Abstract
Fanconi anemia (FA) is a rare genetic disorder characterized by aplastic anemia, cancer/leukemia susceptibility and cellular hypersensitivity to DNA crosslinking agents, such as cisplatin. To date, 12 FA gene products have been identified, which cooperate in a common DNA damage-activated signaling pathway regulating DNA repair (the FA pathway). Eight FA proteins form a nuclear complex harboring E3 ubiquitin ligase activity (the FA core complex) that, in response to DNA damage, mediates the monoubiquitylation of the FA protein FANCD2. Monoubiquitylated FANCD2 colocalizes in nuclear foci with proteins involved in DNA repair, including BRCA1, FANCD1/BRCA2, FANCN/PALB2 and RAD51. All these factors are required for cellular resistance to DNA crosslinking agents. The inactivation of the FA pathway has also been observed in a wide variety of human cancers and is implicated in the sensitivity of cancer cells to DNA crosslinking agents. Drugs that inhibit the FA pathway may be useful chemosensitizers in the treatment of cancer.
Publication history: Republished from Current BioData's Targeted Proteins database (TPdb; http://www.targetedproteinsdb.com).
Similar content being viewed by others
Protein pathway in the disease
Fanconi anemia
Fanconi anemia (FA) is a rare autosomal or X-linked recessive disease characterized by chromosomal instability and cancer susceptibility. All FA complementation groups (discussed below) except FA-B are inherited autosomally [1, 2]. FA prevalence is estimated to be 1–5 per million and the frequency of heterozygous carriers to be 1 in 300 [3, 4]. Notwithstanding the small number of FA patients, this disease constitutes a dynamic research area because the FA pathway plays a crucial role in preventing genomic instability and provides an attractive model for understanding the interplay between DNA repair and ubiquitin biology in tumorigenesis and cancer therapy (reviewed in [5–15]).
The clinical course and the treatment of FA have been extensively reviewed elsewhere [3, 4, 16]. Clinically, FA is characterized by childhood onset aplastic anemia, increased cancer/leukemia susceptibility and developmental defects. Typically, FA patients develop bone marrow failure leading to aplastic anemia during the first decade of life and at least 20% develop malignancies. Most commonly, these include acute myelogenous leukemia and myelodysplastic syndrome, but also head and neck squamous cell carcinoma, gynecological squamous cell carcinoma, esophageal carcinoma, and liver, brain, skin and renal tumors [17–19]. FA subtypes FA-D1 and FA-N are associated with increased predisposition to medulloblastoma, Wilms' tumor and acute leukemia in early childhood, and are clinically different from the other FA subtypes [20–24]. Common developmental defects observed in FA are short stature, developmental disability and abnormalities of the skin, upper extremities, head, eyes, kidneys and ears [3]. In addition, male FA patients often present abnormal gonads [3].
FA cells are hypersensitive to treatment with DNA crosslinking agents such as cisplatin, mitomycin C (MMC), melphalan and diepoxybutane [25], leading to increased chromosome breakage [25, 26].
The Fanconi anemia pathway
Proteins involved in the Fanconi anemia pathway
FA can be divided into at least thirteen complementation groups (FA-A, -B, -C, -D1, -D2, -E, -F, -G, -I, -J, -L, -M and -N), of which the responsible genes for all groups except FA-I have been identified (summarized in Table 1) [6, 23, 24, 27–30]. All FA proteins are required for cellular resistance to DNA crosslinking agents [6] and are considered to cooperate in a common pathway (the FA pathway) that regulates the sensing, signaling and/or repair of interstrand DNA crosslinks (Figure 1). FA proteins are closely related to the breast/ovarian cancer susceptibility genes products BRCA1 and BRCA2, and to their partner proteins, as described below. FANCD1 (mutated in FA-D1) is identical to BRCA2[31] and the most recently identified FA gene, FANCN, is in fact PALB2 (p artner a nd l ocalizer of B RCA2) [23, 24], a crucial regulator of the BRCA2 protein [32]. Additionally, FANCJ is identical to BACH1/BRIP1[29, 30, 33], a DNA helicase that interacts directly with BRCA1. Furthermore, FANCD2, FANCD1/BRCA2, FANCN/PALB2 and BRCA1 colocalize in nuclear foci at the site of DNA damage [32][34, 35] and BRCA1 itself is required for efficient nuclear foci formation of FANCD2 [34, 36]. In light of these interplays between FA and BRCA proteins, the FA pathway is also called the “FA-BRCA pathway” [5] or “FA-BRCA network” [11].
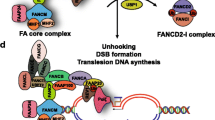
Current model of the Fanconi anemia pathway. (Adapted with modifications from “The molecular pathogenesis of Fanconi anemia: recent progress” Taniguchi T and D'Andrea AD. Blood, Jun 2006; 107: 4223 – 4233. [6]) Eight FA proteins (FANC-A, -B, -C, -E, -F, -G, -L and -M), a FANCM-interacting protein called FAAP24 and an unidentified factor (FAAP100) form a nuclear complex (the FA core complex) with E3 ubiquitin ligase activity. FANCL is the catalytic subunit of the complex and directly interacts with the E2 ubiquitin conjugating enzyme UBE2T through its PHD/RING domain. UBE2T can be inactivated by auto-monoubiquitylation on lysine 91 (K91), but is suggested to form a thioester intermediate with ubiquitin through it's catalytic cysteine 86 (C86) when taking part in the ubiquitylation of FANCD2 (see below). The FA core complex, BLM, RPA and topoisomerase IIIα form a larger super-complex called BRAFT. Note that for diagrammatic purposes the FA core complex is shown here interacting with the FAAP100 subunit, but in reality the quaternary structure of the BRAFT complex is unknown. In response to exogenous DNA damage, or during normal S phase progression, the FANCD2 protein is monoubiquitylated on lysine 561 (K561) in an FA core complex- and UBE2T-dependent manner. DNA damage-induced monoubiquitylation of FANCD2 also requires ATR and RPA. Monoubiquitylation of FANCD2 targets the protein into nuclear foci where it co-localizes with BRCA1, FANCD1/BRCA2, FANCN/PALB2, RAD51, FANCJ/BRIP1 and other proteins. At least some components of the FA core complex (FANCC, FANCE and FANCG) also form nuclear foci. All of these factors are required for cellular resistance to DNA crosslinking agents. Monoubiquitylation of PCNA on lysine 164 (K164) requires RAD6 as an E2 and RAD18 as an E3, but not the FA core complex. In turn, modification of PCNA causes recruitment of translesion synthesis (TLS) DNA polymerases at the site of stalled replication forks. USP1 deubiquitylates both PCNA and FANCD2, negatively regulating both the Fanconi anemia pathway and monoubiquitylation of PCNA.
The FA core complex and monoubiquitylation of FANCD2
Ubiquitin plays a crucial role in the regulation of the FA pathway. Eight FA proteins (FANCA, FANCB, FANCC, FANCE, FANCF, FANCG, FANCL and FANCM), a newly identified FANCM-interacting protein called FAAP24 (FA NCA-a ssociated p olypeptide) [137] and an unidentified factor called FAAP100 form a nuclear protein complex (the FA core complex) required for monoubiquitylation of FANCD2 on lysine 561 [27, 34, 37, 38]. One of the components of the FA core complex, FANCL, has a PHD (plant homeodomain) finger/RING finger domain exhibiting auto-ubiquitin ligase activity in vitro[38][39–41]. FANCL associates through its PHD/RING finger domain with UBE2T, a ubiquitin conjugating enzyme (E2), which is also required for in vivo FANCD2 monoubiquitylation [42]. Taken together, the FA core complex is assumed to constitute a multi-subunit E3 ubiquitin ligase complex for FANCD2, in which FANCL is the catalytic E3 ubiquitin ligase subunit. The catalytic activity of this complex appears to be regulated through the inhibitory auto-monoubiquitylation of UBE2T, stimulated in the presence of FANCL [42]. However, as the direct monoubiquitylation of FANCD2 by the FA core complex has not been reconstituted so far in vitro, the existence of other E2 and E3 enzymes responsible for FANCD2 monoubiquitylation (and somehow controlled by the FA core complex, the E2 activity of UBE2T and the E3 activity of FANCL) cannot be ruled out formally.
Even though a direct DNA binding activity has been demonstrated for unmodified FANCD2 in vitro[43], monoubiquitylation of the protein is required for its translocation to chromatin in vivo and nuclear foci formation at the site of DNA damage, as well as for cellular resistance to DNA crosslinking agents [34, 44]. FANCD2 monoubiquitylation and nuclear foci formation occur in response to DNA damaging agents (ionizing radiation, UV light irradiation, DNA crosslinking agents, hydroxyurea, etc.) and during S phase of the cell cycle even in the absence of exogenous DNA damage [34, 45]. A DNA damage-activated signaling kinase, ATR, and a single-strand DNA binding protein complex, RPA, are required for DNA damage-inducible monoubiquitylation and foci formation of FANCD2, indicating an upstream role for these factors in the activation of the FA pathway [46]. The exact signal and activation cascade required for FANCD2 monoubiquitylation, however, remain elusive. The existence of a specific FANCD2 receptor responsible for recruitment of the monoubiquitylated protein to chromatin has also been proposed [44], but not demonstrated to date.
Roles for the FA core complex in DNA enzymatic processing
The FA core complex is not simply the ubiquitin ligase complex for FANCD2. In addition to its requirement for FANCD2 monoubiquitylation, the FA core complex is required for the translocation into chromatin of monoubiquitylated FANCD2, and for cellular resistance to interstrand DNA crosslinks even if FANCD2 is localized in chromatin, as elegantly demonstrated in chicken DT40 cells using FANCD2–monoubiquitin and FANCD2–histone H2B chimeric proteins [47]. In addition, one component of the FA core complex, FANCM, harbors DNA helicase motifs, a degenerate nuclease motif and in vitro DNA-stimulated ATPase and translocase activities [27]. The newly identified FAAP24 protein, in complex with FANCM, has been shown to preferentially bind to ssDNA and branched DNA structures [137]. Speculatively, FANCM DNA translocase activity could play an important role in displacing the FA core complex along the DNA, allowing DNA damage recognition, or FAAP24 specificity for ssDNA structures may target FANCM and the FA core complex to abnormal, branched DNA structures. In summary, the FA core complex itself can interact with DNA and displays some important functions outside of FANCD2 monoubiquitylation.
Furthermore, the FA core complex forms a larger complex with BLM, RPA and topoisomerase IIIα called BRAFT (for B LM, R PA, F A, and t opoisomerase IIIα). Although the functional relevance of BRAFT is not clear, it harbors a DNA unwinding activity potentially relevant for DNA repair [37].
Negative regulation of the Fanconi anemia pathway by FANCD2 deubiquitylation
FANCD2 monoubiquitylation is a regulated and reversible process. USP1, a deubiquitylating enzyme, removes ubiquitin from monoubiquitylated FANCD2, therefore negatively regulating the FA pathway [48]. Interestingly, USP1 also deubiquitylates monoubiquitylated PCNA (p roliferating c ell n uclear a ntigen), a DNA polymerase processivity factor [49]. PCNA monoubiquitylation leads to the switch from a replicative polymerase to a translesion synthesis (TLS) polymerase at the site of stalled replication forks [50] and participates in the bypass of DNA lesion. Monoubiquitylation of PCNA is dependent on RAD6 (an E2) and RAD18 (an E3) [51], but is independent of the FA core complex. Therefore, these two pathways (the FA pathway and TLS activation through PCNA monoubiquitylation) have different activation mechanisms, but share a common shutoff mechanism.
The Fanconi anemia pathway and DNA repair
Interestingly, some TLS polymerases are also implicated in cellular resistance to interstrand DNA crosslinks [52]. For example, mutations in TLS polymerase-encoding REV3 or REV1 have been shown to be epistatic with FANCC mutations for crosslinker hypersensitivity in chicken DT40 cells [53]. Furthermore, REV1 and FANCD2 colocalize in nuclear foci [53], which could suggest a possible interaction between them. The possible interplay of FA proteins and TLS polymerases in a common pathway regulating the bypass of DNA damage, however, remains to be clarified.
One of the most important issues to clarify concerning the role of the FA pathway in DNA repair is how FA proteins (the FA core complex, FANCD2, FANCD1/BRCA2, FANCN/PALB2, FANCJ/BRIP1/BACH1) and other factors (RAD51, BRCA1, BLM, TLS polymerases etc.) cooperate to sense, signal and repair DNA crosslinks.
In nuclear foci, FANCD2 colocalizes with factors known to be involved in DNA repair and/or DNA damage response, such as BRCA1, FANCD1/BRCA2, FANCN /PALB2, RAD51, BLM, RPA and ATR [32, 34–36, 45, 46]. FANCD2 also partially colocalizes with FANCJ/BRIP1/BACH1 [33] and NBS1, part of the MRE11–RAD50–NBS1 complex implicated in the recognition and early signalization of damaged DNA [54]. Among the subunits of the FA core complex, at least FANCC and FANCE are reported to colocalize with FANCD2 in nuclear foci [47, 55], and FANCG with BRCA2 and RAD51 [56]. All these factors are required for cellular resistance to interstrand DNA crosslinking agents.
Among FA proteins, FANCD1/BRCA2 and FANCJ/BRIP1/BACH1 are neither part of the FA core complex nor required for FANCD2 monoubiquitylation or foci formation [28, 31]. They may work downstream of FANCD2 monoubiquitylation and/or in another pathway. Interestingly, these proteins have been strongly linked to DNA double-strand breaks repair by homologous recombination (HR) [33, 57]. Specifically, FANCD1/BRCA2 regulates HR by controlling the activity of RAD51, the eukaryotic homolog of bacterial RecA [57, 58]. FANCD1/BRCA2 stability and localization in nuclear structures (chromatin and nuclear matrix) is in turn regulated by FANCN/PALB2 [32]. Whether FANCD1/BRCA2, FANCN/PALB2 and RAD51 act downstream or independently of monoubiquitylated FANCD2 and other FA proteins remains unclear. Evidence in favor of a regulatory role for the FA pathway on FANCD1/BRCA2 and RAD51 function comes from reports that monoubiquitylated FANCD2 is required for both the loading of FANCD1/BRCA2 onto damaged chromatin and the increase in FANCD1/BRCA2 and RAD51 nuclear foci in response to DNA damage [35]. However, the requirement for the FA core complex and FANCD2 in efficient RAD51 foci formation in response to DNA damage [35, 59, 60], as well as the efficiency of HR in FA cells (reviewed in reference [6]), remain controversial. FANCD1/BRCA2- and FANCN/PALB2-deficient cells have a clear defect in RAD51 foci formation and HR efficiency [23, 32, 57, 61–63]. In other FA cells, at least mild defects in HR have been documented [33, 53, 62, 64–67], although other reports show normal HR efficiency in these cells [68–70].
FANCJ/BRIP1/BACH1 has DNA-dependent ATPase activity and 5′–3′ DNA helicase activity, and directly binds to the phospho-protein binding BRCT (BR CA1 c arboxyl-t erminal domain [72, 73]) of BRCA1 [71]. How FANCJ/BRIP1/BACH1 interacts with other FA proteins has not been reported, except for partial colocalization with FANCD2 in nuclear foci [33]. Though RAD51 foci formation is normal in FA-J cells (deficient in FANCJ/BRIP1/BACH1) [63], HR efficiency appears to be affected [33].
Taken together, these evidences suggest that FANCD2 plays a role in regulating DNA repair (especially of interstrand DNA crosslinks) in chromatin in cooperation with BRCA1, FANCD1/BRCA2, FANCJ/BRIP1/BACH1, the FA core complex and other factors, but the precise mechanism by which this occurs remains unknown. The highly regulated process of FANCD2 monoubiquitylation could play a crucial role in orienting the repair of DNA damage to HR and/or in attracting TLS polymerases to sites of DNA damage.
Other roles for the Fanconi anemia proteins outside of DNA repair
Several FA proteins function outside of DNA crosslink repair. For example, the phosphorylation of FANCD2 at serine 222 by ATM kinase in response to ionizing radiation is required for the establishment of IR-induced intra-S phase checkpoint, but is not required for resistance to DNA crosslinking agents [74]. Additionally, FANCC (localized in both the cytoplasm and nucleus) is implicated in several cytoplasmic functions, such as JAK/STAT and apoptotic signaling [75–80].
Alterations in the Fanconi anemia pathway in human cancer in the general (non-FA) population
FA patients have an increased risk of developing leukemia and solid tumors, but alterations in the FA pathway have also been reported in a wide variety of human cancers in the general (non-FA) population [81–94] (reviewed in references [6, 10]). Abnormalities in BRCA1 and BRCA2 in cancer have been reviewed elsewhere [95]. Methylation of FANCF, leading to its decreased expression, has been reported in ovarian cancer [81, 82], breast cancer [84], non-small cell lung cancer [85], cervical cancer [86], testicular cancer [87], head and neck squamous cell carcinoma [85], and granulosa cell tumors of the ovary [83]. Inherited and somatic mutations of FANCC and FANCG have been detected in a subset of young onset pancreatic cancer [89, 96]. Additionally, inherited mono-allelic mutations in BACH1/BRIP1/FANCJ and FANCN/PALB2 have been recently implicated in breast cancer predisposition (familial breast cancer) [97][23, 24], suggesting that these genes may be the low penetrance breast/ovarian cancer susceptibility genes researchers have been looking for to explain the non-BRCA1/non-BRCA2 cases. Because the integrity of the FA pathway is crucial for cellular resistance to interstrand DNA crosslinking agents (cisplatin, MMC, melphalan, etc.), tumors with defects in the pathway may be hypersensitive to these agents. In fact, in some cancer cell lines (human ovarian cancer cell lines (TOV-21G and 2008) [81], human myeloma cell lines (8226 and U266) [98] and a human pancreatic cancer cell line (PL11) [99]), the integrity of the FA pathway is a determinant of cisplatin (or melphalan) resistance in vitro or in vivo (mouse xenograft model) [81, 98–100].
Disease models, knockouts, assays
Generation of various knockout murine models for FA (Fanca[101, 102], Fancc[103, 104], Fancg[105, 106], Fancd2[107], Fanca-Fancc double [108], Fancl/Pog[109] and Fancd1/Brca2 [110] ) have been reported. These FA mice display similar phenotypes to that of human FA patients, for example chromosome instability, defective germ cell development and sensitivity to DNA crosslinking agents. Although FA mice do not spontaneously develop bone marrow failure (except for mouse models of the FA-D1 group, harboring homozygous hypomorphic mutations in Fancd1/Brca2[110]), treatment with MMC causes bone marrow failure in Fancc knockout mice [111]. The reason for the absence of spontaneous bone marrow failure in FA mice is still unknown. Several FA mice (Fancd2[107], Fanca[102] and Fancc [112]) are reported to develop tumors including adenocarcinoma, lymphoma, sarcoma and ovarian granulosa cell tumor. In addition, many Brca1- and Brca2- deficient mouse models with cancer susceptibility have been generated (reviewed in reference [113]). FA mice are useful tools to test the applicability of new FA treatment modalities, such as gene therapy [114–120], ex vivo manipulation of hematopoietic stem/progenitor cells [121] and cytokine treatment [122].
Drosophila, Caenorhabditis elegans, Xenopus and zebrafish homologs of FA genes have been more recently identified (Table 1). The FA gene network is conserved from zebrafish [123] and Xenopus[124] to human, while only FANCD2, FANCL and FANCM have Drosophila homologs [125], and FANCD1/BRCA2, FANCD2 (FCD-2), FANCJ and FANCM have homologs in C. elegans[126] (Table 1). Drosophila and C. elegans models will therefore be useful for testing the functions of key proteins in the FA pathway. As only FANCD2, FANCL and FANCM are present in Drosophila, these proteins may constitute the minimal FA pathway machinery. In turn, C. elegans may enable a better understanding of the roles of FANCD1/BRCA2 and FANCJ in the context of the minimal machinery constituted by FANCD2 and FANCM. As with vertebrate mutants, Drosophila fancd2 and fancl mutants, as well as C. elegans fancd2 mutants, show hypersensitivity to DNA crosslinking agents [125, 126]. Drosophila and C. elegans models may, therefore, be useful to dissect the roles and regulations of the FA pathway in a less complex network. The zebrafish model may prove a valuable tool with which to investigate the impact of FA pathway failure on development as fancd2-deficient zebrafish embryos develop similar defects to those found in children with FA, including shortened body length, microcephaly (small head) and microphthalmia (small eyes) [127]. The Xenopus model mostly constitutes a powerful tool with which to investigate the regulation of the FA pathway in vitro through cell-free assay systems using replicating egg extracts [124]. Such assays could also be utilized for screening drugs that modulate the FA pathway.
Disease targets and ligands
The FA pathway is required for cellular resistance to DNA crosslinking agents, including widely used anti-cancer drugs such as cisplatin, MMC and melphalan. As such, inhibition of the FA pathway will lead to sensitization of tumor cells to these drugs. Therefore, the FA pathway is an attractive target for developing small molecule inhibitors that may be clinically useful as chemosensitizers in the treatment of cancer. As described above, the FA pathway involves formation of a multi-subunit protein complex harboring E3 ligase activity, several enzymes (ubiquitin ligase and conjugating enzyme, deubiquitinating enzyme, kinase, ATPase/DNA translocase and ATPase/helicase) and many protein–protein or protein–DNA interactions. All of these components are potential targets for small molecule inhibitors of the FA pathway.
Drug development targeting the FA pathway is still in the early stages, and therefore not much information is available. A high-throughput cell-based screening assay for small molecule inhibitors of the FA pathway has, however, been developed by Alan D'Andrea (Dana-Farber Cancer Institute), Toshiyasu Taniguchi (Fred Hutchinson Cancer Research Center) and their colleagues [128]. In this screen, inhibition of DNA damage-induced FANCD2 nuclear foci formation was utilized as a readout. Continued screening is ongoing and a partial result focusing on one inhibitor, curcumin (diferuloylmethane), has been published [128]. Curcumin is a low-molecular-weight polyphenol derived from the rhizome Curcuma longa and is the principal ingredient in the spice turmeric [129]. Curcumin inhibits FANCD2 monoubiquitylation and nuclear foci formation, although its exact target in the FA pathway has not been identified [128]. However, curcumin sensitizes an ovarian cancer cell line to cisplatin in an FA pathway-dependent manner, suggesting that curcumin sensitization of cisplatin mostly occurs through FA pathway inhibition [128]. In order to establish curcumin as a useful cisplatin chemosensitizer, a detailed isobologram analysis of combinations of curcumin with cisplatin, in vivo studies using mouse models, and identification of the target of curcumin in the FA pathway are required.
New frontiers in drug discovery
Elucidating the mechanism of action of the candidate inhibitors identified in the above-mentioned screen [128] will be crucial for development of specific and effective inhibitors of the FA pathway, and for further understanding the regulation of the FA pathway. Precise analyses of the effects of each drug on individual steps in the FA pathway will be required to identify their specific targets. These analyses will include in vitro ATR kinase assay, in vitro UBE2T and FANCL autoubiquitylation assays, assessment of FA core complex formation and evaluation of nuclear foci formation of DNA repair proteins including BRCA1. A better understanding of the regulatory mechanisms, as well as the identification of new partners of the FA pathway, is also crucial for the identification and development of FA pathway inhibitors. Although multiple groups have been working on structural analysis of FA proteins [130, 131], so far few FA protein crystal structures (BRCA2/FANCD1 [130] and FANCF [131]) have been reported, but such studies could provide useful information for drug design as well as for elucidation of the targets of the candidate inhibitors.
Abbreviations
- FA:
-
Fanconi anemia
- MMC:
-
mitomycin C
- TLS:
-
translesion synthesis
- HR:
-
homologous recombination
- PCNA:
-
proliferating cell nuclear antigen
- PHD:
-
plant homeodomain.
References
Joenje H, Patel KJ: The emerging genetic and molecular basis of Fanconi anaemia. Nat Rev Genet. 2001, 2 (6): 446-457. 10.1038/35076590.
Meetei AR, Levitus M, Xue Y, Medhurst AL, Zwaan M, Ling C, Rooimans MA, Bier P, Hoatlin M, Pals G, et al.: X-linked inheritance of Fanconi anemia complementation group B. Nat Genet. 2004, 36 (11): 1219-1224. 10.1038/ng1458.
Alter BP: Inherited bone marrow failure syndromes. Nathan and Oski's Hematology of Infancy and Childhood. Edited by: Nathan DG, Orkin SH, Ginsburg D, Look AT, Oski FA. 2003, Philadelphia: PA Saunders, 1: 280-365. 6
Auerbach AD, Buchwald M, Joenje H: Fanconi Anemia. The Metabolic and Molecular Bases of Inherited Disease. Edited by: Scriver CR, Sly WS, Childs B, Beaudet AL, Valle D, Kinzler KW, Vogelstein B. 2001, New York: McGraw-Hill, 1: 753-768. 8th edn
D'Andrea AD, Grompe M: The Fanconi anaemia/BRCA pathway. Nat Rev Cancer. 2003, 3 (1): 23-34. 10.1038/nrc970.
Taniguchi T, D'Andrea AD: Molecular pathogenesis of Fanconi anemia: recent progress. Blood. 2006, 107 (11): 4223-4233. 10.1182/blood-2005-10-4240.
Kennedy RD, D'Andrea AD: The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005, 19 (24): 2925-2940. 10.1101/gad.1370505.
Mirchandani KD, D'Andrea AD: The Fanconi anemia/BRCA pathway: a coordinator of cross-link repair. Exp Cell Res. 2006, 312 (14): 2647-2653. 10.1016/j.yexcr.2006.06.014.
Gurtan AM, D'Andrea AD: Dedicated to the core: understanding the Fanconi anemia complex. DNA Repair (Amst). 2006, 5 (9-10): 1119-1125. 10.1016/j.dnarep.2006.05.009.
Mathew CG: Fanconi anaemia genes and susceptibility to cancer. Oncogene. 2006, 25 (43): 5875-5884. 10.1038/sj.onc.1209878.
Venkitaraman AR: Tracing the network connecting BRCA and Fanconi anaemia proteins. Nat Rev Cancer. 2004, 4 (4): 266-276. 10.1038/nrc1321.
Bagby GC, Alter BP: Fanconi anemia. Semin Hematol. 2006, 43 (3): 147-156. 10.1053/j.seminhematol.2006.04.005.
Niedernhofer LJ, Lalai AS, Hoeijmakers JH: Fanconi Anemia (Cross)linked to DNA Repair. Cell. 2005, 123 (7): 1191-1198. 10.1016/j.cell.2005.12.009.
Lyakhovich A, Surralles J: Disruption of the Fanconi anemia/BRCA pathway in sporadic cancer. Cancer Lett. 2006, 232 (1): 99-106. 10.1016/j.canlet.2005.07.038.
Collins N, Kupfer GM: Molecular pathogenesis of Fanconi anemia. Int J Hematol. 2005, 82 (3): 176-183. 10.1532/IJH97.05108.
Tischkowitz M, Dokal I: Fanconi anaemia and leukaemia - clinical and molecular aspects. Br J Haematol. 2004, 126 (2): 176-191. 10.1111/j.1365-2141.2004.05023.x.
Kutler DI, Singh B, Satagopan J, Batish SD, Berwick M, Giampietro PF, Hanenberg H, Auerbach AD: A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood. 2003, 101 (4): 1249-1256. 10.1182/blood-2002-07-2170.
Rosenberg PS, Greene MH, Alter BP: Cancer incidence in persons with Fanconi anemia. Blood. 2003, 101 (3): 822-826. 10.1182/blood-2002-05-1498.
Alter BP: Cancer in Fanconi anemia, 1927-2001. Cancer. 2003, 97 (2): 425-440. 10.1002/cncr.11046.
Wagner JE, Tolar J, Levran O, Scholl T, Deffenbaugh A, Satagopan J, Ben-Porat L, Mah K, Batish SD, Kutler DI, et al.: Germline mutations in BRCA2: shared genetic susceptibility to breast cancer, early onset leukemia, and Fanconi anemia. Blood. 2004, 103 (8): 3226-3229. 10.1182/blood-2003-09-3138.
Offit K, Levran O, Mullaney B, Mah K, Nafa K, Batish SD, Diotti R, Schneider H, Deffenbaugh A, Scholl T, et al.: Shared genetic susceptibility to breast cancer, brain tumors, and Fanconi anemia. J Natl Cancer Inst. 2003, 95 (20): 1548-1551.
Hirsch B, Shimamura A, Moreau L, Baldinger S, Hag-Alshiekh M, Bostrom B, Sencer S, D'Andrea AD: Association of biallelic BRCA2/FANCD1 mutations with spontaneous chromosomal instability and solid tumors of childhood. Blood. 2004, 103 (7): 2554-2559. 10.1182/blood-2003-06-1970.
Xia B, Dorsman JC, Ameziane N, de Vries Y, Rooimans MA, Sheng Q, Pals G, Errami A, Gluckman E, Llera J, et al.: Fanconi anemia is associated with a defect in the BRCA2 partner PALB2. Nat Genet. 2007, 39 (2): 159-161. 10.1038/ng1942.
Reid S, Schindler D, Hanenberg H, Barker K, Hanks S, Kalb R, Neveling K, Kelly P, Seal S, Freund M, et al.: Biallelic mutations in PALB2, which encodes a BRCA2 interacting protein, cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet. 2007, 39 (2): 162-164. 10.1038/ng1947.
Sasaki MS, Tonomura A: A high susceptibility of Fanconi's anemia to chromosome breakage by DNA cross-linking agents. Cancer Research. 1973, 33 (8): 1829-1836.
Auerbach AD: Fanconi anemia diagnosis and the diepoxybutane (DEB) test. Experimental Hematology. 1993, 21 (6): 731-733.
Meetei AR, Medhurst AL, Ling C, Xue Y, Singh TR, Bier P, Steltenpool J, Stone S, Dokal I, Mathew CG, et al.: A human ortholog of archaeal DNA repair protein Hef is defective in Fanconi anemia complementation group M. Nat Genet. 2005, 37 (9): 958-963. 10.1038/ng1626.
Levitus M, Rooimans MA, Steltenpool J, Cool NF, Oostra AB, Mathew CG, Hoatlin ME, Waisfisz Q, Arwert F, De Winter JP, et al.: Heterogeneity in Fanconi anemia: evidence for 2 new genetic subtypes. Blood. 2004, 103 (7): 2498-2503. 10.1182/blood-2003-08-2915.
Levitus M, Waisfisz Q, Godthelp BC, Vries Y, Hussain S, Wiegant WW, Elghalbzouri-Maghrani E, Steltenpool J, Rooimans MA, Pals G, et al.: The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat Genet. 2005, 37 (9): 934-935. 10.1038/ng1625.
Levran O, Attwooll C, Henry RT, Milton KL, Neveling K, Rio P, Batish SD, Kalb R, Velleuer E, Barral S, et al.: The BRCA1-interacting helicase BRIP1 is deficient in Fanconi anemia. Nat Genet. 2005, 37 (9): 931-933. 10.1038/ng1624.
Howlett NG, Taniguchi T, Olson S, Cox B, Waisfisz Q, De Die-Smulders C, Persky N, Grompe M, Joenje H, Pals G, et al.: Biallelic inactivation of BRCA2 in Fanconi anemia. Science. 2002, 297 (5581): 606-609. 10.1126/science.1073834.
Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, Liu X, Jasin M, Couch FJ, Livingston DM: Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006, 22 (6): 719-729. 10.1016/j.molcel.2006.05.022.
Litman R, Peng M, Jin Z, Zhang F, Zhang J, Powell S, Andreassen PR, Cantor SB: BACH1 is critical for homologous recombination and appears to be the Fanconi anemia gene product FANCJ. Cancer Cell. 2005, 8 (3): 255-265. 10.1016/j.ccr.2005.08.004.
Garcia-Higuera I, Taniguchi T, Ganesan S, Meyn MS, Timmers C, Hejna J, Grompe M, D'Andrea AD: Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell. 2001, 7 (2): 249-262. 10.1016/S1097-2765(01)00173-3.
Wang X, Andreassen PR, D'Andrea AD: Functional interaction of monoubiquitinated FANCD2 and BRCA2/FANCD1 in chromatin. Mol Cell Biol. 2004, 24 (13): 5850-5862. 10.1128/MCB.24.13.5850-5862.2004.
Vandenberg CJ, Gergely F, Ong CY, Pace P, Mallery DL, Hiom K, Patel KJ: BRCA1-independent ubiquitination of FANCD2. Mol Cell. 2003, 12 (1): 247-254. 10.1016/S1097-2765(03)00281-8.
Meetei AR, Sechi S, Wallisch M, Yang D, Young MK, Joenje H, Hoatlin ME, Wang W: A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome. Mol Cell Biol. 2003, 23 (10): 3417-3426. 10.1128/MCB.23.10.3417-3426.2003.
Meetei AR, de Winter JP, Medhurst AL, Wallisch M, Waisfisz Q, van de Vrugt HJ, Oostra AB, Yan Z, Ling C, Bishop CE, et al.: A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet. 2003, 35 (2): 165-170. 10.1038/ng1241.
Aravind L, Iyer LM, Koonin EV: Scores of RINGS but no PHDs in ubiquitin signaling. Cell Cycle. 2003, 2 (2): 123-126.
Scheel H, Hofmann K: No evidence for PHD fingers as ubiquitin ligases. Trends Cell Biol. 2003, 13 (6): 285-287. 10.1016/S0962-8924(03)00102-8. author reply 287-288
Bienz M: The PHD finger, a nuclear protein-interaction domain. Trends Biochem Sci. 2006, 31 (1): 35-40. 10.1016/j.tibs.2005.11.001.
Machida YJ, Machida Y, Chen Y, Gurtan AM, Kupfer GM, D'Andrea AD, Dutta A: UBE2T is the E2 in the Fanconi anemia pathway and undergoes negative autoregulation. Mol Cell. 2006, 23 (4): 589-596. 10.1016/j.molcel.2006.06.024.
Park WH, Margossian S, Horwitz AA, Simons AM, D'Andrea AD, Parvin JD: Direct DNA binding activity of the Fanconi anemia D2 protein. J Biol Chem. 2005, 280 (25): 23593-23598. 10.1074/jbc.M503730200.
Montes de Oca R, Andreassen PR, Margossian SP, Gregory RC, Taniguchi T, Wang X, Houghtaling S, Grompe M, D'Andrea AD: Regulated interaction of the Fanconi anemia protein, FANCD2, with chromatin. Blood. 2005, 105 (3): 1003-1009. 10.1182/blood-2003-11-3997.
Taniguchi T, Garcia-Higuera I, Andreassen PR, Gregory RC, Grompe M, D'Andrea AD: S-phase-specific interaction of the Fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood. 2002, 100 (7): 2414-2420. 10.1182/blood-2002-01-0278.
Andreassen PR, D'Andrea AD, Taniguchi T: ATR couples FANCD2 monoubiquitination to the DNA-damage response. Genes Dev. 2004, 18 (16): 1958-1963. 10.1101/gad.1196104.
Matsushita N, Kitao H, Ishiai M, Nagashima N, Hirano S, Okawa K, Ohta T, Yu DS, McHugh PJ, Hickson ID, et al.: A FancD2-monoubiquitin fusion reveals hidden functions of Fanconi anemia core complex in DNA repair. Mol Cell. 2005, 19 (6): 841-847. 10.1016/j.molcel.2005.08.018.
Nijman SM, Huang TT, Dirac AM, Brummelkamp TR, Kerkhoven RM, D'Andrea AD, Bernards R: The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol Cell. 2005, 17 (3): 331-339. 10.1016/j.molcel.2005.01.008.
Huang TT, Nijman SM, Mirchandani KD, Galardy PJ, Cohn MA, Haas W, Gygi SP, Ploegh HL, Bernards R, D'Andrea AD: Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol. 2006, 8 (4): 339-347. 10.1038/ncb1378.
Kannouche PL, Wing J, Lehmann AR: Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol Cell. 2004, 14 (4): 491-500. 10.1016/S1097-2765(04)00259-X.
Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S: RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002, 419 (6903): 135-141. 10.1038/nature00991.
Dronkert ML, Kanaar R: Repair of DNA interstrand cross-links. Mutat Res. 2001, 486 (4): 217-247.
Niedzwiedz W, Mosedale G, Johnson M, Ong CY, Pace P, Patel KJ: The Fanconi anaemia gene FANCC promotes homologous recombination and error-prone DNA repair. Mol Cell. 2004, 15 (4): 607-620. 10.1016/j.molcel.2004.08.009.
Nakanishi K, Taniguchi T, Ranganathan V, New HV, Moreau LA, Stotsky M, Mathew CG, Kastan MB, Weaver DT, D'Andrea AD: Interaction of FANCD2 and NBS1 in the DNA damage response. Nat Cell Biol. 2002, 4 (12): 913-920. 10.1038/ncb879.
Pace P, Johnson M, Tan WM, Mosedale G, Sng C, Hoatlin M, de Winter J, Joenje H, Gergely F, Patel KJ: FANCE: the link between Fanconi anaemia complex assembly and activity. EMBO J. 2002, 21 (13): 3414-3423. 10.1093/emboj/cdf355.
Hussain S, Witt E, Huber PA, Medhurst AL, Ashworth A, Mathew CG: Direct interaction of the Fanconi anaemia protein FANCG with BRCA2/FANCD1. Hum Mol Genet. 2003, 12 (19): 2503-2510. 10.1093/hmg/ddg266.
Moynahan ME, Pierce AJ, Jasin M: BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001, 7 (2): 263-272. 10.1016/S1097-2765(01)00174-5.
Davies AA, Masson JY, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR, West SC: Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell. 2001, 7 (2): 273-282. 10.1016/S1097-2765(01)00175-7.
Digweed M, Rothe S, Demuth I, Scholz R, Schindler D, Stumm M, Grompe M, Jordan A, Sperling K: Attenuation of the formation of DNA-repair foci containing RAD51 in Fanconi anaemia. Carcinogenesis. 2002, 23 (7): 1121-1126. 10.1093/carcin/23.7.1121.
Pichierri P, Averbeck D, Rosselli F: DNA cross-link-dependent RAD50/MRE11/NBS1 subnuclear assembly requires the Fanconi anemia C protein. Hum Mol Genet. 2002, 11 (21): 2531-2546. 10.1093/hmg/11.21.2531.
Stark JM, Pierce AJ, Oh J, Pastink A, Jasin M: Genetic steps of mammalian homologous repair with distinct mutagenic consequences. Mol Cell Biol. 2004, 24 (21): 9305-9316. 10.1128/MCB.24.21.9305-9316.2004.
Nakanishi K, Yang YG, Pierce AJ, Taniguchi T, Digweed M, D'Andrea AD, Wang ZQ, Jasin M: Human Fanconi anemia monoubiquitination pathway promotes homologous DNA repair. Proc Natl Acad Sci U S A. 2005, 102 (4): 1110-1115. 10.1073/pnas.0407796102.
Godthelp BC, Wiegant WW, Waisfisz Q, Medhurst AL, Arwert F, Joenje H, Zdzienicka MZ: Inducibility of nuclear Rad51 foci after DNA damage distinguishes all Fanconi anemia complementation groups from D1/BRCA2. Mutat Res. 2006, 594 (1-2): 39-48.
Yang YG, Herceg Z, Nakanishi K, Demuth I, Piccoli C, Michelon J, Hildebrand G, Jasin M, Digweed M, Wang ZQ: The Fanconi anemia group A protein modulates homologous repair of DNA double-strand breaks in mammalian cells. Carcinogenesis. 2005, 26 (10): 1731-1740. 10.1093/carcin/bgi134.
Hirano S, Yamamoto K, Ishiai M, Yamazoe M, Seki M, Matsushita N, Ohzeki M, Yamashita YM, Arakawa H, Buerstedde JM, et al.: Functional relationships of FANCC to homologous recombination, translesion synthesis, and BLM. Embo J. 2005, 24 (2): 418-427. 10.1038/sj.emboj.7600534.
Yamamoto K, Hirano S, Ishiai M, Morishima K, Kitao H, Namikoshi K, Kimura M, Matsushita N, Arakawa H, Buerstedde JM, et al.: Fanconi anemia protein FANCD2 promotes immunoglobulin gene conversion and DNA repair through a mechanism related to homologous recombination. Mol Cell Biol. 2005, 25 (1): 34-43. 10.1128/MCB.25.1.34-43.2005.
Yamamoto K, Ishiai M, Matsushita N, Arakawa H, Lamerdin JE, Buerstedde JM, Tanimoto M, Harada M, Thompson LH, Takata M: Fanconi anemia FANCG protein in mitigating radiation- and enzyme-induced DNA double-strand breaks by homologous recombination in vertebrate cells. Mol Cell Biol. 2003, 23 (15): 5421-5430. 10.1128/MCB.23.15.5421-5430.2003.
Bridge WL, Vandenberg CJ, Franklin RJ, Hiom K: The BRIP1 helicase functions independently of BRCA1 in the Fanconi anemia pathway for DNA crosslink repair. Nat Genet. 2005, 37 (9): 953-957. 10.1038/ng1627.
Ohashi A, Zdzienicka MZ, Chen J, Couch FJ: Fanconi anemia complementation group D2 (FANCD2) functions independently of BRCA2- and RAD51-associated homologous recombination in response to DNA damage. J Biol Chem. 2005, 280 (15): 14877-14883. 10.1074/jbc.M414669200.
Mosedale G, Niedzwiedz W, Alpi A, Perrina F, Pereira-Leal JB, Johnson M, Langevin F, Pace P, Patel KJ: The vertebrate Hef ortholog is a component of the Fanconi anemia tumor-suppressor pathway. Nat Struct Mol Biol. 2005, 12 (9): 763-771. 10.1038/nsmb981.
Cantor SB, Bell DW, Ganesan S, Kass EM, Drapkin R, Grossman S, Wahrer DC, Sgroi DC, Lane WS, Haber DA, et al.: BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell. 2001, 105 (1): 149-160. 10.1016/S0092-8674(01)00304-X.
Manke IA, Lowery DM, Nguyen A, Yaffe MB: BRCT Repeats As Phosphopeptide-Binding Modules Involved in Protein Targeting. Science. 2003, 302 (5645): 636-639. 10.1126/science.1088877.
Yu X, Chini CC, He M, Mer G, Chen J: The BRCT domain is a phospho-protein binding domain. Science. 2003, 302 (5645): 639-642. 10.1126/science.1088753.
Taniguchi T, Garcia-Higuera I, Xu B, Andreassen PR, Gregory RC, Kim ST, Lane WS, Kastan MB, D'Andrea AD: Convergence of the Fanconi anemia and ataxia telangiectasia signaling pathways. Cell. 2002, 109 (4): 459-472. 10.1016/S0092-8674(02)00747-X.
Fagerlie SR, Koretsky T, Torok-Storb B, Bagby GC: Impaired type I IFN-induced Jak/STAT signaling in FA-C cells and abnormal CD4+ Th cell subsets in Fancc-/- mice. J Immunol. 2004, 173 (6): 3863-3870.
Zhang X, Li J, Sejas DP, Rathbun KR, Bagby GC, Pang Q: The Fanconi anemia proteins functionally interact with the protein kinase regulated by RNA (PKR). J Biol Chem. 2004, 279 (42): 43910-43919. 10.1074/jbc.M403884200.
Saadatzadeh MR, Bijangi-Vishehsaraei K, Hong P, Bergmann H, Haneline LS: Oxidant hypersensitivity of Fanconi anemia type C-deficient cells is dependent on a redox-regulated apoptotic pathway. J Biol Chem. 2004, 279 (16): 16805-16812. 10.1074/jbc.M313721200.
Pang Q, Christianson TA, Keeble W, Koretsky T, Bagby GC: The anti-apoptotic function of Hsp70 in the PKR-mediated death signaling pathway requires the Fanconi anemia protein, FANCC. J Biol Chem. 2002, 277 (51): 49638-49643. 10.1074/jbc.M209386200.
Pang Q, Fagerlie S, Christianson TA, Keeble W, Faulkner G, Diaz J, Rathbun RK, Bagby GC: The Fanconi anemia protein FANCC binds to and facilitates the activation of STAT1 by gamma interferon and hematopoietic growth factors. Mol Cell Biol. 2000, 20 (13): 4724-4735. 10.1128/MCB.20.13.4724-4735.2000.
Brodeur I, Goulet I, Tremblay CS, Charbonneau C, Delisle MC, Godin C, Huard C, Khandjian EW, Buchwald M, Levesque G, et al.: Regulation of the Fanconi anemia group C protein through proteolytic modification. J Biol Chem. 2004, 279 (6): 4713-4720. 10.1074/jbc.M301291200.
Taniguchi T, Tischkowitz M, Ameziane N, Hodgson SV, Mathew CG, Joenje H, Mok SC, D'Andrea AD: Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med. 2003, 9 (5): 568-574. 10.1038/nm852.
Wang Z, Li M, Lu S, Zhang Y, Wang H: Promoter hypermethylation of FANCF plays an important role in the occurrence of ovarian cancer through disrupting Fanconi anemia-BRCA pathway. Cancer Biol Ther. 2006, 5 (3): 256-260. 10.1158/1535-7163.MCT-05-0299.
Dhillon VS, Shahid M, Husain SA: CpG methylation of the FHIT, FANCF, cyclin-D2, BRCA2 and RUNX3 genes in Granulosa cell tumors (GCTs) of ovarian origin. Mol Cancer. 2004, 3: 33-10.1186/1476-4598-3-33.
Olopade OI, Wei M: FANCF methylation contributes to chemoselectivity in ovarian cancer. Cancer Cell. 2003, 3 (5): 417-420. 10.1016/S1535-6108(03)00111-9.
Marsit CJ, Liu M, Nelson HH, Posner M, Suzuki M, Kelsey KT: Inactivation of the Fanconi anemia/BRCA pathway in lung and oral cancers: implications for treatment and survival. Oncogene. 2004, 23 (4): 1000-1004. 10.1038/sj.onc.1207256.
Narayan G, Arias-Pulido H, Nandula SV, Basso K, Sugirtharaj DD, Vargas H, Mansukhani M, Villella J, Meyer L, Schneider A, et al.: Promoter hypermethylation of FANCF: disruption of Fanconi Anemia-BRCA pathway in cervical cancer. Cancer Res. 2004, 64 (9): 2994-2997. 10.1158/0008-5472.CAN-04-0245.
Koul S, McKiernan JM, Narayan G, Houldsworth J, Bacik J, Dobrzynski DL, Assaad AM, Mansukhani M, Reuter VE, Bosl GJ, et al.: Role of promoter hypermethylation in cisplatin treatment response of male germ cell tumors. Mol Cancer. 2004, 3: 16-10.1186/1476-4598-3-16.
Tischkowitz M, Ameziane N, Waisfisz Q, De Winter JP, Harris R, Taniguchi T, D'Andrea A, Hodgson SV, Mathew CG, Joenje H: Bi-allelic silencing of the Fanconi anaemia gene FANCF in acute myeloid leukaemia. Br J Haematol. 2003, 123 (3): 469-471. 10.1046/j.1365-2141.2003.04640.x.
van Der Heijden MS, Yeo CJ, Hruban RH, Kern SE: Fanconi anemia gene mutations in young-onset pancreatic cancer. Cancer Res. 2003, 63 (10): 2585-2588.
van Der Heijden MS, Brody JR, Kern SE: Functional screen of the Fanconi anemia pathway in cancer cells by fancd2 immunoblot. Cancer Biol Ther. 2004, 3 (6): 534-537.
van der Heijden MS, Brody JR, Gallmeier E, Cunningham SC, Dezentje DA, Shen D, Hruban RH, Kern SE: Functional defects in the Fanconi anemia pathway in pancreatic cancer cells. Am J Pathol. 2004, 165 (2): 651-657.
Condie A, Powles RL, Hudson CD, Shepherd V, Bevan S, Yuille MR, Houlston RS: Analysis of the Fanconi anaemia complementation group A gene in acute myeloid leukaemia. Leuk Lymphoma. 2002, 43 (9): 1849-1853. 10.1080/1042819021000009274.
Tischkowitz MD, Morgan NV, Grimwade D, Eddy C, Ball S, Vorechovsky I, Langabeer S, Stoger R, Hodgson SV, Mathew CG: Deletion and reduced expression of the Fanconi anemia FANCA gene in sporadic acute myeloid leukemia. Leukemia. 2004, 18 (3): 420-425. 10.1038/sj.leu.2403280.
Lensch MW, Tischkowitz M, Christianson TA, Reifsteck CA, Speckhart SA, Jakobs PM, O'Dwyer ME, Olson SB, Le Beau MM, Hodgson SV, et al.: Acquired FANCA dysfunction and cytogenetic instability in adult acute myelogenous leukemia. Blood. 2003, 102 (1): 7-16. 10.1182/blood-2002-09-2781.
Turner N, Tutt A, Ashworth A: Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004, 4 (10): 814-819. 10.1038/nrc1457.
Couch FJ, Johnson MR, Rabe K, Boardman L, McWilliams R, de Andrade M, Petersen G: Germ line Fanconi anemia complementation group C mutations and pancreatic cancer. Cancer Res. 2005, 65 (2): 383-386.
Seal S, Thompson D, Renwick A, Elliott A, Kelly P, Barfoot R, Chagtai T, Jayatilake H, Ahmed M, Spanova K, et al.: Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet. 2006, 38 (11): 1239-1241. 10.1038/ng1902.
Chen Q, Van der Sluis PC, Boulware D, Hazlehurst LA, Dalton WS: The FA/BRCA pathway is involved in melphalan-induced DNA interstrand cross-link repair and accounts for melphalan resistance in multiple myeloma cells. Blood. 2005, 106 (2): 698-705. 10.1182/blood-2004-11-4286.
van der Heijden MS, Brody JR, Dezentje DA, Gallmeier E, Cunningham SC, Swartz MJ, DeMarzo AM, Offerhaus GJ, Isacoff WH, Hruban RH, et al.: In vivo therapeutic responses contingent on Fanconi anemia/BRCA2 status of the tumor. Clin Cancer Res. 2005, 11 (20): 7508-7515. 10.1158/1078-0432.CCR-05-1048.
Ferrer M, de Winter JP, Mastenbroek DC, Curiel DT, Gerritsen WR, Giaccone G, Kruyt FA: Chemosensitizing tumor cells by targeting the Fanconi anemia pathway with an adenovirus overexpressing dominant-negative FANCA. Cancer Gene Ther. 2004, 11 (8): 539-546. 10.1038/sj.cgt.7700734.
Cheng NC, van De Vrugt HJ, van Der Valk MA, Oostra AB, Krimpenfort P, de Vries Y, Joenje H, Berns A, Arwert F: Mice with a targeted disruption of the Fanconi anemia homolog fanca. Hum Mol Genet. 2000, 9 (12): 1805-1811. 10.1093/hmg/9.12.1805.
Wong JC, Alon N, McKerlie C, Huang JR, Meyn MS, Buchwald M: Targeted disruption of exons 1 to 6 of the Fanconi Anemia group A gene leads to growth retardation, strain-specific microphthalmia, meiotic defects and primordial germ cell hypoplasia. Hum Mol Genet. 2003, 12 (16): 2063-2076. 10.1093/hmg/ddg219.
Chen M, Tomkins DJ, Auerbach W, McKerlie C, Youssoufian H, Liu L, Gan O, Carreau M, Auerbach A, Groves T, et al.: Inactivation of Fac in mice produces inducible chromosomal instability and reduced fertility reminiscent of Fanconi anaemia. Nature Genetics. 1996, 12 (4): 448-451. 10.1038/ng0496-448.
Whitney MA, Royle G, Low MJ, Kelly MA, Axthelm MK, Reifsteck C, Olson S, Braun RE, Heinrich MC, Rathbun RK, et al.: Germ cell defects and hematopoietic hypersensitivity to gamma-interferon in mice with a targeted disruption of the Fanconi anemia C gene. Blood. 1996, 88 (1): 49-58.
Koomen M, Cheng NC, van de Vrugt HJ, Godthelp BC, van der Valk MA, Oostra AB, Zdzienicka MZ, Joenje H, Arwert F: Reduced fertility and hypersensitivity to mitomycin C characterize Fancg/Xrcc9 null mice. Hum Mol Genet. 2002, 11 (3): 273-281. 10.1093/hmg/11.3.273.
Yang Y, Kuang Y, De Oca RM, Hays T, Moreau L, Lu N, Seed B, D'Andrea AD: Targeted disruption of the murine Fanconi anemia gene, Fancg/Xrcc9. Blood. 2001, 98 (12): 3435-3440. 10.1182/blood.V98.12.3435.
Houghtaling S, Timmers C, Noll M, Finegold MJ, Jones SN, Meyn MS, Grompe M: Epithelial cancer in Fanconi anemia complementation group D2 (Fancd2) knockout mice. Genes Dev. 2003, 17 (16): 2021-2035. 10.1101/gad.1103403.
Noll M, Battaile KP, Bateman R, Lax TP, Rathbun K, Reifsteck C, Bagby G, Finegold M, Olson S, Grompe M: Fanconi anemia group A and C double-mutant mice. Functional evidence for a multi-protein Fanconi anemia complex. Exp Hematol. 2002, 30 (7): 679-688. 10.1016/S0301-472X(02)00838-X.
Agoulnik AI, Lu B, Zhu Q, Truong C, Ty MT, Arango N, Chada KK, Bishop CE: A novel gene, Pog, is necessary for primordial germ cell proliferation in the mouse and underlies the germ cell deficient mutation, gcd. Hum Mol Genet. 2002, 11 (24): 3047-3053. 10.1093/hmg/11.24.3047.
Navarro S, Meza NW, Quintana-Bustamante O, Casado JA, Jacome A, McAllister K, Puerto S, Surralles J, Segovia JC, Bueren JA: Hematopoietic dysfunction in a mouse model for Fanconi anemia group D1. Mol Ther. 2006, 14 (4): 525-535. 10.1016/j.ymthe.2006.05.018.
Carreau M, Gan OI, Liu L, Doedens M, McKerlie C, Dick JE, Buchwald M: Bone marrow failure in the Fanconi anemia group C mouse model after DNA damage. Blood. 1998, 91 (8): 2737-2744.
Carreau M: Not-so-novel phenotypes in the Fanconi anemia group D2 mouse model. Blood. 2004, 103 (6): 2430-10.1182/blood-2003-11-3946.
Moynahan ME: The cancer connection: BRCA1 and BRCA2 tumor suppression in mice and humans. Oncogene. 2002, 21 (58): 8994-9007. 10.1038/sj.onc.1206177.
Rio P, Segovia JC, Hanenberg H, Casado JA, Martinez J, Gottsche K, Cheng NC, Van De Vrugt HJ, Arwert F, Joenje H, et al.: In vitro phenotypic correction of hematopoietic progenitors from Fanconi anemia group A knockout mice. Blood. 2002, 100 (6): 2032-2039.
Gush KA, Fu KL, Grompe M, Walsh CE: Phenotypic correction of Fanconi anemia group C knockout mice. Blood. 2000, 95 (2): 700-704.
Noll M, Bateman RL, D'Andrea AD, Grompe M: Preclinical protocol for in vivo selection of hematopoietic stem cells corrected by gene therapy in Fanconi anemia group C. Mol Ther. 2001, 3 (1): 14-23. 10.1006/mthe.2000.0226.
Galimi F, Noll M, Kanazawa Y, Lax T, Chen C, Grompe M, Verma IM: Gene therapy of Fanconi anemia: preclinical efficacy using lentiviral vectors. Blood. 2002, 100 (8): 2732-2736. 10.1182/blood-2002-04-1245.
Haneline LS, Li X, Ciccone SL, Hong P, Yang Y, Broxmeyer HE, Lee SH, Orazi A, Srour EF, Clapp DW: Retroviral-mediated expression of recombinant Fancc enhances the repopulating ability of Fancc-/- hematopoietic stem cells and decreases the risk of clonal evolution. Blood. 2003, 101 (4): 1299-1307. 10.1182/blood-2002-08-2404.
Yamada K, Olsen JC, Patel M, Rao KW, Walsh CE: Functional correction of Fanconi anemia group C hematopoietic cells by the use of a novel lentiviral vector. Mol Ther. 2001, 3 (4): 485-490. 10.1006/mthe.2001.0287.
Yamada K, Ramezani A, Hawley RG, Ebell W, Arwert F, Arnold LW, Walsh CE: Phenotype correction of Fanconi anemia group a hematopoietic stem cells using lentiviral vector. Mol Ther. 2003, 8 (4): 600-610. 10.1016/S1525-0016(03)00223-5.
Li X, Le Beau MM, Ciccone S, Yang FC, Freie B, Chen S, Yuan J, Hong P, Orazi A, Haneline LS, et al.: Ex vivo culture of Fancc-/- stem/progenitor cells predisposes cells to undergo apoptosis, and surviving stem/progenitor cells display cytogenetic abnormalities and an increased risk of malignancy. Blood. 2005, 105 (9): 3465-3471. 10.1182/blood-2004-06-2483.
Carreau M, Liu L, Gan OI, Hitzler JK, Dick JE, Buchwald M: Short-term granulocyte colony-stimulating factor and erythropoietin treatment enhances hematopoiesis and survival in the mitomycin C-conditioned Fancc(-/-) mouse model, while long-term treatment is ineffective. Blood. 2002, 100 (4): 1499-1501. 10.1182/blood-2001-11-0007.
Titus TA, Selvig DR, Qin B, Wilson C, Starks AM, Roe BA, Postlethwait JH: The Fanconi anemia gene network is conserved from zebrafish to human. Gene. 2006, 371 (2): 211-223. 10.1016/j.gene.2005.11.038.
Sobeck A, Stone S, Costanzo V, de Graaf B, Reuter T, de Winter J, Wallisch M, Akkari Y, Olson S, Wang W, et al.: Fanconi anemia proteins are required to prevent accumulation of replication-associated DNA double-strand breaks. Mol Cell Biol. 2006, 26 (2): 425-437. 10.1128/MCB.26.2.425-437.2006.
Marek LR, Bale AE: Drosophila homologs of FANCD2 and FANCL function in DNA repair. DNA Repair (Amst). 2006, 5 (11): 1317-1326. 10.1016/j.dnarep.2006.05.044.
Collis SJ, Barber LJ, Ward JD, Martin JS, Boulton SJ: C. elegans FANCD2 responds to replication stress and functions in interstrand cross-link repair. DNA Repair (Amst). 2006, 5 (11): 1398-1406. 10.1016/j.dnarep.2006.06.010.
Liu TX, Howlett NG, Deng M, Langenau DM, Hsu K, Rhodes J, Kanki JP, D'Andrea AD, Look AT: Knockdown of zebrafish Fancd2 causes developmental abnormalities via p53-dependent apoptosis. Dev Cell. 2003, 5 (6): 903-914. 10.1016/S1534-5807(03)00339-3.
Chirnomas D, Taniguchi T, de la Vega M, Vaidya AP, Vasserman M, Hartman AR, Kennedy R, Foster R, Mahoney J, Seiden MV, et al.: Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther. 2006, 5 (4): 952-961. 10.1158/1535-7163.MCT-05-0493.
Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al.: Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21 (4B): 2895-2900.
Yang H, Jeffrey PD, Miller J, Kinnucan E, Sun Y, Thoma NH, Zheng N, Chen PL, Lee WH, Pavletich NP: BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science. 2002, 297 (5588): 1837-1848. 10.1126/science.297.5588.1837.
Kowal P, Gurtan AM, Stuckert P, D'Andrea AD, Ellenberger T: Structural determinants of human fancf protein that function in the assembly of a DNA damage signaling complex. J Biol Chem. 2006, on line publication
Rudolf J, Makrantoni V, Ingledew WJ, Stark MJ, White MF: The DNA repair helicases XPD and FancJ have essential iron-sulfur domains. Mol Cell. 2006, 23 (6): 801-808. 10.1016/j.molcel.2006.07.019.
Timmers C, Taniguchi T, Hejna J, Reifsteck C, Lucas L, Bruun D, Thayer M, Cox B, Olson S, D'Andrea AD, et al.: Positional cloning of a novel Fanconi anemia gene, FANCD2. Mol Cell. 2001, 7 (2): 241-248. 10.1016/S1097-2765(01)00172-1.
Meetei AR, Yan Z, Wang W: FANCL replaces BRCA1 as the likely ubiquitin ligase responsible for FANCD2 monoubiquitination. Cell Cycle. 2004, 3 (2): 179-181.
Martin JS, Winkelmann N, Petalcorin MI, McIlwraith MJ, Boulton SJ: RAD-51-dependent and -independent roles of a Caenorhabditis elegans BRCA2-related protein during DNA double-strand break repair. Mol Cell Biol. 2005, 25 (8): 3127-3139. 10.1128/MCB.25.8.3127-3139.2005.
Leveille F, Blom E, Medhurst AL, Bier P, Laghmani el H, Johnson M, Rooimans MA, Sobeck A, Waisfisz Q, Arwert F, et al.: The Fanconi anemia gene product FANCF is a flexible adaptor protein. J Biol Chem. 2004, 279 (38): 39421-39430. 10.1074/jbc.M407034200.
Ciccia A, Ling C, Coulthard R, Yan Z, Xue Y, Meetei AR, Laghmani el H, Joenje H, McDonald N, de Winter JP, et al.: Identification of FAAP24, a Fanconi Anemia Core Complex Protein that Interacts with FANCM. Mol Cell. 2007, 25 (3): 331-343. 10.1016/j.molcel.2007.01.003.
Publication history
Republished from Current BioData's Targeted Proteins database (TPdb; http://www.targetedproteinsdb.com).
Acknowledgements
We thank Anindya Dutta, Alan D. D'Andrea, Nazneen Rahman, Johan de Winter, Hans Joenje and Bing Xia for sharing data prior to publication. We thank Wataru Sakai and Emily Villegas for critical reading of the manuscript. CJ is supported by Fondation Betancourt. TT is a Searle Scholar and a V Scholar. We apologize that we cannot cite some of the important literature on FA due to the limitation of space.
This article has been published as part of BMC Biochemistry Volume 8 Supplement 1, 2007: Ubiquitin-Proteasome System in Disease Part 1. The full contents of the supplement are available online at http://www.biomedcentral.com/1471-2091/8?issue=S1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Jacquemont, C., Taniguchi, T. The Fanconi anemia pathway and ubiquitin. BMC Biochem 8 (Suppl 1), S10 (2007). https://doi.org/10.1186/1471-2091-8-S1-S10
Published:
DOI: https://doi.org/10.1186/1471-2091-8-S1-S10