Abstract
Background
Snake venom composition varies widely both among closely related species and within the same species, based on ecological variables. In terrestrial snakes, such variation has been proposed to be due to snakes' diet. Land snakes target various prey species including insects (arthropods), lizards (reptiles), frogs and toads (amphibians), birds (aves), and rodents (mammals), whereas sea snakes target a single vertebrate class (fishes) and often specialize on specific types of fish. It is therefore interesting to examine the evolution of toxins in sea snake venoms compared to that of land snakes.
Results
Here we describe the expression of toxin genes in the venom glands of two sea snakes, Lapemis curtus (Spine-bellied Sea Snake) and Acalyptophis peronii (Horned Sea Snake), two members of a large adaptive radiation which occupy very different ecological niches. We constructed cDNA libraries from their venom glands and sequenced 214 and 192 clones, respectively. Our data show that despite their explosive evolutionary radiation, there is very little variability in the three-finger toxin (3FTx) as well as the phospholipase A2 (PLA2) enzymes, the two main constituents of Lapemis curtus and Acalyptophis peronii venom. To understand the evolutionary trends among land snakes, sea snakes and sea kraits, pairwise genetic distances (intraspecific and interspecific) of 3FTx and PLA2 sequences were calculated. Results show that these proteins appear to be highly conserved in sea snakes in contrast to land snakes or sea kraits, despite their extremely divergent and adaptive ecological radiation.
Conclusion
Based on these results, we suggest that streamlining in habitat and diet in sea snakes has possibly kept their toxin genes conserved, suggesting the idea that prey composition and diet breadth may contribute to the diversity and evolution of venom components.
Similar content being viewed by others
Background
The composition of snake venoms varies widely both within a species and among closely related species [1–4]. This variation is proposed to be due to changes in the diet of snakes, based on the findings in the variation of intraspecific venom composition in a pit viper, Calloselasma rhodostoma, a land snake [2]. Land snakes depend on a diversity of prey including lizards (reptiles), frogs and toads (amphibians), birds (aves), insects (arthropods), and rodents (mammals)[5, 6]. They probably require a range of toxins that target different groups of prey species since there is variation in venom's ability for immobilization and killing across such a variety of prey. Toxins which are used for systematic prey envenomation found to have several isoforms in their venom gland as evident from global cataloguing of snakes toxin gene expression [3, 7–16] and it has been correlated that variation in prey favors the evolution of multiple isoforms of toxins in venoms [9, 17]. The variety of isoforms is believed to have been achieved through frequent gene duplications accompanied by an accelerated rate of evolution [18–20] similar to the generation of adaptive response in immunoglobulins and major histocompatibility complex genes in response to a wide range of foreign antigens [21]. Thus, a birth-and-death mode of evolution generates diversity in toxins allowing snakes to feed on a variety of prey species [22].
Elapid snakes are a monophyletic clade of approximately 300 species in 61 genera [23]. True sea snakes (Hydrophiinae) and sea kraits (Laticauda spp.) form two elapid clades that have evolved independently but are either rooted within (true sea snakes) or basal to (sea kraits) the terrestrial Australo-Papuan elapids rather than other elapid groups [24–28]. These snakes have adapted to marine life and undergone many changes in foraging behavior, morphology and diet [29]. As a result, although their feeding systems are confined to prey of a single vertebrate class (fishes), they often specialize on particular types or families of fish [30, 31]. With such restrictions in both diet and habitat, one might expect low diversity in toxin components (relative to snakes with broader diets), as has been shown to be the case in the hydrophiinae subfamily [9]. We showed by analyzing the cDNA library of Aipysurus eydouxii that its 3FTx gene is inactivated by a dinucleotide (TT) deletion [32] and the evolution of its PLA2 isoenzymes, unlike those from other snake venoms, is decelerated [33]. As this unique sea snake feeds exclusively fish eggs [31], we suggested that a shift in the diet of A. eydouxii may have resulted in the relaxation of selection pressures on its 3FTx and PLA2 genes
Here, we examined the total gene expression pattern of two other sea snakes, Lapemis curtus and Acalyptophis peronii, which have distinct and different habitats and feeding systems. L. curtus inhabits many different areas like open sea, estuaries, and coral reefs, whereas A. peronii inhabits only sandy areas between coral reefs [34]. L. curtus in contrast to other sea snakes is a generalist feeder and its diet is one of the most diverse of all sea snakes [30, 31, 34–36]. Its prey consists of fishes (90%; 31 different families) and very few invertebrates (10%; squid and cuttlefish) [30, 35, 36]. Additionally, L. curtus cohabits with other sea snakes, and consequently may be overlapping in diet. In contrast, the diet of A. peronii is confined mainly to gobies (one class of sea fish) [34] and it is a diet and habitat specialist. Because these two snakes are members of a large adaptive radiation of the Hydrophis lineage and they might have diverged very rapidly, differences in their venoms might also be widely divergent if they track diet specialization. On the other hand, if diet specialization within a constrained group of prey (e.g., only fish), drives more of a streamlining of venom evolution, then we might expect there to be few or no changes in venom constituents. Therefore, it could be interesting to compare the total toxin gene expression of these two sea snakes.
We constructed cDNA libraries of the venom glands from A. peronii and L. curtus specimens and sequenced about 200 clones of each. Sampling of transcriptoms indicates the presence of any new and/or rare families of toxins and enables analyses of the molecular evolutionary trends among toxin genes. Further, to compare the evolution of toxin genes among land snakes, sea snakes, and sea kraits, we calculated the evolutionary distances using all available sequences of two principle components of the toxin proteome, 3FTx and PLA2.
Results
cDNA libraries of Lapemis curtus and Acalyptophis peronii venom glands
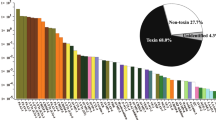
We obtained 4 and 5 μg of mRNA from 30 mg of venom gland tissues of Lapemis curtus and Acalyptophis peronii, respectively. We constructed two separate cDNA libraries using 1 μg of mRNA from each preparation. From the clones containing inserts, we randomly selected 250 and 225 clones, respectively. From these clones we were able to obtain sequences of 214 cDNA clones from L. curtus and 192 cDNA clones for A. peronii. Figure 1 shows the distribution of clones in both venom glands.
Lapemis curtus library
3FTx
To date, three long-chain isoforms of 3FTx (AAL54893, AAL54892 and ABN54806) and four short-chain isoforms of 3FTx (AAL54894, AAL54895, P68416 and ABN54805) [37]have been reported from L. curtus venom. We found cDNA clones encoding both long-chain isoforms (AAL54893 and AAL54892) of 3FTx in the library (41% abundance, Figure 1) and the ratio between the number of clones of isoforms AAL54893 and AAL54892 was ~10:1. We also found cDNA clones encoding a short-chain 3FTx (AAL54894; ~2% abundance, Figure 1) [37]. No variation was observed in the coding sequence of the mature proteins with AAL54893, AAL54892 and AAL54894. However, we could not detect the long chain isoform (ABN54806) and three other short-chain isoforms of 3FTx (AAL54895, P68416 and ABN54805) [37, 38].
PLA2
So far, three isoforms of PLA2 of L. curtus (AAL55556, AAL55555 and AAL54920) have been reported [39]. We were only able to detect one isoform which is completely identical at the nucleotide level with AAL55555 (~10% abundance, Figure 1).
CRISP
Partial sequences of two isoforms (Q8UW25 and Q8UW11) of CRISP from L. curtus venom glands have recently been reported. Our cDNA library contained ~2% clones coding for Q8UW25 isoform without any variation at nucleotide level (Figure 1).
Others
The cDNA library has a singleton presence of a growth factor (AY742212) which shows significant identity to Platelet Derived Growth Factor (PDGF). The partial sequence shows 70% identity to the C-terminus of the predicted PDGF-D isoform from Gallus gallus (chicken). Although growth factors such as NGF [40] and VEGF [41] are known to be present in the venom, this is the first report of PDGF-like protein sequence from the venom gland. However, further studies are needed to confirm the presence of PDGF protein in the venom.
L. curtus library contained ~20% housekeeping genes (Figure 1), including ribosomal RNA, ribosomal proteins and cytochromes. In addition, ~25% of cDNA sequences did not show significant identity to toxins or metabolic genes (Figure 1). BlastX search of these sequences showed poor or only partial identity to any protein sequences with other organisms or no match at all. These unknown sequences in most of the cases are partial, singleton clones. However, their origin (venom gland or marginal contamination of surrounding tissues) still needs to be established.
Acalyptophis peronii library
3FTx
Amino acid sequences of two isoforms of short-chain 3FTx have been reported earlier [42, 43]. Gln43 of the major isoform (AY742211) has changed to Glu43 in the minor isoform (AY742210) [43]. In Acalyptophis peronii library, the short-chain 3FTx was most abundant (~64%) (Figure 1) and there are two isoforms of 3FTx in equal numbers (60 and 62 respectively). These two isoforms (AY742210 and AY742211) have three nucleotide changes in their signal sequences leading to substitution of Thr7 (ACC) and Leu8 (TTG) by Ser7 (TCC) and Pro8 (CCG), respectively. However, no variation was observed in the coding sequence of the mature protein and the deduced protein sequence corresponds only to the major isoform [42]. As we did not obtain clones corresponding to the minor form, we propose that the minor form is most likely due to deamidation of Gln43 [44] and not a separate gene product. Generally in toxin families, it has been observed that the signal peptide regions, 5'UTR and 3'UTR are highly conserved, whereas the mature protein region shows a number of substitutions [19, 45]. In contrast, the two isoforms of short-chain 3FTx differ in their signal peptide region but not in the mature protein in this case. It would be interesting to examine the importance of these substitutions.
PLA2
So far no PLA2 sequences from A. peronii have been reported. We found partial clones having 3' terminal sequences of PLA2 in A. peronii library (~5%; Figure 1). They show 100% identity in the 3'UTR region with L. curtus PLA2 (AAL55556 and AAL54920). Further identification and characterization of full length PLA2 is underway.
Others
The cDNA library contains ~6% clones encoding housekeeping genes (Figure 1). These include NADH dehydrogenase, ribosomal proteins and Ca2+ binding proteins (calglandulin). The latter class of protein has been implicated in toxin secretion [46, 47]. Like the L. curtus library, the A. peronii library also contained ~25% with no homology to any known toxin or housekeeping genes (Figure 1). As earlier, in most cases these sequences are partial, singleton clones and their origin needs to be verified.
Intra and interspecific relationship of 3FTx and PLA2 sequences
The number of available protein sequences encoding 3FTx and PLA2 were higher than cDNA sequences because most of the sequences have been reported from direct protein sequencing. Therefore, we used protein sequences to calculate intra and interspecific pairwise distances for land snakes, sea snakes and sea kraits. It should be noted that due to paucity of the available data the number of species and number of short-chain 3FTx used for the calculations for land snakes, sea snakes and sea kraits were not the same.
For short-chain 3FTx, 37% of the intraspecific distances of both Pseudonaja textilis and Bungarus species (land snakes) are in the range of (0.2–0.3) and (0.7–0.8) respectively, while 63% of the intraspecific distances of sea kraits fall in the range of (0.1–0.2), and most of the intraspecific pairwise distances of sea snakes are in the range of (0.02–0.04) (Figure 2A). Interspecific pairwise distances also appear higher (50% in the range of 0.7 for Bungarus species) for land snakes, and lower for sea snakes (100% in the range of 0.02). Interspecific distances of 3FTx for Pseudonaja species were not calculated because sequences were only available from one species (P. textilis). The higher genetic distances of 3FTx in land snakes indicate higher levels of genetic diversity compared to sea snakes, where sequences were much more conserved. The genetic diversity within sea kraits is intermediate in both intra and interspecific comparisons. For PLA2, 22% of the Australian elapids and 36% of the Bungarus species intraspecific distances fall between (0.1–0.3) and (0.1–0.2) respectively. On the other hand, 97% and 44% of the sea snakes' and sea kraits' intraspecific distances ranged from (0.1–0.2) and (0.2–0.3) respectively (Figure 2B). Interspecific distances of PLA2 for Australian elapids, Bungarus species and sea kraits and sea snakes have comparable values (30%–60% in the range of 0.2–0.3; Figure 2B). But in sea snakes, interspecific distances (58% fall between 0.2–0.3) appear lower than the intraspecific distances. One of the possibilities for this reverse trend can be due to poor phylogenetic resolution among species in the hydrophiinae subfamily [48, 49]
Pairwise intraspecific (white bar) and interspecific (black bar) distances for land snakes, sea snakes and sea kraits. Panel A: 3FTx (1a and 1b: land snakes; Pseudonaja textilis and Bungarus species respectively), 2 and 3: sea kraits and sea snakes respectively. Panel B: PLA2: (4a and 4b: land snakes; Australian elapids and Bungarus species respectively), 5 and 6: sea kraits and sea snakes respectively. RF denotes relative frequency.
Discussion
Snake venoms are a rich and diverse source of pharmacologically active proteins and peptide components [50, 51]. Some of these components are enzymes, whereas others are nonenzymatic proteins or polypeptides. Most of these components are offensive weapons to capture the prey, injection of venom into prey leads to immobilization, death and can subsequently aid in digestion as well [52, 53]. Venom might also be used for defensive purposes to keep possible predators away. Venom systems appear to have evolved to meet some of these goals, a single time in reptile evolution, at the base of the Toxicofera [54, 55].
In this work, we show the high abundance of 3FTx in the venoms of sea snakes (41% for Lapemis curtus and ~64% for Acalyptophis peronii) while PLA2 is a distant second largest group (~10% for L. curtus and ~5% for A. peronii) of sea snake toxins. Overall, both the 3FTx and PLA2 do not possess an abundance of different isoforms generating significant variation in the venom composition. The fact that we did not detect some of the isoforms of these two groups of toxins as previously reported in L. curtus may be either due to regional variation within the species or a sampling artifact since the cDNA library was generated from venom glands of a single snake. However, both groups of toxins appears to be simple and do not have noteworthy diversity in their isoform compositions. It suggests that sea snake venoms genes are quite conserved, and therefore lack the diversity in its venom composition as observed for land snake and sea kraits. However, additional data from gene expression profile, frequency of gene duplication and accelerated evolution profile of sea snakes is needed to further test this hypothesis.
Comparison of intraspecific distances among 3FTx showed that the maximum value for land snakes is 0.8 whereas sea snakes are at 0.03 and sea kraits, 0.2 (Figure 2A). The variation between land and sea snakes is about 30 fold, whereas land snake and sea krait differ only 4 fold. However, this level of variation has not been found in PLA2 genes. In land snakes, the maximum intraspecific distance is 0.2 for land snakes and sea kraits, whereas sea snakes have a maximum value of 0.1, indicating a difference of only 2 fold (Figure 2B). Interspecific distances, for both 3FTx and PLA2, on the other hand, show greater or equal values than the intraspecific differences in land snakes and sea kraits (Figure 2A and 2B). From the genetic distance data, it is obvious that 3FTx is gaining more variability than PLA2. This is probably relevant because envenomation by elapid snakes is usually characterized by rapid neurotoxic complications due to presence of large amounts of neurotoxins [56]. Overall, our calculation for the intra and interspecific variation in both 3FTx and PLA2 appears distinct among land snakes, sea snakes and sea kraits indicating the probable existence of distinct evolutionary patterns that separate these groups.
Interestingly, the conservation of toxin diversity in sea snakes is not confined within species, it extends across different genera. For example, Enhydrina schistosa, a common sea snake, has just two neurotoxins (P25492 and P25493) [57]. The toxin P25492 is identical in sequence to a short-chain neurotoxin found in Lapemis curtus venom [38] and the other toxin, P25493, is identical to the short-chain neurotoxins found in venoms of Hydrophis cyanocinctus [58] and Pelamis platurus [59]. In contrast, among 276 3FTxs reported to date [22], we could not find a single 3FTx common across different genera of land snakes. Conservation of toxin sequences, even across genera of marine snakes is possibly due to a highly constrained niche, and the streamlined nature of their venoms is responsible for the remarkable degree of antivenom cross-reactivity [60].
The analysis of our cDNA libraries indicated that the Lapemis curtus venom is marginally more diverse than that of Acalyptophis peronii. The L. curtus library contains CRISP and growth factor isoforms in addition to 3Ftx neurotoxins and PLA2 enzymes. Chen et al. (AAV98367) reported the presence of a kallikrein toxin in Lapemis curtus venom as well. Recruitment of additional toxin families like CRISP, growth factor, kallikrein toxin may be due to its broad dietary requirements. In contrast, A. peronii venom glands contain only neurotoxins and PLA2 in high concentration and ittargets only gobies as its diet [30, 31, 34–36]. Therefore, evolution of toxin(s) in a generalist (L. curtus) and a restricted feeder (A. peronii) appear to be different. This does not indicate that other toxin classes are not expressed at low levels; more rigorous sequencing may reveal rarer transcripts.
The toxin expression profile data from cDNA library of L. curtus and A. peronii and a relationship between their habitat and diet may suggest that ecological variables presumably played a major role in determining the trajectory of their evolutionary paths along ecological niches (specialist and generalist) and not completely because of a distant phylogenetic relationship between them. There are however, a few specific cases available in the literature, where a relationship between intraspecific variations in venom with respect to dietary preferences has not been found [61, 62, 63]. Do these specific exceptions prove the general rule, or is there a threshold where the evolution of toxins becomes decoupled from feeding ecology and/or diet? These questions remain cogent for the future of toxin evolution research and we propose that sea snakes will remain major players in helping to understand how toxin evolution and feeding ecology are linked.
Conclusion
Global cataloguing of toxin expression shows conserved expression pattern of two main families of toxins, 3FTx and PLA2, in two sea snakes venom giving rise to a simple venom composition relative to land snakes and sea kraits. Genetic distance values of 3FTx and PLA2 toxins show a more diverse trend of evolution for land snakes and sea kraits than to sea snakes. As the diet breadth (prey items) expands from sea snakes to land snakes (sea kraits as intermediate), we suggest that these trends in evolution of toxins may be linked to their diet.
Methods
Collection of venom glands
Lapemis hardwickii has been synonymized with Lapemis curtus [64] so L. curtus is used in this paper. One specimen of L. curtus and another of A. peronii were collected from Albatross Bay in Weipa, Queensland, Australia. Venom glands were dissected from each of these freshly caught snakes. Two glands from each snake were used for the construction of cDNA libraries. Although sample sizes are small for each species, the difficulty in acquiring specimens or keeping individuals in captivity make even these small sample sizes extremely valuable and worthy of study.
Library construction, sequencing and analysis
Total RNA was extracted from the venom glands using RNeasy Mini Kit (Qiagen, Hilden, Germany). The integrity of total RNA was checked by agarose gel electrophoresis. The mRNA was purified using mRNA isolation kit (Roche Applied Science, Mannheim, Germany). The purified total mRNA was used to make the cDNA library following the instructions of the SMART cDNA library construction kit (Clontech, Mountain view, California, USA). The library was packaged using Gigapack gold packaging extract (Stratagene, Cedar Creek, Texas, USA). Individual clones were rescued from randomly selected white plaques and grown in (Luria broth + ampicillin) medium. Plasmids were purified using QIAprep spin miniprep kit (Qiagen, Hilden, Germany). Purified plasmids were sequenced by cycle sequencing reaction using the BigDye Terminator v3.1 kit (Applied Biosystem, Foster City, California, USA) and with an automated DNA sequencer (Model 3100A, Applied Biosystem, Foster City, California, USA). Sequences were compared to cDNA and protein sequences in NCBI database using BLAST program (BlastN and BlastX) and identical (or similar) clones were clustered. Each cluster was aligned using the program ClustalW in European Bioinformatics Institute site.
Calculation of genetic distances
Genetic distances were compared by calculating intra and interspecific pairwise distances for the 3FTx and the PLA2 enzymes. All available protein sequences of 3FTx (short-chain isoforms) and PLA2 of land snakes, sea kraits and sea snakes were retrieved [see additional file 1]. Redundant sequences and signal peptides were removed and aligned. Aligned sequences were analyzed in PAUP* version 4.0 program [65] using the pairwise distance algorithm (uncorrected distances, kimura-2 parameters) for both within and between species. The pairwise distances were then plotted as a group for land snakes, sea snakes and sea kraits.
Accession numbers
Nucleotide sequence data reported here have been deposited in GenBank under accession numbers [GenBank: AY742212, GenBank: AY742210, GenBank: AY742211].
References
Chippaux JP, Boche J, Courtois B: Electrophoretic patterns of the venoms from a litter of Bitis gabonica snakes. Toxicon. 1982, 20: 521-523. 10.1016/0041-0101(82)90019-8.
Daltry JC, Wuster W, Thorpe RS: Diet and snake venom evolution. Nature. 1996, 379: 537-540. 10.1038/379537a0.
Fry BG, Wickramaratna JC, Hodgson WC, Alewood PF, Kini RM, Ho H, Wuster W: Electrospray liquid chromatography/mass spectrometry fingerprinting of Acanthophis (death adder) venoms: taxonomic and toxinological implications. Rapid Commun Mass Spectrom. 2002, 16: 600-608. 10.1002/rcm.613.
Jayanthi GP, Gowda TV: Geographical variation in India in the composition and lethal potency of Russell's viper (Vipera russelli) venom. Toxicon. 1988, 26: 257-264. 10.1016/0041-0101(88)90216-4.
Shine R: Habitats, diets, and sympatry in snakes: a study from Australia. Can J Zool. 1977, 55: 1118-1128-
Shine R: Constraints, Allometry, and adaptation: food habits and reproductive biology of Australian Brownsnakes (Pseudonaja: Elapidae). Herpetologica. 1989, 45: 195-207.
Bazaa A, Marrakchi N, El Ayeb M, Sanz L, Calvete JJ: Snake venomics: comparative analysis of the venom proteomes of the Tunisian snakes Cerastes cerastes, Cerastes vipera and Macrovipera lebetina. Proteomics. 2005, 5: 4223-4235. 10.1002/pmic.200402024.
Francischetti IM, My-Pham V, Harrison J, Garfield MK, Ribeiro JM: Bitis gabonica (Gaboon viper) snake venom gland: toward a catalog for the full-length transcripts (cDNA) and proteins. Gene. 2004, 337: 55-69. 10.1016/j.gene.2004.03.024.
Fry BG, Wuster W, Ryan Ramjan SF, Jackson T, Martelli P, Kini RM: Analysis of Colubroidea snake venoms by liquid chromatography with mass spectrometry: evolutionary and toxinological implications. Rapid Commun Mass Spectrom. 2003, 17: 2047-2062. 10.1002/rcm.1148.
Juarez P, Sanz L, Calvete JJ: Snake venomics: characterization of protein families in Sistrurus barbouri venom by cysteine mapping, N-terminal sequencing, and tandem mass spectrometry analysis. Proteomics. 2004, 4: 327-338. 10.1002/pmic.200300628.
Junqueira-de-Azevedo IL, Ho PL: A survey of gene expression and diversity in the venom glands of the pitviper snake Bothrops insularis through the generation of expressed sequence tags (ESTs). Gene. 2002, 299: 279-291. 10.1016/S0378-1119(02)01080-6.
Kashima S, Roberto PG, Soares AM, Astolfi-Filho S, Pereira JO, Giuliati S, Faria M, Xavier MA, Fontes MR, Giglio JR, Franca SC: Analysis of Bothrops jararacussu venomous gland transcriptome focusing on structural and functional aspects: I--gene expression profile of highly expressed phospholipases A2. Biochimie. 2004, 86: 211-219. 10.1016/j.biochi.2004.02.002.
Li S, Wang J, Zhang X, Ren Y, Wang N, Zhao K, Chen X, Zhao C, Li X, Shao J, Yin J, West MB, Xu N, Liu S: Proteomic characterization of two snake venoms: Naja naja atra and Agkistrodon halys. Biochem J. 2004, 384: 119-127. 10.1042/BJ20040354.
Nawarak J, Sinchaikul S, Wu CY, Liau MY, Phutrakul S, Chen ST: Proteomics of snake venoms from Elapidae and Viperidae families by multidimensional chromatographic methods. Electrophoresis. 2003, 24: 2838-2854. 10.1002/elps.200305552.
Serrano SM, Shannon JD, Wang D, Camargo AC, Fox JW: A multifaceted analysis of viperid snake venoms by two-dimensional gel electrophoresis: an approach to understanding venom proteomics. Proteomics. 2005, 5: 501-510. 10.1002/pmic.200400931.
Tsai IH, Chen YH, Wang YM: Comparative proteomics and subtyping of venom phospholipases A2 and disintegrins of Protobothrops pit vipers. Biochim Biophys Acta. 2004, 1702: 111-119.
Fry BG, Wuster W: Assembling an arsenal: origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences. Mol Biol Evol. 2004, 21: 870-883. 10.1093/molbev/msh091.
Deshimaru M, Ogawa T, Nakashima K, Nobuhisa I, Chijiwa T, Shimohigashi Y, Fukumaki Y, Niwa M, Yamashina I, Hattori S, Ohno M: Accelerated evolution of crotalinae snake venom gland serine proteases. FEBS Lett. 1996, 397: 83-88. 10.1016/S0014-5793(96)01144-1.
Ogawa T, Oda N, Nakashima K, Sasaki H, Hattori M, Sakaki Y, Kihara H, Ohno M: Unusually high conservation of untranslated sequences in cDNAs for Trimeresurus flavoviridis phospholipase A2 isozymes. Proc Natl Acad Sci U S A. 1992, 89: 8557-8561. 10.1073/pnas.89.18.8557.
Ogawa T, Chijiwa T, Oda-Ueda N, Ohno M: Molecular diversity and accelerated evolution of C-type lectin-like proteins from snake venom. Toxicon. 2005, 45: 1-14. 10.1016/j.toxicon.2004.07.028.
Nei M, Gu X, Sitnikova T: Evolution by the birth-and-death process in multigene families of the vertebrate immune system. Proc Natl Acad Sci U S A. 1997, 94: 7799-7806. 10.1073/pnas.94.15.7799.
Fry BG, Wuster W, Kini RM, Brusic V, Khan A, Venkataraman D, Rooney AP: Molecular evolution and phylogeny of elapid snake venom three-finger toxins. J Mol Evol. 2003, 57: 110-129. 10.1007/s00239-003-2461-2.
Golay P, Smith HM, Broadley DG, Dixon JR, McCarthy C, Rage JC, Schatti B, Toriba M: Endoglyphs and other major venomous snake of the world. A checklist. 1993, Aïre-Genèva, Azemiops S.A., 1-478.
Keogh JS: Molecular phylogeny of elapid snakes and a consideration of their biogeographic history. Biological journal of the Linnean Society. 1998, 63: 117-203. 10.1006/bijl.1997.0178.
Keogh JS, Shine R, Donnellan S: Phylogenetic relationship of terrestrial Australo-Papuan elapid snake (subfamily Hydrophiinae) based on cytochrome b and 16S rRNA sequences. Mol Phylogenet Evol. 1998, 10: 67-81. 10.1006/mpev.1997.0471.
Schwaner TD, Baverstock PR, Dessauer HC, Mengden GA: Immunological evidence for the phylogenetic relationship of Australian elapid snakes. Biology of Australasian Frog and Reptiles. Edited by: G G, R S and H E. 1985, Sydney, Surrey Beatty & Sons, 177-184.
Slowinski JB, Keogh JS: Phylogenetic relationships of elapid snakes based on cytochrome b mtDNA sequences. Mol Phylogenet Evol. 2000, 15: 157-164. 10.1006/mpev.1999.0725.
Voris HK: A phylogeny of the sea snake (Hydrophiidae). Fieldiana (Zoology). 1977, 70: 79-166.
Heatwole H: In Sea Snakes. 1999, Miami, Florida, Krieger Publishing, 2nd edition
Glodek GS, Voris HK: Marine snake diets: prey composition, diversity and overlap. Copeia. 1982, 3: 661-666. 10.2307/1444667.
Voris HK, Voris HH: Feeding strategies in marine snakes: an analysis of evolutionary, morphological, behavioral and ecological relationship. Amer Zool. 1983, 23: 411-425.
Li M, Fry BG, Kini RM: Eggs-only diet: its implications for the toxin profile changes and ecology of the marbled sea snake (Aipysurus eydouxii). J Mol Evol. 2005, 60: 81-89. 10.1007/s00239-004-0138-0.
Li M, Fry BG, Kini RM: Putting the brakes on snake venom evolution: the unique molecular evolutionary patterns of Aipysurus eydouxii (Marbled sea snake) phospholipase A2 toxins. Mol Biol Evol. 2005, 22: 934-941. 10.1093/molbev/msi077.
Greer AE: Encyclopedia of Australian Reptiles: Australian museum online. 2004, [http://www.amonline.net.au/herpetology/research/encyclopedia.pdf]
Cogger HG: Reptiles and amphibians of Australia. 2000, New South Wales, Reed New Holland, Frenchs Forest, 15th March 2005, [http://www.amonline.net.au/herpetology/research/index.htm]6th
Shine R: Australian Snakes- a natural history. 1993, Chatswoods, New South Wales, Reed Books
Zhong XF, Peng LS, Wu WY, Wei JW, Yang H, Yang YZ, Xu AL: Identification and functional characterization of three postsynaptic short-chain neurotoxins from Hydrophiinae, Lapemis hardwickii gray. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 2001, 33: 457-462.
Fox JW, Elzinga M, Tu AT: Amino acid sequence of a snake neurotoxin from the venom of Lapemis hardwickii and the detection of a sulfhydryl group by laser Raman spectroscopy. FEBS Lett. 1977, 80: 217-220. 10.1016/0014-5793(77)80443-2.
Yang WL, Wei JW, Zhong XF, Zhao GJ, Peng LS, Wu WY, Xu AL: Diversity of PLA2 genes from sea snake Lapemis hardwickii gray venom. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai). 2001, 33: 345-350.
Bailey GS, Banks BE, Pearce FL, Shipolini RA: A comparative study of nerve growth factors from snake venoms. Comp Biochem Physiol B. 1975, 51: 429-438. 10.1016/0305-0491(75)90034-6.
Tokunaga Y, Yamazaki Y, Morita T: Specific distribution of VEGF-F in Viperinae snake venoms: isolation and characterization of a VGEF-F from the venom of Daboia russelli siamensis. Arch Biochem Biophys. 2005, 439: 241-247. 10.1016/j.abb.2005.05.020.
Mori N, Tu AT: Isolation and primary structure of the major toxin from sea snake, Acalyptophis peronii, venom. Arch Biochem Biophys. 1988, 260: 10-17. 10.1016/0003-9861(88)90418-3.
Mori N, Tu AT: Amino-acid sequence of the minor neurotoxin from Acalyptophis peronii venom. Biol Chem Hoppe Seyler. 1988, 369: 521-526.
Bischoff R, Kolbe HV: Deamidation of asparagine and glutamine residues in proteins and peptides: structural determinants and analytical methodology. J Chromatogr B Biomed Appl. 1994, 662: 261-278. 10.1016/0378-4347(94)00203-7.
Nakashima K, Ogawa T, Oda N, Hattori M, Sakaki Y, Kihara H, Ohno M: Accelerated evolution of Trimeresurus flavoviridis venom gland phospholipase A2 isozymes. Proc Natl Acad Sci U S A. 1993, 90: 5964-5968. 10.1073/pnas.90.13.5964.
Goncalves LR, Yamanouye N, Nunez-Burgos GB, Furtado MF, Britto LR, Nicolau J: Detection of calcium-binding proteins in venom and Duvernoy's glands of South American snakes and their secretions. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1997, 118: 207-211. 10.1016/S0742-8413(97)00130-8.
Junqueira-de-Azevedo IL, Pertinhez T, Spisni A, Carreno FR, Farah CS, Ho PL: Cloning and expression of calglandulin, a new EF-hand protein from the venom glands of Bothrops insularis snake in E. coli. Biochim Biophys Acta. 2003, 1648: 90-98.
Lukoschek V, Keogh JS: Molecular phylogeny of sea snakes reveals a rapidly diverged adaptive radiation. Biological journal of the Linnean Society. 2006, 15867150:
Rasmussen AR: Phylogenetic analysis of the "true" aquatic elapid snakes Hydrophiinae (sensu Smith et al., 1977) indicates two independent radiations into water. Steenstrupia. 2002, 27: 47-63.
Harvey AL: Sanke Toxins. 1991, New York, Prgamon Press
Lee CY: Snake Venoms. Handbook of Experimental Pharmacology. 1979, Springer-Verlag, Berlin
Dufton MJ: Kill and cure: the promising future for venom research. Endeavour. 1993, 17: 138-140. 10.1016/0160-9327(93)90104-B.
Kardong KV: Snake toxins and venom: an evolutionary perspective. Herpetologica. 1996, 52: 36-46.
Fry BG, Vidal N, Norman JA, Vonk FJ, Scheib H, Ramjan SF, Kuruppu S, Fung K, Blair HS, Richardson MK, Hodgson WC, Ignjatovic V, Summerhayes R, Kochva E: Early evolution of the venom system in lizards and snakes. Nature. 2006, 439: 584-588. 10.1038/nature04328.
Vidal N, Hedges SB: The phylogeny of squamate reptiles (lizards, snakes, and amphisbaenians) inferred from nine nuclear protein-coding genes. C R Biol. 2005, 328: 1000-1008.
Hodgson WC, Wickramaratna JC: In vitro neuromuscular activity of snake venoms. Clin Exp Pharmacol Physiol. 2002, 29: 807-814. 10.1021/bi00774a034.
Fryklund L, Eaker D: Amino acid sequences of the two principal neurotoxins of Enhydrina schistosa venom. Biochemistry. 1972, 11: 4633-4640. 10.1021/bi00774a034.
Liu CS, Blackwell RQ: Hydrophitoxin b from Hydrophis cyanocinctus venom. Toxicon. 1974, 12: 543-546. 10.1016/0041-0101(74)90047-6.
Wang CL, Liu CS, Hung YO, Blackwell RQ: Amino acid sequence of pelamitoxin a, the main neurotoxin of the sea snake, Pelamis platurus. Toxicon. 1976, 14: 459-466. 10.1016/0041-0101(76)90063-5.
Chetty N, DU A, Hodgson WC, Winkel K, Fry BG: The in vitro neuromuscular activity of Indo-Pacific sea-snake venoms: efficacy of two commercially available antivenoms. Toxicon. 2004, 44: 193-200. 10.1016/j.toxicon.2004.05.022.
Greene HW: The evolution of feeding mechanisms in snakes. Tucson Herp Soc News. 1988, 8: 65-69.
Greene HW: The evolution of feeding mechanisms in snakes. Tucson Herp Soc News. 1988, 8: 75-78.
Williams V, White J, Schwaner TD, Sparrow A: Variation in venom proteins from isolated populations of tiger snakes (Notechis ater niger, N. scutatus) in South Australia. Toxicon. 1988, 26: 1067-1075. 10.1016/0041-0101(88)90205-X.
Gritis PA, Voris HK: Variability and significance of parietal and ventral scales in the marine snakes of the genus Lapemis (serpentes: Hydrophiidae), with comments on the occurence of spiny scales in the genus. Fieldiana (Zoology) New Series. 1990, 56: 1-13.
Swofford DL: PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). 2003, Sinauer Associates
Acknowledgements
This work was supported from the grants from Biomedical Research Council, Agency for Science and Technology Research, Singapore (RMK) and the Australian Geographic Society, Australia & Pacific Science Foundation, Australian Research Council, CASS Foundation (BGF).). We acknowledge the suggestion and help of Dr. Rudolph Meier, Mr. Shiyang Kwong for the data analysis. We would also like to thank Dave Donald for his invaluable help in collecting specimens in Weipa.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The author(s) declares that there are no competing interests.
Authors' contributions
SP has performed the experiments, data analysis, writing and extension of the theme of the manuscript. DB has helped to examine the phylogenetic aspect of the concept. BGF is responsible for the sample collection and writing of the manuscript. RMK contributed the developing the concept and writing of the manuscript. All the authors contributed to editing the manuscript and approved of its final form.
Electronic supplementary material
12862_2007_466_MOESM1_ESM.doc
Additional file 1: Calculation of genetic distance for 3FTx and the PLA2 enzymes. The data compares genetic distances among land snakes, sea snakes and sea kraits. (DOC 52 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Pahari, S., Bickford, D., Fry, B.G. et al. Expression pattern of three-finger toxin and phospholipase A2 genes in the venom glands of two sea snakes, Lapemis curtus and Acalyptophis peronii: comparison of evolution of these toxins in land snakes, sea kraits and sea snakes. BMC Evol Biol 7, 175 (2007). https://doi.org/10.1186/1471-2148-7-175
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2148-7-175