Abstract
Background
An important component of sexual selection arises because females obtain viability benefits for their offspring from their mate choice. Females choosing extra-pair fertilization generally favor males with exaggerated secondary sexual characters, and extra-pair paternity increases the variance in male reproductive success. Furthermore, females are assumed to benefit from 'good genes' from extra-pair sires. How additive genetic variance in such viability genes is maintained despite strong directional selection remains an evolutionary enigma. We propose that sexual selection is associated with elevated mutation rates, changing the balance between mutation and selection, thereby increasing variance in fitness and hence the benefits to be obtained from good genes sexual selection. Two hypotheses may account for such elevated mutation: (1) Increased sperm production associated with sperm competition may increase mutation rate. (2) Mutator alleles increase mutation rates that are revealed by the expression of condition-dependent secondary sexual characters used by choosy females during their mate choice. M Petrie has independently developed the idea that mutator alleles may account for the maintenance of genetic variation in viability despite strong directional selection.
Results
A comparative study of birds revealed a positive correlation between mutation rate at minisatellite loci and extra-pair paternity, but not between mutation rate and relative testes mass which is a measure of relative sperm production. Minisatellite mutation rates were not related to longevity, suggesting a meiotic rather than a mitotic origin of mutations.
Conclusion
We found evidence of increased mutation rate in species with more intense sexual selection. Increased mutation was not associated with increased sperm production, and we suggest that species with intense sexual selection may maintain elevated mutation rates because sexual selection continuously benefits viability alleles expressed in condition-dependent characters. Sexual selection may increase mutational input, which in turn feeds back on sexual selection because of increased variance in viability traits.
Similar content being viewed by others
Background
Sexual selection arises from competition among individuals of the chosen sex for access to individuals of the choosy sex, and females (usually the choosy sex) may either obtain direct or indirect fitness benefits from their mate choice [1]. Indirect fitness benefits may consist of genetically based attractiveness of sons or genetic viability of offspring [e. g., [2, 3]]. The maintenance of indirect fitness benefits in the presence of directional selection by choosy females poses a theoretical problem [1, 4–6], because characters subject to directional selection tend to become fixed leaving little genetic variation. However, empirical studies indicate that genetic variation is a very small, but overall highly significant component of fitness [7]. The mean heritability estimate of fitness weighted by sample size is 0.138 [7–9]. Even very small heritabilities of fitness can be extremely important for maintaining female preferences for good genes on an evolutionary time scale.
Genetic variability in fitness may mainly be maintained by mutations, which usually have slightly deleterious effects [review in [10]]. Mutation rates in sexually reproducing organisms may be minimized because associations between mutability genes and beneficial genetic variants tend to be broken up by recombination [11]. Sex results in recombination disrupting associations between mutator alleles and beneficial alleles arising due to mutation, the only factor maintaining associations being strong linkage [12, 13]. Such hitch-hiking effects will only remain in a population until the beneficial allele has gone to fixation. However, mutation rate may be greater in species with intense sexual selection. Here, we propose two alternative hypotheses for the evolution of elevated mutation rates in sexually reproducing organisms. (1) Intense sperm competition may lead to an elevated male mutation rate. (2) Intense sexual selection may result in beneficial viability alleles being expressed in condition-dependent secondary sexual characters.
Sperm competition may maintain high mutation rates for two different reasons. First, increased sperm production is preceded by an increase in the number of mitotic germline cell divisions in the testes, likely resulting in a higher number of replication-dependent mutations per generation. This observed difference in mutation rate between the sexes [14–16] is likely to be due to similar differences in germline mitoses. Secondly, increased germline mitotic and meiotic rates may constrain the fidelity of replication, repair or recombination. Thus, mutational input for viability traits may increase as a direct consequence of intense sperm competition, and this may suffice to maintain genetic variability in fitness.
The second hypothesis suggests that under intense directional sexual selection elevated mutation rates can be maintained through mutator alleles if the sexual selection process continuously favours individuals carrying the beneficial viability alleles. Strong linkage between mutator alleles and beneficial alleles may not be necessary in this situation because most mutants are removed each generation due to skew in mating success caused by the intense sexual selection. The reason is that males carrying mutations obtain few or no matings. Therefore, females may be able to continuously choose mates with high, genetically based viability. That is the case if the variability in expression of secondary sexual characters increases with the mutational input, and if most deleterious mutants are lost each generation due to strong skew in male mating success caused by the effects of mate choice. Thus, the only requirements for such a mechanism to work is that good genes sexual selection is important, and that a considerable amount of variance in fitness is due to mutation. Similar arguments have been put forward for the evolution of sex [17, 18].
Sperm competition is an important component of sexual selection [19]. Numerous studies of paternity have shown that the expression of secondary sexual characters is the single-most important correlate of paternity in birds [review in [20]]. Furthermore, extra-pair paternity generally increases the variance in male mating success considerably, since males successful in siring offspring in their own nest also are successful at other nests, as shown in several specific studies [e. g., [21–23]]. Thus, there is a general increase in the standardized variance in male success caused by extra-pair paternity, and this variance is directly related to the expression of secondary sexual characters [review in [24]].
Here we describe (1) patterns of the relationship between mutation rate and extra-pair paternity, a component of sexual selection, by (i) investigating the relationship between minisatellite mutation rates and the frequency of extra-pair paternity in birds. (ii) We investigate whether intense sperm competition preceded or followed the evolution of high levels of genetic variability. (iii) We investigate the relationship between mutation rate and relative testes size as a measure of sperm production. (iv) We investigate the relationship between mutation rate and longevity across species to determine whether the increased mutation rate in certain species is simply caused by a greater average age of males in such species. (2) In the discussion we describe the potential mechanisms accounting for those patterns, and (3) we briefly discuss the evolutionary implications of these patterns.
Methods
Data sets
Information on extra-pair paternity, minisatellite mutation rates per band and generation, minisatellite probes, body mass, sexual dichromatism and survival rate were obtained from the literature (data set and sources are reported in the Appendix [See Additional file: 1]). Mutation rate estimates were not confounded by estimates of extra-pair paternity since paternity exclusion routinely has been based on both a large number (usually at least three) of novel minisatellite bands with respect to the putative father, but not the putative mother, and a low band sharing coefficient with the putative father, but not the putative mother. Whenever possible we calculated the mutation rate based on the number of novel bands and the average number of bands scored in the study for the offspring that were not extra-pair offspring. The latter precaution was taken since a single or few novel band(s) in an individual due to mutation cannot readily be distinguished among a large number of novel bands due to extra-pair paternity. As an illustration of this approach we provide the calculations based on two studies. Westneat [25] estimated extra-pair paternity in the indigo bunting Passerina cyanea. He reported paternity for a total of 63 offspring of which 22 were considered to be fathered by extra-pair males based on a large number of novel bands (on average 8.2 novel bands) and low band sharing coefficients with the presumed father, but not the mother. Of the remaining 41 nestlings, 28 had 0 novel bands, 10 had one novel band and 3 had two novel bands, in total 16 novel bands. The mean number of bands scored per individual was 37.5 bands, which gives 41 × 37.5 = 1537.5 bands for these offspring. Mutation rate is therefore 16 / 1537.5 = 0.010407 in this study. Negro et al. [26] reported extra-pair paternity in the lesser kestrel Falco naumanni for 87 nestlings of which 3 were extra-pair offspring with four unmatched bands with the father. Among the remaining 84 nestlings there were three cases of a single novel band. Since the number of bands scored was on average 10.9 per individual, mutation rate was 3 / (10.9 × 84) = 0.003277. If multiple mutation estimates were available for a single species, a mean estimate weighted by sample size was used in the analyses. Minisatellite mutation rates had a significant repeatability [27] among populations of the same species of 0.76 (F = 7.41, d.f. = 7,10, P = 0.0027). Mutation rates were also significantly repeatable among probes for the same population (R = 0.65, F = 4.75, d.f. = 8,9, P = 0.016). There were no significant differences in mutation rates depending on type of restriction enzymes used (Alu: F = 0.04, d.f. = 1,60, P = 0.83; Hae: F = 0.001, d.f. = 1,60, P = 0.97) or minimum size of fragments scored (F = 1.45, d.f. = 1,60, P = 0.23). There was no significant difference in mutation rate between different molecular laboratories (F = 1.46, d.f. = 35,41, P = 0.12). Likewise, there was no temporal change in mutation rate with year of publication, as expected if techniques improved with time (r = 0.10, t = 0.90, P = 0.37).
Extra-pair paternity was defined as the percentage of offspring sired by males other than the attending male. For species with cooperative breeding systems, extra-pair paternity was defined as the percentage of offspring sired by males other than the attending males. This definition was justified since, for example, dunnocks Prunella modularis have a high degree of shared paternity in polygynandrous groups, but no extra-pair paternity in monogamous groups [28]. In addition, most extra-pair paternity in cooperative groups of superb fairy wrens Malurus cyaneus is caused by extra-group males with a preferred phenotype, while within group males hardly account for any extra-pair paternity [29]. If sexual selection was driving multiple mating by females, we should also expect female dunnocks in monogamous groups to engage in extra-pair copulations. They do not. In addition, shared paternity among multiple males within a group will decrease rather than increase the variance in male mating success, causing a reduction in the intensity of sexual selection. However, female superb fairy wrens engage in extra-pair copulations independent of group composition. These observations suggest that mixed paternity within groups of cooperative breeders is unrelated to sexual selection, but is caused by within-group competition for paternity. Extra-pair paternity estimates had a highly significant repeatability [27] of 0.68 [30], even though several species showed marked intraspecific variation in extra-pair paternity.
Sexual dichromatism was estimated as the difference between mean male and female color score in the visual spectrum made by three independent scorers based on inspection of field guides [31, 32]. Such scores are repeatable among scorers, and correlate with extra-pair paternity in birds [30–32].
Testes mass for adult birds from the main breeding season were obtained from [33] and unpublished information.
Survival rate was estimated as the annual adult survival rate from detailed population studies (data reported in the Appendix [See Additional file: 1]).
A second data set on genetic variation and extra-pair paternity is fully reported in [30].
Comparative methods
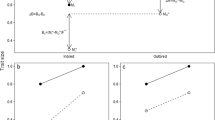
We investigated the relationship between mutation rate and extra-pair paternity using a maximum likelihood test analysing the relationship between two continuous characters [34]. This method implemented in the computer program Continuous (available at http://www.ams.rdg.ac.uk/zoology/pagel/mppubs.html) allows one to test the hypothesis that two continuous characters co-evolve and to test whether the changes in one character precede those in another, all while taking account of the phylogenetic relationships. The proportion of extra-pair paternity was squareroot-arcsine-transformed, mutation rate was log10(x + 0.001)-transformed, testes mass and body mass were log10-transformed and survival rate was squareroot-arcsine-transformed. The correlation between the two variables [34] was calculated from a phylogenetic hypothesis of birds (Fig. 1). This hypothesis was a composite phylogeny based on information in the following references [35–38].
We tested for an association between mutation rate and the proportion of polymorphic allozyme loci, respectively, and extra-pair paternity. This was done by investigating whether transitions to higher mutation rates and a larger proportion of polymorphic loci preceded or followed transitions to more frequent extra-pair paternity using a continuous time Markov model with the computer program Continuous [34]. The phylogeny for this analysis is given in Fig. 2.
Phylogenetic relationships of bird species with information on extra-pair paternity and the frequency of polymorphic loci. Adapted from [26].
Results
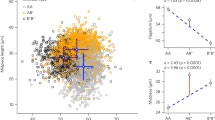
Minisatellite mutation rates derived from paternity studies were significantly positively related to sexual selection, as estimated from extra-pair paternity (Fig. 3). A phylogenetic correlation analysis revealed that mutation rates were positively related to extra-pair paternity (in likelihood ratio = 3.07, d.f. = 1, P = 0.013). The estimated phylogenetically corrected correlation was 0.279, thus accounting for 7.8% of the variance. The data suggested that extra-pair paternity had a smaller variance (0.01289) than the mutation rate (0.04749). Regressions of variables onto path length (the length of path in the phylogeny in Fig. 1) provides information on the rate of change in the variable in question. Regressions of the two variables onto path length revealed the following regression models: Mutation rate = -2.553 + 0.041 Path length and Extra-pair paternity = 0.133 + 0.012 Path length. Both of these regression coefficients were small and of a similar magnitude, which makes it difficult to determine which variable was changing first. These conclusions were independent of any particular phylogeny since we obtained qualitatively similar results with a standard taxonomy [39].
Interspecific variation in sexual dichromatism has arisen by sexual selection and is positively correlated with extra-pair paternity in several comparative studies [30–32], and extra-pair paternity has been predicted to be related to adult survivorship [40]. The effect of extra-pair paternity on mutation rate while controlling for sexual dichromatism in a multiple regression analysis did not affect the previous conclusion, since the correlation between mutation rate and extra-pair paternity gave a likelihood ratio = 3.42, d.f. = 1, P = 0.0089. The estimated phylogenetically corrected correlation coefficient was 0.293, thus accounting for 8.6% of the variance.
Relative testes mass, calculated as residuals from a regression of log-transformed testes mass on log-transformed body mass, was not significantly correlated with mutation rate: ln likelihood ratio = 0.36, d.f. = 1, P = 0.39. The estimated phylogenetically corrected correlation coefficient was 0.105. This conclusion was insensitive to outliers since an analysis base on ranked values gave a similar result (ln likelihood ratio = 0.28, d.f. = 1, P = 0.45, estimated phylogenetically corrected correlation coefficient = 0.093). The conclusion was independent of a particular phylogeny since we obtained similar results with the standard taxonomy [39]. Testes mass controlled for body mass is correlated with sperm production rate in mammals [41], although similar data are unavailable for other taxa.
A previous study of extra-pair paternity in birds revealed more frequent extra-pair paternity in species with a high genetic variability [30]. A temporal order test [42] demonstrated that an increase in extra-pair paternity preceded an increase in genetic variability as estimated from the frequency of polymorphic allozyme loci. This analysis was prompted by a previous study that unable to distinguish between the order of evolution of extra-pair paternity and genetic variability [30]. The positive correlation found in the previous study was corroborated using the phylogenetic correlation analysis, which was positive and significant (ln likelihood ratio = 2.65, d.f. = 1, P = 0.021, estimated phylogenetically corrected correlation coefficient = 0.375). Regressions of the two variables onto path length revealed the following regression models: Polymorphism = 0.455 - 0.002 Path length and Extra-pair paternity = 0.053 + 0.045 Path length. This implies that extra-pair paternity have changed much more rapidly with path length than polymorphism, although the variance in extra-pair paternity was only slightly larger than the variance in polymorphism. It is more likely that the variable that changes more is also the one that changed first. Watterson & Guess [43] have shown theoretically that the most common neutral alleles tend to be the oldest, supporting our interpretation that the variable that changes more is also the one that changed first. Thus an increase in extra-pair paternity is likely to generate greater genetic variability.
Minisatellite mutation rates were not higher in long-lived than in short-lived species. Mutation rate was not significantly related to adult survival rate corrected for the effects of allometry based on a phylogenetically corrected estimate (ln likelihood ratio = 1.07, d.f. = 1, P = 0.14). This is contrary to the prediction based on the hypothesis that differences in mutation rates among species were due to germline mutations accumulating during lifetime [44, 45]. The estimate of the phylogenetically corrected correlation coefficient was 0.174.
Discussion
The lek paradox arises from the observation that females are often very choosy in their mate choice, even when there are no apparent material benefits to be obtained from such a choice [3, 5]. Genetic benefits of mate choice have the theoretical difficulty that any highly beneficial allele rapidly will go to fixation [3, 4]. However, fitness itself seems to have a small, but highly significant heritability [7–9], and estimates of the magnitude of genetic benefits obtained by females also seem generally to be important [46]. Since mechanisms that generate new genetic variability must balance the rate at which alleles go to fixation, the maintenance of large amounts of genetic variability for fitness must be addressed. Although mechanisms that maintain significant additive genetic variance in fitness have been proposed [e. g., [3]], it remains unknown to which extent they suffice to account for the relatively large heritabilities of fitness [7].
In this paper we have shown that there is a positive correlation of an intermediate effect size between a measure of sexual selection (extra-pair paternity) and mutation rate for near neutral molecular markers (minisatellites) (Fig. 3). Estimates of minisatellite mutation rates showed statistically significant repeatabilities among populations, but also among probes used in single population samples. Thus, the data used for the present study provided reliable estimates. This is the first comparative demonstration of a significant predictor of mutation rates of any genetic system in any group of organisms, and such a novel pattern is interesting in itself independent of the exact mechanisms proposed. The novel pattern reported here suggests that mutational input is not necessarily independent of the intensity of sexual selection, but that it may even increase with an increasing intensity of sperm competition. An analysis of extra-pair paternity and genetic variability in birds, using the proportion of polymorphic loci as an estimate of genetic variability, also demonstrated a positive relationship between sexual selection and genetic variability. Furthermore, this analysis indicated that it was extra-pair paternity that evolved first, followed by a change in genetic variation. This finding suggests that sexual selection initially may be related to good genes effects, and that sexual selection subsequently may be driving the evolution of genetic variability through an increase in germline mutation rate, rather than vice versa. Rather than depleting genetic variation in good genes, sexual selection may increase such genetic variability through an increase in germline mutation rate. If mutational input is dependent on the intensity of sexual selection, as indicated by the present study, this provides a novel mechanism that may help resolve the lek paradox.
Extra-pair paternity has been shown to increase the standardised variance in male reproductive success within own nests by on average a factor 5.19 across a sample of 8 species of birds, with a maximum value of 15.20 [24]. While this suggests that sperm competition increases at least one component of variance in male mating success in these species, obviously we cannot know if that is the case in all species. If extra-pair paternity has evolved for reasons other than sexual selection, for which there is little or no empirical evidence [review in [24]], these additional factors would only increase the level of noise in the relationship between mutation rate and extra-pair paternity. Thus, the estimate of the relationship between mutation rate and sexual selection reported here is likely to be conservative. However, positive relationships between sexual dichromatism and the rate of extra-pair paternity across species of birds suggest that the expression of secondary sexual characters has coevolved with extra-pair paternity [32].
We proposed two alternative hypotheses for the evolution of elevated mutation rates. Sperm competition generally results in an increase in sperm production rates and size of ejaculates [41, 47–50, 52], since males may win at sperm competition by providing more sperm than competitors [52–55]. How does an increase in sperm production relate to minisatellite mutation rates in birds? The most straightforward connection is that the minisatellite mutation rate is positively correlated with the number of germline cell divisions, due to mutations during replication. Indeed, most human minisatellite loci show a strong male-biased mutation rate [56–58], as do avian minisatellites [59, 60]. However, sequence analyses of human minisatellite mutations unravel inter-allelic recombination or gene conversion-like events, most likely of meiotic origin [61–64]. A meiotic mechanism may also govern mutations in avian minisatellites, since mutation rates were unrelated to longevity. Studies of humans and mice indicate various mechanisms [65], suggesting that replication, repair or recombination may be more error-prone when mitotic and meiotic rates are elevated. However, we found no significant relationship between minisatellite mutation rate and the relative size of testes (Fig. 4). Thus, it is possible that factors unrelated to replication are responsible for the observed positive relationships between minisatellite mutation rates and sperm competition.
The second hypothesis is that linkage between mutator alleles and beneficial alleles arising though mutation may allow females to continuously select more viable males as expressed in their condition-dependent secondary sexual characters. This hypothesis can account for the association between mutation rate and sexual selection. It is also consistent with sex-biased mutation rates for minisatellites, and the lack of association between survival rate and mutation. This hypothesis cannot be rejected with available data.
The increased mutation rates among species with frequent extra-pair paternity, suggests that mutational input may increase in species subject to intense sexual selection as a direct consequence of sperm competition. Most minisatellite loci have little or no effect on phenotype [66], and they thus offer nothing directly on which females may base their mate choice. Since minisatellite mutation rates are correlated with extra-pair paternity, then standard mutations (which are often replication dependent) should show a greater effect on the expression of secondary sexual characters. Therefore, there is no reason to assume that an increased mutation rate is restricted to neutral genetic markers such a minisatellites.
The observation that sexual selection is particularly intense in birds with a high minisatellite mutation rate has implications for the resolution of the lek paradox of how females can choose mates for indirect fitness benefits without alleles going to fixation [5, 6]. Our analyses indicate that the balance between mutation and sexual selection is not constant, as previously assumed, since mutational input for minisatellites, and, therefore, presumably also for other parts of the genome, is positively correlated with the intensity of sexual selection arising from sperm competition. Hence selection of mates based on indirect fitness benefits can be maintained perhaps not because of the presence of "good" genes, but because females are attempting to avoid "bad" genes arising from deleterious mutations as expressed in condition-dependent secondary sexual characters. Females may be able to chose sexual partners with reduced levels of mutagenesis by having evolved preferences for males with signals based on antioxidants such as carotenoids. Carotenoids are often involved in sexual signalling, but they also play an important role for high quality sperm [67]. Hence, females may choose the partner with the lowest level of mutagenesis by preferring males with the most exaggerated carotenoid-based signals, resulting in the maintenance of high mutation rates. This effect of sexual selection related to deleterious mutations with viability effects may be widespread, as sperm competition occurs commonly in many animal taxa [19], and similar phenomena are reported from other kingdoms.
Conclusions
We tested the hypothesis that sexual selection is associated with increased germline mutation rates, using a comparative analysis of a data set for minisatellite mutation rates in birds. Species with more intense sexual selection as revealed by higher levels of extra-pair paternity had significantly elevated mutation rates, accounting for more than 10% of the variance. We found no evidence of mutation rate being related to relative testes mass, which reflects sperm production rate. In addition, we found no evidence of mutation rates being higher in species with higher survival rates. These observations suggest that the increased mutation rate in species with intense sperm competition has a meiotic basis. An analysis of extra-pair paternity and genetic variability in birds, using the proportion of polymorphic loci as an estimate of genetic variability, indicated that extra-pair paternity changed before there was a change in genetic variation. Thus, sexual selection may be driving the evolution of genetic variability, presumably through an increased mutation rate. High mutation rates are associated with sexual selection, and they may be maintained through mate choice for sires with extravagantly expressed secondary sexual characters reliably revealing beneficial viability alleles.
References
Andersson M: Sexual selection. Princeton: Princeton University Press. 1994
Fisher RA: The genetical theory of natural selection. Oxford: Clarendon Press. 1930
Hamilton WD, Zuk M: Heritable true fitness and bright birds: A role for parasites?. Science. 1982, 218: 384-387.
Charlesworth B: 1987 The heritability of fitness. In: Sexual selection: Testing the alternatives. Edited by: Bradbury JW, Andersson MB. 1987, New York: Wiley, 21-40.
Taylor PD, Williams GC: 1982 The lek paradox is not resolved. Theor Popul Biol. 1982, 22: 392-409.
Kirkpatrick M, Ryan MJ: The evolution of mating preferences and the paradox of the lek. Nature. 1991, 350: 33-38. 10.1038/350033a0.
Burt A: Perspective: The evolution of fitness. Evolution. 1995, 49: 1-8.
Kruuk LEB, et al: Heritability of fitness in a wild mammal population. Proc Natl Acad Sci USA. 2000, 97: 698-703. 10.1073/pnas.97.2.698.
Merilä J, Sheldon BC: Lifetime reproductive success and heritability in nature. Am Nat. 2000, 155: 301-310. 10.1086/303330.
Lynch M, Blancard J, Houle D, Kibota T, Schultz S, Vassilieva L, Willis J: Perspective: Spontaneous deleterious mutation. Evolution. 1999, 53: 645-663.
Taddei F, Radman M, Maynard Smith J, Toupance B, Gouyon PH, Godelle B: Role of mutator alleles in adaptive evolution. Nature. 1997, 387: 700-702. 10.1038/42696.
Partridge L, Barton NH: Evolving evolvability. Nature. 2000, 407: 457-458. 10.1038/35035173.
Johnson T: Beneficial mutations, hitchhiking and the evolution of mutation rates in sexual populations. Genetics. 1999, 151: 1621-1631.
Miyata H, Hayashida H, Kuma K, Mitsuyasa K, Yasunaga T: Male-driven molecular evolution: A model and nucleotide sequence analysis. Cold Spring Harbor Symp Quant Biol. 1987, 52: 863-867.
Shimmin LC, Chang BHJ, Li W-H: Male-driven evolution in DNA sequences. Nature. 1993, 362: 745-747. 10.1038/362745a0.
Ellegren H, Fridolfsson A-K: Male-driven evolution of DNA sequences in birds. Nature Genetics. 1997, 17: 182-184.
Siller S: Sexual selection and the maintenance of sex. Nature. 2001, 411: 689-692. 10.1038/35079578.
Agrawal AF: Sexual selection and the maintenance of sexual reproduction. Nature. 2001, 411: 692-695. 10.1038/35079590.
Birkhead TR, Møller AP, Editors: Sperm competition and sexual selection. London: Academic Press. 1998
Møller AP, Ninni P: Sperm competition and sexual selection: a meta-analysis of paternity studies of birds. Behav Ecol Sociobiol. 1998, 43: 345-358. 10.1007/s002650050501.
Yezerinac SM, Weatherhead PJ: Extra-pair mating, male plumage cloration and sexual selection in yellow warblers (Dendroica petechia). Proc R Soc Lond B. 1997, 264: 527-532. 10.1098/rspb.1997.0075.
Whittingham LA, Lifjeld JT: Extra-pair fertilizations increase the opportunity for sexual selection in the monogamous house martin Delichon urbica. J Avian Biol. 1995, 26: 283-288.
Sheldon BC, Ellegren H: Sexual selection resulting from extrapair paternity in collared flycatchers. Anim Behav. 1999, 57: 285-298. 10.1006/anbe.1998.0968.
Møller AP: Sperm competition and sexual selection. In: Sperm competition and sexual selection. Edited by: Birkhead TR, Møller AP. 1998, London: Academic Press, 55-90.
Westneat DF: Genetic parentage in the indigo bunting: a study using DNA fingerprinting. Behav Ecol Sociobiol. 1990, 27: 67-76.
Negro JJ, Villarroel M, Tella JM, Kuhnlein U, Hiraldo F, Donazar JA, Bird DM: DNA fingerprinting reveals a low incidence of extra-pair fertilizations in the lesser kestrel. Anim Behav. 1996, 51: 935-943. 10.1006/anbe.1996.0097.
Falconer DS, Mackay TFC: Introduction to quantitative genetics. New York: Longman. 1996, 4
Burke TA, Davies NB, Bruford MW, Hatchwell BJ: Parental care and mating behaviour of polygynandrous dunnocks Prunella modularis related to paternity by DNA fingerprinting. Nature. 1989, 338: 249-251. 10.1038/338249a0.
Dunn PO, Cockburn A: Extrapair mate choice and honest signaling in cooperatively breeding superb fairy-wrens. Evolution. 1999, 53: 938-946.
Petrie M, Doums C, Møller AP: The degree of extra-pair paternity increases with genetic variability. Proc Natl Acad Sci USA. 1998, 95: 9390-9395. 10.1073/pnas.95.16.9390.
Møller AP: Immune defence, extra-pair paternity and sexual selection in birds. Proc R Soc Lond B. 1997, 264: 561-566. 10.1098/rspb.1997.0080.
Møller AP, Birkhead TR: The evolution of plumage brightness in birds is related to extra-pair paternity. Evolution. 1994, 48: 1089-1100.
Møller AP: Sperm competition, sperm depletion, paternal care and relative testes size in birds. Am Nat. 1991, 137: 887-906.
Pagel M: Inferring evolutionary processes from phylogenies. Zool Scripta. 1997, 26: 331-349.
Sibley SG, Ahlquist JE: Phylogeny and classification of birds: A study in molecular evolution. New Haven and London: Yale University Press. 1990
Sheldon FH, Slikas B, Kinnarney M, Gill FB, Zhao E, Silverin B: DNA-DNA hybridization evidence of phylogenetic relationships among major lineages of Parus. Auk. 1992, 109: 173-185.
Sheldon FH, Winkler DW: Intergeneric phylogenetic relationships of swallows estimated by DNA-DNA hybridization. Auk. 1993, 110: 798-824.
Patten MA, Fugate M: Systematic relationship among the emberizid sparrows. Auk. 1998, 115: 412-424.
Howard R, Moore A: A complete checklist of the birds of the world. London: Academic Press. 1991, Second
Mauck RA, Marschall EA, Parker PG: Adult survival and imperfect assessment of parentage: Effects on male parenting decisions. Am Nat. 1999, 154: 99-109. 10.1086/303216.
Møller AP: Ejaculate quality, testes size and sperm production in mammals. Funct Ecol. 1989, 3: 91-96.
Pagel M: Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc R Soc Lond B. 1994, 255: 37-45.
Watterson GA, Guess HA: Is the most frequent allele the oldest? Theor. Popul Biol. 1977, 11: 141-160.
Medawar PB: An unsolved problem in biology. London: HK Lewis. 1952
Charlesworth B: Evolution in age-structured populations. Cambridge: Cambridge University Press. 1980
Møller AP, Alatalo RV: Good genes effects in sexual selection. Proc R Soc Lond B. 1999, 266: 85-91. 10.1098/rspb.1999.0607.
Short RV: Sexual selection and its component parts, somatic and genital selection, as illustrated by man and the great apes. Adv Study Behav. 1979, 9: 131-158.
Harcourt AH, Harvey PH, Larson SG, Short RV: Testes weight, body weight and breeding system in primates. Nature. 1981, 293: 55-57.
Møller AP: Ejaculate quality, testis size and sperm competition, in primates. J Human Evol. 1988, 17: 479-488.
Møller AP: Testes size, ejaculate quality and sperm competition in birds. Biol J Linn Soc. 1988, 33: 273-283.
Møller AP, Briskie JV: Extra-pair paternity, sperm competition and the evolution of testis size in birds. Behav Ecol Sociobiol. 1995, 36: 357-365. 10.1007/s002650050158.
Parker GA: Sperm competition and its evolutionary consequences in insects. Biol Rev. 1970, 45: 525-567.
Parker GA: Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J theor Biol. 1982, 96: 281-294.
Parker GA: Sperm competition games: raffles and roles. Proc R Soc Lond B. 1990, 242: 121-126.
Parker GA: Sperm competition games: sneaks and extra-pair copulations. Proc R Soc Lond B. 1990, 242: 127-133.
Jeffreys AJ, Royle NJ, Wilson V, Wong Z: Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988, 322: 278-281. 10.1038/332278a0.
Vergnaud G, Mariat D, Apiou F, Aurias A, Lathrop M, Lauthier V: The use of synthetic tandem repeats to isolate new VNTR loci: cloning of a human hypermutable sequence. Genomics. 1991, 11: 135-144.
Henke J, Henke L: Recent observations on human minisatellite mutations. Int J Legal Med. 1995, 107: 204-208.
Verheyen GR, Kempenaers B, Burke T, Van den Broeck M, Van Broeckhoven C, Dhondt A: Identification of hypervariable single locus minisatellite probes in the blue tit Parus caeruleus. Molec Ecol. 1994, 3: 137-143.
Wetton JH, Parkin DT: A suite of falcon single-locus minisatellite probes: a powerful alternative to DNA fingerprinting. Molec Ecol. 1997, 6: 119-128. 10.1046/j.1365-294X.1997.00161.x.
Buard J, Vergnaud G: Complex recombination events at the hypervariable minisatellite CEB1 (D2S90). EMBO J. 1994, 13: 3203-3210.
Jeffreys AJ, Tamaki K, MacLeod A, Monckton DG, Neil DL, Armour JAL: Complex gene conversion events in germline mutations at human minisatellites. Nature Genetics. 1994, 6: 136-145.
Jeffreys AJ, Neumann R: Somatic mutation processes at a human minisatellite. Human Molec. Genet. 1997, 6: 129-132. 10.1093/hmg/6.1.129.
Jeffreys AJ, Neil DL, Neumann R: Repeat instability at human minisatellites arising from meiotic recombination. EMBO J. 1998, 17: 4147-4157. 10.1093/emboj/17.14.4147.
Bois P, Stead JDH, Bakshi S, Williamson J, Neumann R, Moghadaszadeh B, Jeffreys AJ: Isolation and characterization of mouse minisatellites. Genomics. 1998, 50: 317-330. 10.1006/geno.1998.5329.
Avise JC: Molecular markers: Natural history and evolution. New York: Chapman and Hall. 1994
Blount J, Møller AP, Houston DC: Antioxidants, showy males and sperm quality. Ecol Letters. 2001, 4: 393-396. 10.1046/j.1461-0248.2001.00255.x.
Acknowledgements
We are grateful for data provided by M. Martín-Vivaldi and M. Petrie, comments by H. Ellegren, T. Martin, T. Mousseau, M. Raymond, B. Sheldon, P. Sniegowski and F. Taddei, and M. Pagel and J. Soler for crucial assistance with the analyses. JJC was supported by Spanish DGICYT (PB95-0110).
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
APM conceived the original idea and drafted the manuscript.
JJC conducted the analyses and drafted the manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Møller, A., Cuervo, J. Sexual selection, germline mutation rate and sperm competition. BMC Evol Biol 3, 6 (2003). https://doi.org/10.1186/1471-2148-3-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2148-3-6