Abstract
Background
We are interested in understanding how the twenty neurons of the C. elegans pharynx develop in an intricate yet reproducible way within the narrow confines of the embryonic pharyngeal primordium. To complement an earlier study of the pharyngeal M2 motorneurons, we have now examined the effect of almost forty mutations on the morphology of a bilateral pair of pharyngeal neurosecretory-motor neurons, the NSMs.
Results
A careful description of the NSM morphology led to the discovery of a third, hitherto unreported process originating from the NSM cell body and that is likely to play a proprioceptive function. We found that the three NSM processes are differently sensitive to mutations. The major dorsal branch was most sensitive to mutations that affect growth cone guidance and function (e.g. unc-6, unc-34, unc-73), while the major sub-ventral branch was more sensitive to mutations that affect components of the extracellular matrix (e.g. sdn-1). Of the tested mutations, only unc-101, which affects an adaptin, caused the loss of the newly described thin minor process. The major processes developed synaptic branches post-embryonically, and these exhibited activity-dependent plasticity.
Conclusion
By studying the effects of nearly forty different mutations we have learned that the different NSM processes require different genes for their proper guidance and use both growth cone dependent and growth cone independent mechanisms for establishing their proper trajectories. The two major NSM processes develop in a growth cone dependent manner, although the sub-ventral process relies more on substrate adhesion. The minor process also uses growth cones but uniquely develops using a mechanism that depends on the clathrin adaptor molecule UNC-101. Together with the guidance of the M2 neuron, this is the second case of a pharyngeal neuron establishing one of its processes using an unexpected mechanism.
Similar content being viewed by others
Background
The development of organ innervation requires numerous axon guidance cues that are evolutionarily conserved and more easily studied in simple organisms. Research in this area has led to a general model where receptors on growth cones, which are motile structures at the tips of growing axons, respond to a wide variety of cues that include long-range signals such as netrins or secreted forms of semaphorins, or contact-dependent cues that include ephrins, Slit and many components of the extracellular matrix [1–3]. Different growth cones may express different receptors and therefore respond to different molecular cues as they explore their environment. Growth cones migrate towards attractants and away from repellents, and in this way establish unique axon trajectories. This model has great explanatory power and undoubtedly is generally correct. However it poses a conundrum: the diversity of neuron types and trajectories seems vast, yet the known guidance signals and receptors are few. In an effort to explain how the limited guidance toolbox can generate a wide variety of neuron trajectories, and also in the hope of discovering novel mechanisms of axon guidance, we are pursuing an in-depth explanation of how the pharyngeal neurons develop in C. elegans.
The C. elegans pharynx is an isolated neuro-muscular organ composed of only 80 cells. It is located at the anterior end of the worm where its primary function is to rhythmically pump and grind bacterial suspensions (food) before transferring the product to the intestine. Twenty neurons innervate the pharynx in a highly reproducible fashion, and most of these may be ablated without severe effects on viability or pharyngeal pumping [4]. There are few interactions between the pharynx and other somatic tissues, either during morphogenesis or during its physiological function.
The pharyngeal nervous system can be divided into four principal parts: two subventral nerve cords, one dorsal nerve cord, and one circular pharyngeal nerve ring located within the posterior half of the metacorpus [5]. One interesting aspect of the system is that the neurons extend trajectories within muscle cell folds, rather than between a basement membrane and hypodermal cells, as do most body neurons. This unique topology may rely on hitherto uncharacterized mechanisms for guiding axonal development. In a published study we have previously described a genetic analysis of the axon guidance of the bilaterally symmetrical motorneurons M2L and M2R that are located within pharynx of C. elegans [6]. In that study we discovered that the M2 neurons establish most of their trajectories in a growth cone-independent manner, a finding that raised the question of whether other pharyngeal neurons also utilize such mechanisms.
To address this question and better understand how the pharyngeal nervous system develops, we studied the genetic pathways that regulate guidance of another pair of pharyngeal neurons, the NSMs (Pharyngeal neurosecretory-motor neurons), that can be visualized using the pTPH-1::GFP reporter [7]. The cell bodies of the NSML and NSMR serotonergic neurons are located sub-ventrally in the pharyngeal metacorpus, and each extends posteriorly one bifurcating major process and one thin minor process ([5]; this article). The NSMs have neurohumoral functions and were postulated to sense bacteria in the pharyngeal lumen by their proprioceptive endings and to communicate with the rest of the worm's body via secretion into the pseudocoelomic fluid [5]. Ablation of the NSMs significantly decreases the enhanced slowing response that starved worms exhibit upon encountering food, suggesting that they contribute to this behavior [8]. Our study shows that different branches of the same neuron respond differently to the same set of guidance cues. In particular, the minor (dendritic) process of the NSM establishes its trajectory in a manner that is dependent on the clathrin adaptor complex, whereas the major (axonal) process requires more typical growth cone and guidance molecules for its proper guidance and branching.
Results and Discussion
Expression profiling of the pTPH-1::GFPreporter
Pharyngeal expression of the pTPH-1::GFP reporter is limited to the NSM neurons in larvae and adults; outside the pharynx, expression is also seen in the nerve ring and some other extra-pharyngeal neurons e.g. HSN, ADF (Fig. 1). During embryogenesis, expression of pTPH-1::GFP within the pharyngeal primordium can first be observed at approximately 300 min. of development (Fig. 2). When the pharyngeal primordium begins to elongate at two-fold stage, strong expression of the pTPH-1::GFP reporter is observed both in the muscle cells of the procorpus and metacorpus. In the late three-fold stage, as the pharynx elongates and matures, pharyngeal expression of the pTPH-1::GFP reporter becomes restricted to the NSM neurons; pharyngeal muscle expression weakens and eventually disappears completely by the time of hatching [7]. Because of the embryonic expression of pTPH-1::GFP in pharyngeal muscles, the NSM processes have already established their trajectories by the time that they can be unambiguously scored using this reporter. It is therefore not possible to directly study the NSM neurons as they are developing using the pTPH-1::GFP reporter, although it is useful to examine the final morphology of these neurons. Note that none of the mutants tested in the course of the present study affected the levels of pTPH-1::GFP expression (see below).
Morphology of the NSM neurons. (A) Cartoon of one NSM neuron, NSML (B) Flattened stack of confocal images of an adult worm expressing pTPH-1::GFP. The main structures shown in panels A and B are the cell body (red circle; filled triangle), the major sub-ventral (blue; filled arrow) and dorsal (green; open triangle) and the minor (red line; open arrow) processes. The scale bar represents 20 μm.
Time course of expression of the pTPH-1::GFP reporter. Transgenic worms were photographed at different developmental stages: (A) ~330 minutes-old embryo; (B) Two-fold stage; (C) Two-and-a-half-fold stage; (D) Three-fold stage; and (E) Young adult. The following GFP-positive cells or structures are indicated: pharyngeal muscle (open arrows), NSM cell body (filled triangle), and the major sub-ventral (filled arrow) and dorsal (open triangle) processes. The minor process is not visible in these images. Scale bar represents 20 μm.
Morphology of the NSM neurons
The NSML and NSMR neurons are bilateral homologues. The cell body of each neuron resides within a subventral pm4 muscle cell in the metacorpus and extends one major and one minor process (Fig. 1). The major process bifurcates near the cell body to produce one long subventral process that runs posteriorly within the subventral nerve cord of the isthmus, and a shorter dorsal process running posteriorly in the dorsal nerve cord. To reach the dorsal cord, these processes first pass through the pharyngeal nerve ring. Both dorsal processes then run together posteriorly within the dorsal nerve cord of the isthmus. Both dorsal and subventral processes run near the outside edge of the nerve cords, very close to the pseudocoelom, and make synapses to the pharyngeal basement membrane and muscle cells. Within the isthmus these processes form en passant synaptic swellings and short synaptic branches at the basal zone, contacting the basement membrane to release vesicles into the pseudocoelom. The dorsal process terminates half way through the isthmus whereas the subventral process ends just anterior to the terminal bulb. The minor dendritic process is thin and synapse-free, extending under the cuticle of the pharyngeal lumen within the isthmus. This thin process had not previously been reported as a separate entity in a detailed ultrastructural description of the pharynx [5], but careful examination of archival electron micrographs confirmed their presence and shapes (Fig. 3). The thin process likely plays a proprioceptive function because it is devoid of organelles and is embedded in close contact with the cuticle lining the pharyngeal lumen.
Thin NSM processes seen in serial thin sections by TEM. (A) Profiles of NSML (large arrowheads) and NSMR (arrows) in the region of the pharyngeal nerve ring. Each neuron shows several profiles within this section as it travels around the ring, including some profiles that are beyond the edge of the panel. NSMR sends a large process inward from the ring to the cuticle, diving between two portions of the right ventral pharyngeal muscle pm4 (4), to which it is attached by adherens junctions (called desmosomes in [5]; compare to their Figure 9). A few small vesicles are present within NSMR near the cuticle. The thin process of NSML (top left arrowhead) has already migrated laterally, passing underneath pharyngeal muscle pm5 (5), and is also filled with clear vesicles. (B) Less than one micron posterior to panel A, the thin processes of NSML (arrowhead) and NSMR (arrow) are migrating laterally to positions lying between pm5 (5) and the marginal cells (mc2), and both thin processes are filled with clear vesicles. Large adherens junctions secure pm5 to mc2 at their inner borders. The region shown is subject to severe distortion during each pharyngeal pumping cycle, and the inner portion of these cells, together with the NSM processes will move laterally to positions outside the view of this panel with every contraction. Thin cuticle strands extend within the pharyngeal lumen; these are part of the sieve (S). (C) Far posterior to panel B, this section lies about halfway along the pharyngeal isthmus. The minor processes of NSML and NSMR are now thinner and contain few or no vesicles. Each lies within a groove between pm5 and mc2 cells, well secured by the local adherens junctions. Again this portion of the thin process is subject to maximum shearing forces during pharyngeal pumping, and is presumed to be stretch-sensitive. (D) A section at the border of the posterior pharyngeal bulb, where the minor processes of NSML and NSMR are about to end, becoming even thinner and still in close contact to pm5 and mc2 cells and their luminal adherens junctions. This is also the posterior limit of pm5 and mc2 cells. (E) Closeup of NSMR from a section close to panel A, which shows several vesicles restricted to the portion of the process closest to the pharyngeal cuticle. (F) Closeup of NSML from the same section shown in B, to show the clear vesicles and nearby adherens junction just to the left of NSML. All panels were scanned from archival TEM images (negatives or prints) of Albertson and Thomson (1976) stored in the Hall archive [5]. Scale bars: A-D share the same 1 μm scale bar; the scale bars in E and F represent 0.5 μm.
Morphology of the NSM synaptic branches over time and in response to serotonin
C. elegans neurons are typically simple and unbranched [9]. For this reason, it was of particular interest to examine the synaptic branches found on the NSM major processes during development and to investigate whether their number or shapes change in response to activity. We found that synaptic branches develop post-embryonically: they are mostly absent at the L1 stage but are numerous by the L4 stage and continue to increase in number between 1-day and 4-day old adults (Fig. 4). When synaptic swellings were counted in L1 larvae, we found that they are present in numbers similar to the number of synaptic branches in 4-days old adults, namely 18.4 ± 0.7 and 18.1 ± 1.0, respectively. This indicates that it is the morphology of the synaptic branches that changes during development/aging, rather than the number of synapses per se. To test if pharyngeal muscle activity could feedback onto the NSM neurons to regulate the dynamics of the synaptic branches, we grew worms in the presence of 50 μM levamisole from the L1 larval stage to 1-day old adults. Levamisole is an acetylcholine analogue that causes muscles to contract constitutively, thus causing the animals to also grow more slowly. It had no effect on the number of synaptic branches for developmentally age-matched animals, suggesting that there is no feedback between contraction and the number of synaptic branches. We next tested for the possibility that the increase in synaptic branches may be a feedback response to the need for more efficient release of serotonin by the serotonergic NSM neurons. We found that growing worms from L1 to 1-day old adults in the presence of 7.5 mM serotonin caused a significant reduction in the number of synaptic branches (Fig. 4). This result supports the notion that the synaptic branches increase to meet a need for more efficient serotonin secretion into the pseudocoelom, and represent an example of synaptic plasticity in C. elegans.
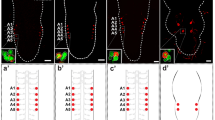
Development of the NSM synaptic branches. Flattened confocal stacks are shown for larval stages L1 (A) and L4 (B). Note the prominent synaptic branches at the L4 but not L1 stage. The graph shows the average number of synaptic branches from at least 11 individuals for each age or condition. 1d-lev and 1d-ser refer to animals grown in levamisole or serotonin from the L1 stage, respectively, and scored as 1-day old adults. Asterisks indicate significant differences with p < 0.05 between the indicated groups.
Estimating the natural variation in NSM morphology
We have examined the morphology of the NSM neurons in almost forty different genetic backgrounds, and grouped the most commonly observed phenotypes into distinct classes (Fig. 5 and Table 1). In wild-type control worms, more than 95% of the NSM neurons were scored as normal, with only 4% showing a short dorsal process and 1% showing a short sub-ventral process (Table 2). As control mutations that should not have specific effects on the NSM neurons we used unc-30 and unc-46. These mutations affect differentiation or GABA secretion in GABAergic neurons and should not have direct effects on NSM development since the NSMs are serotonergic neurons. Not surprisingly, the unc-30 and unc-46 mutations had little effect on the NSMs: 93 and 91% of the NSM neurons were scored as normal in these two mutants, and the rare abnormal neurons were cases where the length of the processes appeared as either too short or too long, compared to the wild-type (Table 2). The average and standard deviation for the three control genetic backgrounds is 93± 2% of NSM neurons being scored as normal. We considered that any mutation that causes defects in more than 15% of the scored NSM neurons (twice as many as in the controls) is having a significant effect.
The major NSM process develops in a growth cone dependent manner
Several mutations known to impair growth cone function, e.g. unc-51, unc-73, unc-76 and unc-119, caused defects in the major NSM process and affected both the long subventral and, usually more severely, the short dorsal branch. In particular, both branches of the major process are often either absent or show premature termination in the unc-73 mutant, which affects a guanine nucleotide exchange factor involved in Rac signaling within growth-cones [10]. In this mutant, 27% of the dorsal processes and 10% of the sub-ventral processes are absent. unc-119 encodes a novel protein important for regulating axon branching and fasciculation [11, 12]. The mutant exhibited a high frequency of defects in the dorsal branch which was missing in 22% and too short in 41% of cases.
In the unc-51 mutant background only the dorsal process is markedly affected. unc-51 encodes a Serine/threonine-protein kinase involved in membrane recycling within growth cones [13]. In the mutant, 29% of the NSM neurons lack a dorsal process while 38% have defects in the termination of the dorsal or subventral process along the anterior-posterior axis, indicating that unc-51 is important both for initiation of the dorsal processes and for their proper termination (Table 1). unc-76, which encodes a FEZ protein (fasciculation and elongation protein [14, 15], displays a similar phenotype. Other growth cone defective mutants such as unc-14 and unc-115 also show defects in the dorsal process but at a lower frequency. unc-14 is suggested to be a regulator of unc-51 in axonal elongation and guidance [13], whereas unc-115 encodes an actin-binding LIM Zn-finger protein [16].
Netrin signalling guides the major NSM processes
unc-6 encodes a C. elegans netrin ligand that guides many axons along the dorso-ventral axis [17, 18]. It can act as an attractant or a repellent, depending on type of receptor that is expressed on the growth cone. unc-40 encodes a netrin transmembrane receptor important for dorso-ventral guidance [19] and also determines the polarity of neurons [20], whereas the unc-5 co-receptor is required for netrin-mediated repulsion [21]. unc-6 is also important for anterior-posterior guidance [20, 22]. In the unc-6 mutant background, we found that 32% of the NSM neurons have no dorsal process and 24% have a short dorsal process, suggesting that unc-6 guides the dorsal process in establishing its trajectories and in determining its lengths. Mutation in the unc-5 gene mainly results in premature termination of both the dorsal and sub-ventral process of the NSM neurons: 26% of the dorsal processes are short and 13% are absent. In unc-40 mutants, 26% of the dorsal and 8% of the sub-ventral processes are absent. Interestingly, 4% of the NSM neurons show anterior misguidance defects, indicating that unc-40 gene is not only important for proper guidance of the major process but also in determining the polarity of the NSM neurons.
sax-3 determines the axonal polarity of the NSM neurons independently of slt-1
Slit-Robo signaling is involved in midline guidance in both invertebrate and vertebrates [23]. In C. elegans, mutations in the ROBO homolog sax-3 cause cell migration and midline guidance defects [24]. In sax-3 mutants the NSM neurons often project one axon branch anteriorly (37%) or exhibit a morphology so abnormal, with cell bodies frequently misplaced, that it is not easily classified (19%). 12% of the NSM neurons in these mutants lack the dorsal processes and 3% have a short sub-ventral process. These results indicate that sax-3 is important in establishing the anterior-posterior polarity of the NSM neurons, or their correct positioning within the primordium, and for axon initiation.
slt-1 is the only known ligand for sax-3 in C. elegans and is involved in dorsal-ventral and midline guidance [25]. The slt-1 mutant caused the dorsal and sub-ventral and processes to be short in 16% and 22% of cases, respectively. The fact that the sax-3 mutation caused more severe and qualitatively different defects than the slt-1 mutation indicates that it can bind at least one other ligand during NSM neuron development.
Other guidance genes necessary for the major NSM processes
unc-34 is homologous to the signal transduction protein ENABLED that is required for axon guidance and plays a vital role in downstream signalling for both the netrin and slit pathways [26]. In unc-34 mutants, 77% of the dorsal processes are absent indicating that this gene is essential for the initiation of the dorsal process from the NSM cell body. Another guidance gene, unc-69 is also important for the proper development of the major process. unc-69 encodes a protein with a short coiled-coil domain that is generally important for axon outgrowth and proper synaptic formation [27, 28]. In unc-69 mutants, the initiation (28%) and termination (38%) of the dorsal process are primarily affected, with similar weaker effects on the sub-ventral process.
The ephrins are a highly conserved family of proteins that are involved in axon guidance in vertebrates whereas in C. elegans they seem to play only a minor role in axon guidance but have critical functions in multiple aspects of epidermal morphogenesis [29, 30]. To find out if ephrins are involved in NSM guidance, we scored the NSMs in mutants for the sole ephrin receptor (vab-1). Our results indicate that ephrins play only a minor role, if any, in the development of the dorsal processes of the NSM neurons: vab-1 mutant exhibit 7% small dorsal process, 1% missing dorsal process and no sub-ventral defects.
Another important axon guidance molecule in C. elegans is unc-129, which encodes a member of the TGF-beta family of secreted growth factor signalling molecules that acts as a dorso-ventral guidance cue [31, 32]. The dorsal and subventral processes of the NSM neurons showed a variety of initiation and termination defects that affected 43% of NSM neurons.
Finally, kal-1, which encodes an extracellular protein with a fibronectin III domain that likely acts as a guidance cue [33], had a slightly stronger effect on the sub-ventral processes (20% absent or short) than on the dorsal one (16% absent or short).
Extracellular matrix (ECM) molecules and the major NSM process
ECM molecules provide instructive signals for many neurons in C. elegans, and are also important also for the maintenance of their morphologies [34]. We studied the NSM trajectories in the mec-1, sax-7, sdn-1, unc-23, unc-52 and unc-112 mutants. Our findings indicate that ECM or ECM-adhesion genes are involved in both the initiation and the termination of the major NSM processes. sdn-1 is a type I transmembrane heparan sulfate proteoglycan thought to be involved in cell-cell or cell-matrix adhesion [35]. Of all the mutations studied, sdn-1 caused the most severe defects on the sub-ventral process, with 32% of them being absent, and another 5% being too short. The large transmembrane ECM adhesion molecule sax-7 caused severe defects in both the long dorsal and the subventral processes, with 37% and 34% being affected, respectively. mec-1 and unc-112 encode two extracellular proteins that are also involved mainly in development of the sub-ventral process. MEC-1, is an ECM protein with multiple EGF and Kunitz domains and is involved in organizing channel complexes within mechanosensory assemblies [36], and UNC-112 is a membrane-associated structural/signalling protein that has been implicated in linking the actin cytoskeleton to the ECM [37]. In mec-1 mutants, 8% of the NSM sub-ventral processes are absent and another 4% are short. In unc-112 mutants, 9% of the NSM sub-ventral processes are short. Other ECM genes such as unc-23, unc-52 exhibit low penetrance defects in the major process of the NSM neurons. Both these mutants lead to initiation and termination problems in the dorsal and sub-ventral processes of the NSM, which often caused thickened endings. unc-61 encodes a C. elegans septin that is thought to regulate the contact points between the cytoskeleton and the cell cortex, in this way possibly influencing attachment between the cell and the extracellular matrix [38–40]. In the unc-61 mutant, 19% of the dorsal processes were too long or absent, and 5% of the subventral branches were truncated.
Synapses are essential for the normal development of the NSM major process
Some C. elegans neurons require proper localization of active synapses for their morphology and maintenance [41]. We found that the trajectories of the dorsal NSM process is often defective in the syg-1 mutant, but that both of the major processes are generally not affected by mutations in unc-101 or unc-104. SYG-1 is an immunoglobulin super-family protein that determines the position of specific synapses in C. elegans [42, 43]. 26% of NSM neurons in syg-1 mutants have a shorter dorsal process and 24% have no dorsal process, indicating that formation of synapses is essential both for the initiation and the proper guidance of the dorsal process. The weak effects of unc-101 and unc-104 on the major processes perhaps reflect their intracellular functions [44–47]. For example, while the unc-104 mutant does have reduction in the formation of synapses, its function seems to reside principally in synaptic vesicle trafficking rather than synapse formation per se [45, 47].
NSM function mutations
The NSM neurons are serotonergic, and their physiological activity depends on the tph-1 and mod-5 genes that respectively encode a tryptophane hydroxylase and a serotonin transporter [7, 48]. Short dorsal (17%) and subventral (7%) processes were found in the mod-5 mutant. This result supports the idea that functioning synapses may play a role in the development or maintenance of the NSM neurons.
Proper cell positioning is required for determining the polarity of the NSM processes
Unsurprisingly, we found that the NSM processes depend on correct cellular context to establish their trajectories: the NSM neurons were grossly abnormal in the pharyngeal mutant pha-2. pha-2 encodes a homeobox transcription factor necessary for the successful differentiation of the pm5 muscle cells that make up the isthmus, and also for the establishment of the correct morphologies of several pharyngeal structures, including the isthmus and metacorpus [49, 50]. In pha-2 mutants, the isthmus is short and thick due to misplaced cell nuclei. In these mutants, 45% of NSM neurons abnormally send a major process anteriorly, and 21% of the dorsal processes are absent; another 27% of the neurons show other defects such as multiple branching and misplaced cell bodies. Our results indicate that pha-2 is not only required for the proper positioning of the NSM cell bodies within the pharynx but also determines the axonal polarity of the NSM neurons. mnm-5 is a mutant in which the cell bodies of the pharyngeal M2 neurons are often misplaced into the metacorpus, rather than being located in their normal position within the posterior bulb [6]. The molecular identity of mnm-5 is currently unknown. In this mutant, 8% of the NSM dorsal processes are missing, and 11% of the NSM cell bodies are misplaced or misshaped, and send grossly misguided branches.
Development of the NSM minor process depends on unc-101
Our study has focused mostly on the major processes because the minor process was very difficult to score reliably due to its weak GFP signal. In spite of this, we found that the thin process is rather resistant to mutations that affect growth cones, although low penetrance defects such as occasional truncations are observed in these mutants, suggesting that some growth cone function is directly or indirectly required for its elongation. Interestingly, the NSM minor process was uniquely sensitive to the unc-101 mutation. Neurons are polarized cells that contain distinct sets of proteins in their axons and dendrites and the polarized movement of proteins is a result of selective transport. In C. elegans, clathrin-associated proteins are known to determine the polarity between axons and dendrites. unc-101 encodes a mu1 subunit of the AP-1 clathrin adaptor complex that is involved in polarized dendrite transport and for determining the axon-dendrite polarity in olfactory neurons [44, 46, 51]. In unc-101 mutants, the NSM neurons have no minor process whereas the major process is completely normal (Fig. 6). This result suggests that unc-101 is essential for polarized transport of proteins that are important for initiation and development of the minor process of the NSM neuron. Together with the proximal trajectory of the M2 neurons, which is established without the use of a growth cone [6], this is the second case of a pharyngeal neuron establishing part of its arborization through an unexpected mechanism. It may be that the NSM minor process is established by the stretching of the axon between the NSM cell body and a substrate that moves away during development, as is the case for the M2 axon [6, 52] and the dendrites of some sensory neurons [53]. This would explain the resilience of the minor process to growth cone mutations. Alternatively, and given that the trajectory of the minor process follows precisely the pm5/mc border (Fig. 3), it is possible that a physically confined adhesion pathway accounts for the developmental robustness of this minor process. While dendrites and axons utilize many of the same guidance cues and mechanisms [54, 55], significant differences in their development have previously been noted. For example, dendrites in Drosophila are more critically dependent on the secretory pathway during their elongation [56], a finding that echoes the role of unc-101 during the development of the NSM dendrite.
The thin NSM process is absent in unc-101 mutants. (A-B) and (C-D) show a cartoon summary and flattened stacks of confocal images of an adult wild-type and unc-101 worm, respectively. Note that the minor process (open arrow) is present in wild-type but absent in the mutant. The major sub-ventral (filled arrow) and dorsal (open triangle) processes are also shown. The asterisks indicate the ADF cells that lie just outside the pharynx.
Closing remarks
By studying the effects of nearly forty different mutations we have learned that the different processes of the NSM neurons require different genes for their proper guidance and use growth cone dependent mechanisms for establishing their trajectories (summarized in Fig. 7). We also found that the NSM minor process is unique in that its development depends on the clathrin adaptor molecule UNC-101.
One obvious limitation of our work is that we do not know if the tested mutations have a direct or secondary effect on the NSM neurons. We also did not test for cell-autonomous versus non-autonomous functions. Another limitation in our study was that the pTPH-1::GFP reporter that we used allowed us only to examine the NSM neurons in their final, or near final configurations. It is possible, although unlikely, that some observed phenotypes were due to the degeneration of neurons that had successfully developed prior to observation. Despite these limitations, it was reassuring that genes acting within a distinct biochemical pathway, i.e. the various classes shown in Table 2, caused similar NSM defects. This allows us to draw specific conclusions regarding the development of the NSM neurons, and broad conclusions regarding the development of nervous systems in general.
In a broad perspective, and also taking into account our earlier work on the guidance of the pharyngeal neuron M2 [6, 52], several conclusions emerge from our genetic analyses that are relevant to all developing nervous systems. One is that neuronal process outgrowth is extremely robust: i) with the exception of the effect of unc-101 on the NSM minor process, which is a highly unusual branch type, no mutation completely impaired the development of a particular axon; and ii) almost all of the tested axon guidance mutants could perturb the establishment of the studied axon trajectories. The various axons must each be capable of responding to multiple, redundant cues to different effect in order to initiate and guide its unique trajectory. Thus M2 and NSM both depend on many of the same guidance pathways (e.g. unc-6, slt-1), yet produce very different morphologies. We have previously shown that the M2 growth cone requires an interaction with the M3 neuron for its proper programming [52]. Each cell must reprogram its growth cone in specific ways according to its cell identity, often multiple times over the course of its migration. Such growth cone reprogramming has been amply documented elsewhere, as in the developing grasshopper limb when the Ti1 pioneer axon contacts specific guidepost cells then proceeds to the next target [57, 58], or when commissural neurons cross the midline in Drosophila and their growth cones are reprogrammed to prevent them from crossing again [59]. The diversity of axon pathways apparently results not from an immense diversity of molecular cues, but from differences in the relative timing or strength of a growth cone's responses and choice(s) among a limited number of cues. Conclusions more specific to NSM development are presented below.
Conclusion
1. The serotonergic NSM neurons harbour short synaptic branches that develop post-embryonically, and whose number can be regulated by serotonin levels.
2. The growth of the NSM processes, just like that of the M2 neuron previously studied [6], relies on multiple guidance cues, as well as on growth cone dependent and independent mechanisms.
3. Of thirty-nine mutations tested, only ten produced less than 15% abnormal NSM neurons, yet only three caused highly penetrant defects in more than 80% of NSM neurons (pha-2, unc-34, and unc-101). Of these, only unc-34 and unc-101 are likely to directly affect the growth of the NSM processes, rather than its guidance. Thus NSM development integrates multiple (redundant) pathways and is a robust mechanism usually capable of withstanding failure in any one pathway.
4. Of the twenty-nine mutants with significant effects on the NSM neurons, twenty-five mutants had more severe effects on the major dorsal than the subventral NSM process, including mutations that impair growth cone function (e.g. unc-51, unc-73, unc-76) or guidance cues and their interpretation (e.g. unc-6, unc-34). Thus the dorsal process probably requires more complex guidance decisions relative to the sub-ventral process, as one would expect from its more intricate trajectory.
5. Among the mutations studied, only four (kal-1, sdn-1, slt-1, and mec-1) had more severe effects on the major sub-ventral branch. Of these, sdn-1 had the most severe effect, suggesting that adhesion or substrate choice is a relatively important parameter influencing the growth of that branch.
6. Only one mutation, unc-101, strongly affected the minor NSM process. Given that UNC-101 is an adaptin molecule orthologous to the mu1-I subunit of adaptor protein complex 1 (AP-1), this suggests that the minor branch does not depend on growth cones for its establishment, but rather relies on the targeted deposition of material from clathrin-coated vesicles or on the clathrin-dependent endocytosis of a signal from the environment by the outgrowing dendrite.
Methods
Nematode strains and cultivation
All strains were maintained using standard methods [60], and were grown at 20°C, unless otherwise stated. List of mutants and transgenic strains used in this study:
Linkage group (LG) I: kal-1(gb503), mod-5(mg280), mab-20 (bx24), unc-14(e57), unc-73(e936), unc-40(e271), unc-101(m1).
LG II: tph-1 (mg280), unc-52(e1421), unc-104(rh1016), vab-1(dx31).
LG III: eat-4(ky5), mnm-5(et5), unc-69(e587), unc-119(e2498).
LG IV: efn-2(ev658), pha-3(ad607), sax-7(ok1244), unc-5(e35), unc-30(e318), unc-44(e362), unc-129(ev55), zag-1(ok214), zdls13.
LG V: max-1 (cz1632), mec-1(e1066), unc-23(e611), unc-46 (e177), unc-51 (e369), unc-61 (e228), unc-76 (e911), unc-112 (r367), vab-8(ev411).
LG X: fax-1(gm83), pha-2 (ad472), sax-3 (ky123), sdn-1(zh20), slt-1 (eh15), syg-1(Ky652), tag-53 (gk163), unc-115(e222), wrk-1(ok695), zig-4 (gk34).
zdls13 carries the tph-1::gfp transcriptional reporter expressed in the NSM, HSN and ADF neurons, and was a gift from Scott Clark. This is strictly a promoter reporter expressing no part of the TPH-1 protein.
Phenotype scoring and documentation
Worms carrying zdls13 were mounted on 2% agarose pads containing a drop of 100 mM levamisole. NSM pharyngeal neuron trajectories were scored at 400× and 1 000× magnification using a Leica DML or a Zeiss Axioplan compound microscope equipped with FITC filter sets, used with excitation 450–490 nm. Each NSM neuron of any given animal was scored into the following categories: Wild-type (cell body is properly placed in metacorpus and both the major and the minor processes show no guidance defect), short-dorsal process (the dorsal process terminates prematurly in the isthmus), short-subventral process (the length of the subventral process is the same or shorter than the dorsal process), long-dorsal process (the dorsal process is as long as the subventral process), no-dorsal process (dorsal process does not extend posteriorly), no-subventral process (the subventral process is absent), anterior-misguidance (one of the processes extends anteriorly within the procorpus instead of growing posteriorly), no-minor process (the minor process is absent) and others (includes defects that are not categorized). Each strain was scored independently by two of the authors and the scoring results were found to be similar and therefore pooled in Table 2.
Confocal microscopy
Worms were mounted on dried 2% agarose pads (in H2O), paralyzed by one drop of levamisole (100 mM) and examined by a Zeiss LSM 510 META System connected to an inverted Zeiss Axiovert 200 microscope.
Levamisole and serotonin treatments
For some experiments L1 larvae were grown in the presence of 50 mM levamisole or 7.5 mM serotonin until they became 1-day old adults. They were then mounted on agarose pads, imaged by confocal microscopy and the number of synaptic branches counted by analysing Z-stacks of the images.
Electron microscopy
Archival prints of the wild type adult pharynx were examined from four serially sectioned animals from the MRC collection (JSA, N2W, N2T and N2U) and several others in the Hall collection to follow the trajectory of the minor dendritic branch and of the multiple short synaptic branches of the two major axons.
References
Dickson BJ: Molecular Mechanisms of axon guidance. Science. 2002, 298: 1959-1964. 10.1126/science.1072165.
Silhankova M, Korswagen HC: Migration of neuronal cells along the anterior-posterior body axis of C. elegans: Wnts are in control. Curr Opin Genet Dev. 2007, 17 (4): 320-325. 10.1016/j.gde.2007.05.007.
Yu TW, Bargmann CI: Dynamic regulation of axon guidance. Nature Neurosci Suppl. 2001, 4: 1169-1176. 10.1038/nn748.
Avery L, Horvitz HR: Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron. 1989, 3: 473-485. 10.1016/0896-6273(89)90206-7.
Albertson DG, Thomson JN: The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976, 275: 299-325. 10.1098/rstb.1976.0085.
Mörck C, Axäng C, Pilon M: A genetic analysis of axon guidance in the C. elegans pharynx. Dev Biol. 2003, 260: 158-175. 10.1016/S0012-1606(03)00238-0.
Sze JY, Victor M, Loer C, Shi Y, Ruvkun G: Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000, 403 (6769): 560-564. 10.1038/35000609.
Sawin ER, Ranganathan R, Horvitz HR: C. elegans locomotory rate is modulated by the environment through a dopaminergic pathwat and by experience through a serotonergic pathway. Neuron. 2000, 26: 619-631. 10.1016/S0896-6273(00)81199-X.
White JG, Southgate E, Thomsom JN, Brenner FRS: The structure of the nervous system of the nematode Caenorhabditis elegans. Phil Trans R Soc Lond. 1986, 314: 1-340. 10.1098/rstb.1986.0056.
Steven R, Kubiseski TJ, Zheng H, Kulkarni S, Mancillas J, Ruiz A, Hogue CWV, Pawson T, Culotti JG: UNC-73 activates the Rac GTPase and is required for cell growth cone migrations in C. elegans. Cell. 1998, 92: 1-20. 10.1016/S0092-8674(00)81406-3.
Knobel KM, Davis MW, Jorgensen EM, Bastiani MJ: UNC-119 suppresses axon branching in C. elegans. Development. 2001, 128: 4079-4092.
Maduro M, Pilgrim D: Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics. 1995, 141 (3): 977-988.
Ogura KI, Wicky C, Magnenat L, Tobler H, Mori I, Müller F, Ohshima Y: Caenorhabditis elegans unc-51 gene required for axonal elongation encodes a novel serine/threonine kinase. Genes Dev. 1994, 8: 2389-2400. 10.1101/gad.8.20.2389.
Bloom L, Horvitz HR: The Caenorhabditis elegans gene unc-76 and its human homologs define a new gene family involved in axonal outgrowth and fasciculation. P N A S. 1997, 94: 3414-3419. 10.1073/pnas.94.7.3414.
Kuroda S, Nakagawa N, Tokunaga C, Tatematsu K, Tanizawa K: Mammalian homologue of the Caenorhabditis elegans UNC-76 protein involved in axonal outgrowth is a protein kinase C zeta-interacting protein. J Cell Biol. 1999, 144 (3): 403-411. 10.1083/jcb.144.3.403.
Lundquist EA, Herman RK, Shaw JE, Bargmann CI: UNC-115, a conserved protein with predicted LIM and actin-binding domains, mediates axon guidance in C. elegans. Neuron. 1998, 21 (2): 385-392. 10.1016/S0896-6273(00)80547-4.
Hedgecock EM, Culotti JG, Hall DH: The unc-5, unc-6 and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990, 2: 61-85. 10.1016/0896-6273(90)90444-K.
Ishii N, Wadsworth WG, Stern BD, Culotti JG: UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992, 9: 873-881. 10.1016/0896-6273(92)90240-E.
Gitai Z, Yu TW, Lundquist EA, Tessier-Lavigne M, Bargmann CI: The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parralel, Rac and UNC-115/AbLIM. Neuron. 2003, 37: 53-65. 10.1016/S0896-6273(02)01149-2.
Levy-Strumpf N, Culotti JG: VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat Neurosci. 2007, 10 (2): 161-168. 10.1038/nn1835.
Leung-Hagesteijn C, Spence AM, Stern BD, Zhou Y, Su MW, Hedgecock EM, Culotti JG: UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type I domains, guides cell and pioneer axon migrations in C. elegans. Cell. 1992, 71: 289-299. 10.1016/0092-8674(92)90357-I.
Watari-Goshima N, Ogura K, Wolf FW, Goshima Y, Garriga G: C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat Neurosci. 2007, 10 (2): 169-176. 10.1038/nn1834.
Brose K, Tessier-Lavigne M: Slit proteins: key regulators of axon guidance, axonal branching, and cell migration. Curr Opin Neurobiol. 2000, 10 (1): 95-102. 10.1016/S0959-4388(99)00066-5.
Zallen JA, Kirch SA, Bargmann CI: Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development. 1999, 126: 3679-3692.
Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, Moulder G, Barstead R, Tessier-Lavigne M, Bargmann CI: C. elegans Slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron. 2001, 32: 25-38. 10.1016/S0896-6273(01)00448-2.
Yu TW, Hao JC, Lim W, Tessier-Lavigne M, Bargmann CI: Shared receptors in axon guidance: SAX-3/Robo signals via UNC-34/Enabled and a Netrin-independent UNC-40/DCC function. Nature Neurosci. 2002, 5: 1147-1154. 10.1038/nn956.
Luo S, Nonet ML: Regulators of kinesin involved in polarized trafficking and axon outgrowth. J Biol. 2006, 5 (4): 8-10.1186/jbiol41.
Su CW, Tharin S, Jin Y, Wightman B, Spector M, Meili D, Tsung N, Rhiner C, Bourikas D, Stoeckli E, Garriga G, Horvitz HR, Hengartner MO: The short coiled-coil domain-containing protein UNC-69 cooperates with UNC-76 to regulate axonal outgrowth and normal presynaptic organization in Caenorhabditis elegans. J Biol. 2006, 5 (4): 9-10.1186/jbiol39.
Quinn CC, Wadsworth WG: Axon guidance: ephrins at WRK on the midline. Curr Biol. 2006, 16 (22): R954-5. 10.1016/j.cub.2006.10.021.
Wang X, Roy PJ, Holland SJ, Zhang LW, Culotti JG, Pawson T: Multiple ephrins control cell organization in C. elegans using kinase-dependent and -independent functions of the VAB-1 Eph receptor. Mol Cell. 1999, 4: 903-913. 10.1016/S1097-2765(00)80220-8.
Colavita A, Krishna S, Zheng H, Padgett RW, Culotti JG: Pioneer axon guidance by UNC-129, a C. elegans TGF-b. Science. 1998, 281: 706-709. 10.1126/science.281.5377.706.
Ikegami R, Zheng H, Ong SH, Culotti J: Integration of semaphorin-2A/MAB-20, ephrin-4, and UNC-129 TGF-beta signaling pathways regulates sorting of distinct sensory rays in C. elegans. Dev Cell. 2004, 6 (3): 383-395. 10.1016/S1534-5807(04)00057-7.
Rugarli EI, Di Schiavi E, Hilliard MA, Arbucci S, Ghezzi C, Facciolli A, Coppola G, Ballabio A, Bazzicalupo P: The Kallmann syndrome gene homolog in C. elegans is involved in epidermal morphogenesis and neurite branching. Development. 2002, 129 (5): 1283-1294.
Sobeih MM, Corfas G: Extracellular factors that regulate neuronal migration in the central nervous system. Int J Dev Neurosci. 2002, 20 (3-5): 349-357. 10.1016/S0736-5748(02)00040-0.
Minniti AN, Labarca M, Hurtado C, Brandan E: Caenorhabditis elegans syndecan (SDN-1) is required for normal egg laying and associates with the nervous system and the vulva. J Cell Sci. 2004, 117 (Pt 21): 5179-5190. 10.1242/jcs.01394.
Emtage L, Gu G, Hartwieg E, Chalfie M: Extracellular proteins organize the mechanosensory channel complex in C. elegans touch receptor neurons. Neuron. 2004, 44 (5): 795-807. 10.1016/j.neuron.2004.11.010.
Schaller MD: UNC112. A new regulator of cell-extracellular matrix adhesions?. J Cell Biol. 2000, 150 (1): F9-F11. 10.1083/jcb.150.1.F9.
Axäng C, Rauthan M, Hall DH, Pilon M: The twisted pharynx phenotype in C. elegans. BMC Dev Biol. 2007, 7: 61-10.1186/1471-213X-7-61.
Finger FP, Kopish KR, White JG: A role for septins in cellular and axonal migrations in C. elegans. Dev Biol. 2003, 261: 220-234. 10.1016/S0012-1606(03)00296-3.
Nguyen TQ, Sawa H, Okano H, White JG: The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J Cell Science. 2000, 113: 3825-3837.
Chisholm AD, Jin Y: Neuronal differentiation in C. elegans. Curr Opin Cell Biol. 2005, 17 (6): 682-689. 10.1016/j.ceb.2005.10.004.
Shen K, Bargmann CI: The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003, 112 (5): 619-630. 10.1016/S0092-8674(03)00113-2.
Shen K, Fetter RD, Bargmann CI: Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004, 116 (6): 869-881. 10.1016/S0092-8674(04)00251-X.
Dwyer ND, Adler CE, Crump JG, L'Etoile ND, Bargmann CI: Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001, 31 (2): 277-287. 10.1016/S0896-6273(01)00361-0.
Hall DH, Hedgecock EM: Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell. 1991, 65: 837-847. 10.1016/0092-8674(91)90391-B.
Lee J, Jongeward GD, Sternberg PW: unc-101, a gene required for many aspects of Caenorhabditis elegans development and behavior, encodes a clathrin-associated protein. Genes Dev. 1994, 8: 60-73. 10.1101/gad.8.1.60.
Otsuka AJ, Jeyaprakash A, Garcia-Anoveros J, Tang LZ, Fisk G, Hartshorne T, Franco R, Born T: The C. elegans unc-104 gene encodes a putative kinesin heavy chain-like protein. Neuron. 1991, 6 (1): 113-122. 10.1016/0896-6273(91)90126-K.
Ranganathan R, Sawin ER, Trent C, Horvitz HR: Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J Neurosc. 2001, 21: 5871-5884.
Mörck C, Axäng C, Goksör M, Pilon M: Misexpression of acetylcholinesterases in the C. elegans pha-2 mutant accompanies ultrastructural defects in pharyngeal muscle cells. Dev Biol. 2006, 297: 446-460. 10.1016/j.ydbio.2006.05.020.
Mörck C, Rauthan M, Wågberg F, Pilon M: pha-2 encodes the C. elegans ortholog of the homeodomain protein HEX and is required for the formation of the pharyngeal isthmus. Dev Biol. 2004, 272: 403-418. 10.1016/j.ydbio.2004.05.011.
Sakaguchi-Nakashima A, Meir JY, Jin Y, Matsumoto K, Hisamoto N: LRK-1, a C. elegans PARK8-related kinase, regulates axonal-dendritic polarity of SV proteins. Curr Biol. 2007, 17 (7): 592-598. 10.1016/j.cub.2007.01.074.
Rauthan M, Mörck C, Pilon M: The C. elegans M3 neuron guides the growth cone of its sister cell M2 via the Krüppel-like zinc finger protein MNM-2. Dev Biol. 2007, 311: 185-199. 10.1016/j.ydbio.2007.08.037.
Sulston JE, Schierenberg E, White JG, Thomson JN: The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983, 100: 64-119. 10.1016/0012-1606(83)90201-4.
Jan YN, Jan LY: The control of dendrite development. Neuron. 2003, 40 (2): 229-242. 10.1016/S0896-6273(03)00631-7.
Kim S, Chiba A: Dendritic guidance. Trends Neurosci. 2004, 27 (4): 194-202. 10.1016/j.tins.2004.02.011.
Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN: Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007, 130 (4): 717-729. 10.1016/j.cell.2007.06.032.
Palka J, Whitlock KE, Murray MA: Guidepost cells. Curr Opin Neurobiol. 1992, 2 (1): 48-54. 10.1016/0959-4388(92)90161-D.
O'Connor TP, Duerr JS, Bentley D: Pioneer growth cone steering decisions mediated by single filopodial contacts in situ. J Neurosci. 1990, 10 (12): 3935-3946.
Dickson BJ, Gilestro GF: Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu Rev Cell Dev Biol. 2006, 22: 651-675. 10.1146/annurev.cellbio.21.090704.151234.
Sulston JE, Hodgkin JA: Methods. The Nematode Caernorhabditis elegans. Edited by: Wood WB. 1988, Cold Spring Harbor, NY, Cold Spring Harbor Laboratory Press, 587-606.
Rankin CH, Wicks SR: Mutations of the Caenorhabditis elegans brain-specific inorganic phosphate transporter eat-4 affect habituation of the tap-withdrawal response without affecting the response itself. J Neurosci. 2000, 20 (11): 4337-4344.
Much JW, Slade DJ, Klampert K, Garriga G, Wightman B: The fax-1 nuclear hormone receptor regulates axon pathfinding and neurotransmitter expression. Development. 2000, 127: 703-712.
Roy PJ, Zheng H, Warren CE, Culotti JG: mab-20 encodes Semaphorin-2a and is required to prevent ectopic cell contacts during epidermal morphogenesis in Caenorhabditis elegans. Development. 2000, 127: 755-767.
Huang X, Cheng HJ, Tessier-Lavigne M, Jin Y: MAX-1, a novel PH/MyTH4/FERM domain cytoplasmic protein implicated in netrin-mediated axon repulsion. Neuron. 2002, 34: 563-576. 10.1016/S0896-6273(02)00672-4.
Zallen JA, Yi BA, Bargmann CI: The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell. 1998, 92: 217-227. 10.1016/S0092-8674(00)80916-2.
Rhiner C, Gysi S, Frohli E, Hengartner MO, Hajnal A: Syndecan regulates cell migration and axon guidance in C. elegans. Development. 2005, 132 (20): 4621-4633. 10.1242/dev.02042.
Ogura K, Shirakawa M, Barnes TM, Hekimi S, Ohshima Y: The UNC-14 protein required for axonal elongation and guidance in Caenorhabditis elegans interacts with the serine/threonine kinase UNC-51. Genes Dev. 1997, 11 (14): 1801-1811. 10.1101/gad.11.14.1801.
Waterston RH, Thomson JN, Brenner S: Mutants with altered muscle structure of Caenorhabditis elegans. Dev Biol. 1980, 77 (2): 271-302. 10.1016/0012-1606(80)90475-3.
Withee J, Galligan B, Hawkins N, Garriga G: Caenorhabditis elegans WASP and Ena/VASP proteins play compensatory roles in morphogenesis and neuronal cell migration. Genetics. 2004, 167 (3): 1165-1176. 10.1534/genetics.103.025676.
Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG: UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996, 87 (2): 187-195. 10.1016/S0092-8674(00)81337-9.
Boontrakulpoontawee P, Otsuka AJ: Mutational analysis of the Caenorhabditis elegans ankyrin gene unc-44 demonstrates that the large spliceoform is critical for neural development. Mol Genet Genomics. 2002, 267 (3): 291-302. 10.1007/s00438-002-0661-x.
Schuske K, Palfreyman MT, Watanabe S, Jorgensen EM: UNC-46 is required for trafficking of the vesicular GABA transporter. Nat Neurosci. 2007, 10 (7): 846-853. 10.1038/nn1920.
Kondo K, Makovec B, Waterston RH, Hodgkin J: Genetic and molecular analysis of eight tRNA(Trp) amber suppressors in Caenorhabditis elegans. J Mol Biol. 1990, 215 (1): 7-19. 10.1016/S0022-2836(05)80090-7.
Rogalski TM, Gilchrist EJ, Mullen GP, Moerman DG: Mutations in the unc-52 gene responsible for body wall muscle defects in adult Caenorhabditis elegans are located in alternatively spliced exons. Genetics. 1995, 139 (1): 159-169.
Lansbergen G, Grigoriev I, Mimori-Kiyosue Y, Ohtsuka T, Higa S, Kitajima I, Demmers J, Galjart N, Houtsmuller AB, Grosveld F, Akhmanova A: CLASPs Attach Microtubule Plus Ends to the Cell Cortex through a Complex with LL5[beta]. Developmental Cell. 2006, 11 (1): 21-32. 10.1016/j.devcel.2006.05.012.
Baumeister R, Liu Y, Ruvkun G: Lineage-specific regulators couple cell lineage asymmetry to the transcription of the Caenorhabditis elegans POU gene unc-86 during neurogenesis. Genes Dev. 1996, 10 (11): 1395-1410. 10.1101/gad.10.11.1395.
Rogalski TM, Mullen GP, Gilbert MM, Williams BD, Moerman DG: The UNC-112 gene in Caenorhabditis elegans encodes a novel component of cell-matrix adhesion structures required for integrin localization in the muscle cell membrane. J Cell Biol. 2000, 150 (1): 253-264. 10.1083/jcb.150.1.253.
George SE, Simokat K, Hardin J, Chisholm AD: The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell. 1998, 92 (5): 633-643. 10.1016/S0092-8674(00)81131-9.
Wightman B, Clark SG, Taskar AM, Forrester WC, Maricq AV, Bargmann CI, Garriga G: The C. elegans gene vab-8 guides posteriorly directed axon outgrowth and cell migration. Development. 1996, 122 (2): 671-682.
Wolf FW, Hung MS, Wightman B, Way J, Garriga G: vab-8 is a key regulator of posteriorly directed migrations in C. elegans and encodes a novel protein with kinesin motor similarity. Neuron. 1998, 20 (4): 655-666. 10.1016/S0896-6273(00)81006-5.
Boulin T, Pocock R, Hobert O: A novel Eph receptor-interacting IgSF protein provides C. elegans motoneurons with midline guidepost function. Curr Biol. 2006, 16 (19): 1871-1883. 10.1016/j.cub.2006.08.056.
Clark SG, Chiu C: C. elegans ZAG-1, a Zn-finger-homeodomain protein, regulates axonal development and neuronal differentiation. Development. 2003, 130: 3781-3794. 10.1242/dev.00571.
Acknowledgements
We thank the C. elegans Genetics Center (funded by the NIH Center for Research Resources) for providing many of the strains used in this study, and the Swegene Centre for Cellular Imaging at Gothenburg for the use of imaging equipment. Original annotated prints of serial section electron micrographs from the work of Albertson and Thomson [5] and White et al [9] are stored in the Hall lab at AECOM. These materials were donated to the Hall archive courtesy of Drs. Jonathan Hodgkin and John White from the MRC/LMB in Cambridge, England. This work was supported by grants to MP from the Swedish funding agencies Vetenskaprådet, Cancerfonden and Åhlens Stiftelse, and by a National Institute of Health (USA) grant to DHH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors' contributions
CA and MR contributed equally, carried out most of the experiments and helped with the writing of the manuscript. DHH provided electron microscopy results, and helped writing the manuscript. MP helped in experimental design and interpretations, and took the main responsibility for writing the article.
Claes Axäng, Manish Rauthan contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Axäng, C., Rauthan, M., Hall, D.H. et al. Developmental genetics of the C. eleganspharyngeal neurons NSML and NSMR. BMC Dev Biol 8, 38 (2008). https://doi.org/10.1186/1471-213X-8-38
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-213X-8-38