomical discussion on Morus plants. Bull Imp Sericult Exp Stat. 1917, 3: 1-62.
Koidzumi G: Synopsis specierum generis Mori. Bull Imp Sericult Exp Stat. 1923, 11: 1-50.
Hotta T: Taxonomical studies on the Morus plants and their distributions in Japan and its viscinties. Japanese Society for Promotion of Science, Ueno Park, Tokyo. 1958, 1-161.
Katsumata T: Mulberry species in west Jawa and their peculiarities. J Seric Sci Jpn. 1972, 42: 213-23.
Airy Shaw HK: A dictionary of flowering plants and ferns. Edited by: Willis JC, Airy Shaw HK. 1973, Cambridge University Press, London, 761-8th
Hirano H: Thremmetological studies of protein variation in mulberry. Bull Sericul Exp Sta. 1980, 28: 67-186.
Wang ZY, Tanksley SD: Restriction fragment length polymorphism in Oryza sativa L. Genome. 1989, 32: 1113-8.
Jana S, Pietrzak LN: Comparative assessment of genetic diversity in wild and primitive cultivated barley in a centre of diversity. Genetics. 1988, 119: 981-90.
Chengyin L, Weihua L, Mingjum L: Relationship between the evolutionary relatives and the variation of esterase isozymes in tea plant. J Tea Sci. 1992, 12: 15-20.
Jelinski DE, Cheliak WM: Genetic diversity and spatial subdivision of Populus tremuloides (Salicaceae) in a heterogeneous landscape. Am J Bot. 1992, 79: 728-36.
Botstein D, White RL, Skolnik M, Davis RW: Construction of a linkage map in man using restriction fragment length polymorphism. Am J Hum Genet. 1980, 32: 314-31.
Tanksley SD, Young ND, Paterson AH, Bonierbale MW: RFLP mapping in plant breeding: New tools for an old science. Biotechnology. 1989, 7: 257-64.
Miller JC, Tanksley SD: Effects of restriction enzymes, probe source, and probe length on detecting restriction fragment length polymorphism in tomato. Theor Appl Genet. 1990, 80: 385-9.
Wang ZY, Second G, Tanksley SD: Polymorphism and phylogenetic relationships among species in the genus Oryza as determined by analysis of nuclear RFLPs. Theor Appl Genet. 1992, 83: 565-81.
Beckmann JS, Soller M: Restriction fragment length polymorphism and genetic improvement of agricultural species. Euphytica. 1986, 35: 111-24.
Williams JG, Kubelik AR, Livak J, Rafalski JA, Tingey SV: DNA polymorphism amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990, 18: 6531-5.
Welsh J, McClelland M: Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990, 18: 7213-8.
Zietkiewicz E, Rafalski A, Labuda D: Genome fingerprinting by simple sequence repeat (SSR-) anchored polymerase chain reaction amplification. Genomics. 1994, 20: 176-83. 10.1006/geno.1994.1151.
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, et al: AFLP: a new technique for DNA Fingerprinting. Nucleic Acids Res. 1995, 23: 4407-14.
Weber JL, May PE: Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989, 44: 388-96.
Wu KS, Tanksley SD: Abundance, polymorphism and genetic mapping of microsatellites in rice. Mol Gen Genet. 1993, 241: 225-35.
Akkaya MS, Bhagwat AA, Cregan PB: Length polymorphism of simple sequence repeat DNA in soybean. Genetics. 1992, 132: 1131-9.
Morgante M, Olivieri AM: PCR-amplified microsatellites as markers in plant genetics. Plant J. 1993, 3: 175-82. 10.1046/j.1365-313X.1993.t01-9-00999.x.
Plaschke J, Ganal MW, Roder MS: Detection of genetic diversity in closely related bread wheat using microsatellite markers. Theor Appl Genet. 1995, 91: 1001-7.
Beckmann JS, Soller M: Toward a unified approach to genetic mapping of eukaryotes based on sequence tagged microsatellite sites. Biotechnology. 1990, 8: 930-2.
Roder MS, Plaschke J, Konig SU, Boner A, Sorrells ME, Tanksley SD, Ganal MW: Abundance, variability and chromosomal location of microsatellites in wheat. Mol Gen Genet. 1995, 246: 327-33.
Tsumura Y, Ohba K, Strauss SH: Diversity and inheritance of inter-simple sequence repeat polymorphism in Douglas-fir (Pseudotsuga menziesii) and Sugi (Cryptomeria japonica). Theor Appl Genet. 1996, 92: 40-5.
Nagaoka T, Ogihara Y: Applicability of inter-simple sequence repeat polymorphisms in wheat for use as DNA markers in comparison to RFLP and RAPD markers. Theor Appl Genet. 1997, 94: 597-602. 10.1007/s001220050456.
Nagaraju J, Kathirvel M, Kumar RR, Siddiq EA, Hasnain SE: Genetic analysis of traditional and evolved Basmati and non-Basmati rice varieties by using fluorescence-based ISSR-PCR and SSR markers. Proc Natl Acad Sci U S A. 2002, 99: 5836-41. 10.1073/pnas.042099099.
Sharma A, Sharma R, Machii H: Assessment of genetic diversity in a Morus germplasm collection using fluorescence-based AFLP markers. Theor Appl Genet. 2000, 101: 1049-55. 10.1007/s001220051579.
Bhattacharya E, Ranade SA: Molecular distinction amongst varieties of Mulberry using RAPD and DAMD profiles. BMC Plant Biol. 2001, 1: 3-10.1186/1471-2229-1-3.
Vijayan K, Chatterjee SN: ISSR profiling of Indian cultivars of mulberry (Morus spp.) and its relevance to breeding programs. Euphytica. 2003, 131: 53-63. 10.1023/A:1023098908110.
Lou CF, Zhang YZ, Zhou JM: Polymorphisms of genomic DNA in parents and their resulting hybrids in mulberry Morus. Sericologia. 1998, 38: 437-49.
Morgante M, Hanafey M, Powell W: Microsatellites are preferentially associated with nonrepetitive DNA in plant genomes. Nat Genet. 2002, 30: 194-200. 10.1038/ng822.
Thormann CE, Ferreira ME, Camargo LEA, Tivang JG, Osborn TC: Comparison of RFLP and RAPD Markers to Estimating Genetic Relationships Within and Among Cruciferous Species. Theor Appl Genet. 1994, 88: 973-80.
Rieseberg LH: Homology among RAPD fragments in interspecific comparisons. Mol Ecol. 1996, 5: 99-105.
Wolff K, Peters-Van Rijn J: Rapid detection of genetic variability in Chrysanthemum (Dendranthema Grandiflora Tzvelev) using random primers. Heredity. 1993, 71: 335-41.
Sambrook J, Fritsch EF, Maniatis T: Molecular cloning A laboratory manual. 1989, New York: Cold Spring Harbor Laboratory, Cold Spring Harbor, 2
Yap IV, Nelson RJ: WINBOOT: A program for performing bootstrap analysis of binary data to determine the confidence limits of UPGMA-based dendrograms. 1996, IRRI Discussion paper Series No. 14, International Rice Research Institute, Manila, Philippines
Acknowledgements
The authors thank the Central Silk Board (Ministry of Textiles – Govt. of India), Bangalore and the Department of Biotechnology, Govt. of India, New Delhi for financial assistance (to JN) for the study. We also thank Prof. Marian Goldsmith for critical reading of the manuscript and useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
AAK carried out RAPD and part of the ISSR work, GMN designed ISSR primers and carried out part of the ISSR work, GVN did DNA extraction and identified appropriate mulberry samples for the study, SK carried out data analysis and manuscript revision, TK carried out sample collection and JN conceived the study, and participated in its design, co-ordination and interpretation of the results.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Awasthi, A.K., Nagaraja, G., Naik, G. et al. Genetic diversity and relationships in mulberry (genus Morus) as revealed by RAPD and ISSR marker assays. BMC Genet 5, 1 (2004). https://doi.org/10.1186/1471-2156-5-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2156-5-1