Abstract
Background
Houttuynia cordata Thunb (HCT) is commonly used in Taiwan and other Asian countries as an anti-inflammatory, antibacterial and antiviral herbal medicine. In this study, we investigated the anti-human lung cancer activity and growth inhibition mechanisms of HCT in human lung cancer A549 cells.
Results
In order to investigate effects of HCT on A549 cells, MTT assay was used to evaluate cell viability. Flow cytometry was employed for cell cycle analysis, DAPI staining, and the Comet assay was used for DNA fragmentation and DNA condensation. Western blot analysis was used to analyze cell cycle and apoptotic related protein levels. HCT induced morphological changes including cell shrinkage and rounding. HCT increased the G0/G1 and Sub-G1 cell (apoptosis) populations and HCT increased DNA fragmentation and DNA condensation as revealed by DAPI staining and the Comet assay. HCT induced activation of caspase-8 and caspase-3. Fas/CD95 protein levels were increased in HCT-treated A549 cells. The G0/G1 phase and apoptotic related protein levels of cyclin D1, cyclin A, CDK 4 and CDK 2 were decreased, and p27, caspase-8 and caspase-3 were increased in A549 cells after HCT treatment.
Conclusions
The results demonstrated that HCT-induced G0/G1 phase arrest and Fas/CD95-dependent apoptotic cell death in A549 cells
Similar content being viewed by others
Background
In Taiwan, 26 individuals per 100,000 died from lung cancer each year, based on reports from the “People Health Bureau of Taiwan”. Surgery, radiotherapy, and chemo-therapy are used for treating lung cancer patients [1–3]. However, those treatments are not satisfactory. Induction of cell cycle arrest and/or apoptosis in lung cancer cells has been considered an influential treatment strategy [4–6]. Many researchers have focused on selectively killing cancer cells or reducing cell number through the induction of cell cycle arrest and apoptosis [6, 7].
Morphological changes in apoptotic cells include cell membrane blebbing, DNA or chromatin condensation, and caspase activation [8–10]. Previous studies have demonstrated that the cell membrane death receptor played an important role in apoptosis [11, 12]. Death receptor signaling is mediated through FasL and Fas/CD95 receptor protein interaction followed by activation of caspase-8 [13–16]. The activation of caspase-3 by caspase-8 is responsible for the cleavage of cellular substrates [13–20]. Cleavage of cellular substrates degrades the chromosomes into fragments during apoptosis [13–16, 21].
Hottuynia cordata Thunb (HCT), also called E-Sung-Cho is a Chinese herb used to treat several different diseases (e.g., bovine mastitis, influenza etc.) [22–24]. In addition, HCT has value in treating allergic inflammation [25, 26], viral infections and anaphylaxis [27–29]. Many studies reported HCT extract has anti-leukemia [30, 31] and anti-colon cancer activity [32, 33]. HCT inhibits the growth of HER2/neu-overexpressing breast cancer cells [34]. In this study, we determined if HCT would have anti-human lung cancer activity and if such effects would be associated with inhibition of cell growth in the human lung cancer line A549.
Methods
Preparation of HCT
Ethanol extract of Houttuynia cordata Thunb (yield: 6.73% of dry wt.) was obtained by 48 h incubation at room temperature. The ethanol extract was filtered through a 0.45 μm filter (Osmonics, Minnetonka, MN, USA), lyophilized and kept at 4°C. The dried extract was re-solubilized in PBS before use as previously described [32, 33].
Chemicals and reagents
RPMI-1640 cell culture medium (Gibco BRL, Life Technologies, MD, USA), DAPI (4,6-diamidino-2-phenylindole dihydrochloride), low-melting agarose, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), and DMSO (dimethyl sulfoxide) were purchased from Sigma (St. Louis, MO, USA). FBS (Fetal bovine serum), penicillin/streptomycin, PI (propidium iodide) and trypsin-EDTA were obtained from Life Technologies (Carlsbad, CA, USA). Proteinase K was purchased from Roche Diagnostics Gmbh (Mannheim, Germany). Ac-DEVE-pNA and Ac-IETD-pNA were purchased from R&D Systems Inc., (MN, USA). All other chemicals used were of analytical grade.
Cell culture
Human lung cancer A549 cells were obtained from the Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan), originally from the American Type Culture Collection (ATCC, USA). Cells were maintained in RPMI-1640 containing 100 mL/L FBS with 100,000 U/L penicillin and 100 mg/L streptomycin.
Cell viability
A549 cells were plated onto 96-well plates and incubated with HCT (0, 125, 250 and 500 μg/ml) for 24 and 48 h. MTT was added to each well then incubated for an additional 4 h at 37°C. The blue formazan product was dissolved in 100 μL of DMSO. The plates were read at O.D.570 nm using a spectrophotometric plate reader (Bio-Rad, Tokyo, Japan). The experiments were performed in triplicate (n = 3). Cell viability was calculated as O.D. of drug-treated sample/O.D. of none treated sample × 100% as previously described [32, 35].
Cell cycle transition and apoptosis determination
For cell cycle and apoptosis determination, A549 cells were plated onto 24-well plates and incubated with HCT (0, 125, 250 and 500 μg/ml) for 24 h. Cells were fixed gently in 70% ethanol at 4°C and then re-suspended in phosphate-buffered saline (PBS) containing 40 μg/ml PI, 0.1 mg/ml RNase and 0.1% Triton X-100 for 30 min at 37°C. Cell cycle transition and apoptosis were then analyzed by flow cytometry (FACS Calibur™; Becton Dickinson, NJ, USA) as previously described [35].
DAPI staining
A549 cells were plated onto 24-well plates and treated with HCT (0 and 500 μg/ml) for 24 h. After HCT treatment, cells were fixed in 4% paraformaldehyde and then incubated with 1 μg/ml of DAPI staining solution in darkness. The apoptotic cells were observed by fluorescence microscopy (Zeiss, Oberköchen, Germany) as previously described [32, 35].
Comet assay
A549 cells were plated onto 24-well plates and incubated with HCT (0 and 500 μg/ml) for 24 h, 1 × 104 cells were mixed with 150 μL 0.75% low-melting agarose held at 37°C and layered onto a pre-treated slide with 1.5% regular agarose. After agarose were solidified on a chilled plate, the slides were transferred to the same lysis buffer, held at room temperature for 4 h and stained with propidium iodide as previously described [36].
Western blotting
A549 cells were plated onto T-75 flasks and treated with HCT (0, 125, 250 and 500 μg/ml) for 24 h. Total cell lysates were prepared as previously described. Thirty μg of total protein applied to SDS-PAGE and transferred onto a polyvinylidene fluoride membrane (PVDF; Millipore). After blocking, the blots were incubated with the appropriate dilution of specific monoclonal antibodies for cyclin D1, cyclin A, CDK 4, CDK 2, p27, caspase-8 and caspase-3 (Santa Cruz Biotechnology, USA). Blots were washed and then incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, USA). Protein expressions were detected using enhanced chemiluminescence kits (Amersham, ECL Kits) as previously described [37].
Caspase-8 and caspase-3 activities assays
A549 cells were plated onto T-75 flasks and incubated with HCT (0, 125, 250 and 500 μg/ml) for 24 h. A549 cells were collected in a protein lysis buffer (50 mM Tris–HCl, 1 mM EDTA, 10 mM EGTA, 10 mM digitonin and 2 mM DTT). Cell lysates were centrifuged at 15,000 × g at 4°C and then incubated with caspase-3 and caspase-8 specific substrates (Ac-DEVE-pNA and Ac-IETD-pNA) with reaction buffer in a 96-well plate at 37°C for 1 h. Caspase activity was determined by measuring O.D. 405 nm of the released pN using a spectrophotometric plate reader (Bio-Rad, Tokyo, Japan) as previously described. The experiments were performed in triplicate [37].
Immunostaining assay for Fas/CD95 protein levels
Fas/CD95 cell surface antigen expression was measured by flow cytometry. A549 cells were plated onto 24 well and treated with HCT (0, 125, 250 and 500 μg/ml) for 12 h. Cells were collected and rinsed in PBS. Fas/CD95 was analyzed by direct immune-fluorescence staining. FITC-conjugated anti-Fas/CD95 and its FITC- conjugated isotype mAb (BD Biosciences Pharmingen, San Diego, CA, USA) were analyzed using a flow cytometer as previously described [38].
Statistical analysis
The experiments were performed in triplicate (n = 3) and all data were expressed as the mean ± standard error. Student’s t-test was used for single variable comparison. For multiple variable comparisons, data were analyzed by one-way ANOVA followed by Dunnett’s test. P < 0.05 was considered significant.
Results
Effect of HCT on viability of A549 cells
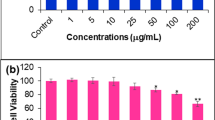
Cell viability of HCT (0, 125, 250 and 500 μg/ml) treated A549 cells was determined by MTT assay for 24 and 48 h. As shown in Figure 1A, HCT reduced cell viability in a concentration- and time-dependent manner (***p < 0.001, compared to HCT 0 μg/ml group). The inhibition of HCT in A549 viability was 15.73%, 27.13%, and 58.57% for 125, 250, and 500 μg/ml at 24 h; 29.32%, 50.81%, and 69.60% for 125, 250, and 500 μg/ml at 48 h, respectively. HCT also induced morphological changes seen as cell shrinkage and rounding (Figure 1B).
Effects of HCT on cell viability and morphological changes in human lung cancer A549 cells. (A) The A549 cells were treated with HCT (0, 125, 250 and 500 μg/ml) for 24 and 48 h, the viable cells were determined by MTT assay as described in Materials and Methods. The experiments were performed in triplicate (n = 3). ***p < 0.001 was considered significantly different in comparison with the control. (B) The cells’ morphological changes were examined and photographed by phase-contrast microscopy.
Effect of HCT on cell-cycle distribution and apoptosis in A549 cells
To evaluate the effects of HCT on cell-cycle distribution and apoptosis, A549 cells were treated with HCT (0, 125, 250 and 500 μg/ml). Figure 2A and B showed that HCT caused an arrest of cell-cycle transition in the G0/G1 phase, and the proportion of cells in the G0/G1 phase increased markedly in a concentration-dependent manner. As shown in Figure 2B, the cell cycle distribution of G0/G1 by HCT (0, 125, 250 and 500 μg/ml) treatment was 43.48%, 45.06%, 58.59%, and 63.25%, respectively. In addition, A549 cells treated with HCT (0, 125, 250 and 500 μg/ml) showed a significant increase in the sub-G1 apoptotic cell population, and the apoptotic cell population was 4.53%, 13.07%, 18.77% and 31.89%, respectively (Figure 3C). These results indicated HCT inhibited cell growth.
Effects of HCT on cell-cycle transition in human lung cancer A549 cells. (A) The A549 cells were treated with HCT (0, 125, 250 and 500 μg/ml) for 24 h, and then were harvested for determination the distribution of cell cycle by flow cytometry and (B) quantitative results were expressed as described in Materials and Methods. The experiments were performed in triplicate (n = 3). Data represents mean ± S.D. of three experiments. ***p < 0.001 was considered significantly different in comparison with the control.
Effects of HCT on DNA condensation, damage and apoptosis in human lung cancer A549 cells. (A) A549 cells stained with DAPI to observe DNA condensation after 24 h of treatment with 500 μg/ml of HCT. (B) A549 cells were examined DNA damage by Comet assay after 24 h of treatment with 500 μg/ml of HCT. Cells were photographed under fluoresce microscopy (x200) as described in Materials and Methods. (C) A549 cells were treated with HCT (0, 125, 250 and 500 μg/ml) for 24 h, and then were harvested for determination the sub-G1 (apoptosis) cell population by flow cytometry as described in Materials and Methods. (D) The bar diagram of the length of Comet tail. The experiments were performed in triplicate (n = 3). *p < 0.05, ***p < 0.001 was considered significantly different in comparison with the control.
Effects of HCT on G0/G1 phase-associated protein levels
We investigated the protein levels of the G0/G1 phase. Figure 4 showed that HCT caused an increase in the protein level of p27 and decreased protein levels of CDK4, CDK2, Cyclin D1 and Cyclin A in A549 cells. HCT (0, 125, 250 and 500 μg/ml) treatment increased p27 expression ratio by 1, 1.8, 1.6, and 1.4, respectively. Among the protein levels of G0/G1 phase, decrease of CDK4 was the most significant one. HCT decreased protein levels of CDK4 by 0%, 20%, 70%, and 90%, respectively.
Effects of HCT on G 0 /G 1 relative protein levels in A549 cells. A549 cells were treated with HCT (0, 125, 250 and 500 μg/ml) for 24 h and then performed western blotting analysis for Cyclin D1, Cyclin A, CDK4, CDK2, and p27 in HCT treated cells as described in Materials and Methods. The experiments were performed in triplicate (n = 3).
Effect of HCT on DNA condensation and damage in A549 cells
Effects of HCT on nuclear morphological change were examined by DAPI staining and DNA damage by the Comet assay. As shown in Figure 3A and B, cells exhibited nuclear shrinkage and DNA condensation after a 24 h-incubation with HCT (500 μg/ml) (Figure 3A). Cells exposed to HCT had an increase in DNA damage as seen in the Comet assay (Figure 3B), and the length of Comet tail was 2.39 fold increase (Figure 3D). The results suggest that HCT induces morphological changes and DNA damage in A549 cells.
Effects of HCT on caspase-8 and caspase-3 activities
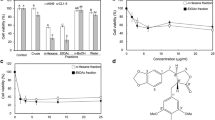
We determined if HCT would stimulate the caspase-9, caspase-8 and caspase-3 activity in A549 cells. Figure 5, panel A and panel B, show that HCT increased caspase-8 and caspase-3 activities in a concentration-dependent manner but caspase-9 activity was unaffected (data not shown). HCT (125, 250 and 500 μg/ml) treatment increased caspase-8 activity by 51.96%, 124.51%, and 208.82%, respectively; increased caspase-3 activity by 85.29%, 150%, and 256.86%, respectively. Our results showed that HCT-induced apoptosis was mediated through the activation of caspase-8 and caspase-3. Thus, we determined whether the Fas/CD95 (death receptor protein) contributes to HCT-induced apoptosis. Figure 6, panel A showed that Fas/CD95 protein level was increased in a concentration-dependent manner in A549 cells. Figure 6, panel B also showed that caspase-8 and caspase-3 protein levels increased in a concentration-dependent manner when A549 cells were treated with HCT. HCT (125, 250 and 500 μg/ml) treatment increased caspase-8 protein expression by 1.1, 1.6, and 2.1 fold, respectively; increased caspase-3 expression ratio by 2.2, 1.6, and 1.9 fold, respectively. To further verify the involvement of caspase-8 and caspase-3 in HCT-induced apoptosis, cells were pretreated with the caspase-8 inhibitor (z-IETD-fmk) and the caspase-3 inhibitor (z-DEVE-fmk). It can be seen in Figure 7, panels A and B showed that the caspase-3 and caspase-8 inhibitors inhibited the apoptotic effects of HCT.
Effects of HCT on caspases-8 and caspase-3 activities in human lung cancer A549 cells. The A549 cells were treated with HCT (0, 125, 250 and 500 μg/ml) for 24 h, and then total cell extracts were incubated with (A) caspases-8 specific substrate (Ac-IETD-pNA) and (B) caspase-3 specific substrate (Ac-DEVE-pNA) respectively. The release of pNA was measured at 405 nm by a spectrophotometer as described in Materials and Methods. The experiments were done in triplicate (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 was considered significantly different in comparison with the control.
Effects of HCT on A549 cells in the Fas/CD95-dependent apoptotic pathway. The A549 cells were treated with HCT (0, 125, 250 and 500 μg/ml) for 12 h, and (A) Fas/CD95 protein expression levels was detected by immune staining and analyzed by flow cytometry as described in Materials and Methods. The experiments were performed in triplicate (n = 3). **p < 0.01, ***p < 0.001 was considered significantly different in comparison with the control. (B) Caspase-8 and caspase-3 protein expression levels in HCT-examined cells were analyzed by Western blotting as described in Materials and Methods.
Effects of caspases-3 and caspase-8 specific inhibitor on cell viability in HCT- treated human lung cancer A549 cells. Cells were pretreated with (A) the caspase-3 inhibitor (z-DEVE-fmk) and (B) the caspase-8 inhibitor (z-IETD-fmk) for 1 h after exposure to HCT (0, 125, 250 and 500 μg/ml) for 24 h exposure, viable cells were determined by MTT assay as described in Materials and Methods. The experiments were performed in triplicate (n = 3). The experiments were performed in triplicate. **p < 0.01, ***p < 0.001 was considered significantly different in comparison with the control.
Discussion
The death receptor apoptotic pathway has previously been proposed as an anti-cancer drug target in human lung cancer [39, 40]. Traditional Chinese medicine (TCM) that could stimulate the death receptor apoptotic pathway should have therapeutic potential in human lung cancer treatment [41–43]. HCT has been used as TCM in Taiwan for many years [44]. The pharmacological activities of HCT include immuno-regulatory, anti-inflammatory, anti-micro bacterial, anti-viral, and anti-cancer effects [24, 25, 27–32]. HCT was reported to be active against leukemia, colorectal cancer and HER2/neu-overexpressing breast cancer cells [30–34]. However, the anti-lung cancer effects have not been well-studied. We previously reported that 450 μg/ml of HCT had anti-cancer activity in human primary colorectal cancer cells from patients [33] and HT29 human colon adenocarcinoma cells [32]. In the present study, HCT had anti-lung cancer activity (Figure 1), and this activity was concentration-, and time-dependent. HCT contains quercetin and quercetin 3-β-D-glucoside (isoquercitrin) which have anti-viral activity [28, 45]. Chou SC, et. al. indicated that quercitrin and isoquercitrin isolated from methanolic extracts of Houttuynia cordata showed excellent DPPH radical scavenging activities with IC50 values of 31 μM and 63 μm, respectively [46]. Also, HCT contains chlorogenic acid [47] which has anti-leukemia effects. In this study, we suggested that ethanolic extract of HCT have anti-lung cancer activity.
Previous studies have shown that HCT induces human lymphoblastic leukemic Molt-4 cell death through an endoplasmic reticulum stress pathway [31]. Banjerdpongchai R, et. al. suggested that ethanolic extract of HCT induces human leukemic HL-60 and Molt-4 cell apoptosis through a mitochondrial apoptotic pathway [30].
Evidences showing the link between apoptosis and the cell cycle are compelling. Regulation of cell cycle traverse involves activations of cyclin-dependent kinases (CDKs). CDKs (e.g. CDK1, CDK2, CDK4/6) is paired with the cyclins (e.g. cyclins A, B, E, D1-3) [48]. Activation of CDKs by cyclins leads to phosphorylation of the retinoblastoma protein (pRb) [49] and leads to diminished binding of pRb to the E2F transcription factor, which various genes necessary for cell cycle progression. The activities of CDKs are antagonized by CDK inhibitors. CDK inhibitors directly interact with CDKs and negatively regulate the activity of CDKs. p27kip1, belongs to the Cip/Kip (kinase inhibitor protein) family, inhibits most CDKs [50]. Dysregulation of cell cycle is one of the most potent stimuli for apoptosis induction [51]. p27kip1[52], cyclin D1 [53], has been shown to influence the apoptotic process. Direct inhibition of CDKs, induction of endogenous CDK inhibitors (e.g. p27kip1) or down-regulation of cyclins required for CDK activation (e.g. cyclin D1) are mechanisms of inhibition of cell cycle progression [48]. Our results showed that HCT increased p27 expression, decreased cyclin D1, cyclin A, CDK 4, and CDK 2 which leaded to cycle G0/G1 arrest. HCT induced apoptosis through caspase8/caspase-3 activation (Figure 5A,B) and up-regulated the protein levels of Fas/CD95 (Figure 6A), caspase-8 and caspase-3 (Figure 6B), while HCT did not affect the caspase-9 activity (data not shown) in A549 cells. In addition, HCT induced growth inhibition was significantly attenuated by the specific caspase-3 and caspase-8 inhibitors (Figure 7A, B). One interpretation of our findings is that HCT induces A549 cell apoptosis and activates caspase-8, and −3 through the Fas/CD95-mediated death receptor apoptotic pathway.
Conclusions
In conclusion, this study demonstrated that HCT has anti-lung cancer activity by modulating G0/G1 arrest and stimulating the Fas/CD95 protein level, which leads to caspase-8 and caspase-3 activation resulting in the induction of apoptosis in human lung cancer A549 cells (Figure 8).
Abbreviations
- HCT:
-
Houttuynia Cordata Thunb
- DAPI:
-
4,6-Diamidino-2-Phenylindole Dihydrochloride
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- FBS:
-
Fetal Bovine Serum
- PBS:
-
Phosphate-Buffered Saline
- DMSO:
-
Dimethyl Sulfoxide
- PI:
-
Propidium Iodide
- PVDF:
-
Polyvinylidene Fluoride Membrane.
References
Vallieres E, Peters S, Van Houtte P, Dalal P, Lim E: Therapeutic advances in non-small cell lung cancer. Thorax. 2011, 67 (12): 1097-1101.
Neal JW, Gubens MA, Wakelee HA: Current management of small cell lung cancer. Clin Chest Med. 2011, 32 (4): 853-863. 10.1016/j.ccm.2011.07.002.
Liao Z, Lin SH, Cox JD: Status of particle therapy for lung cancer. Acta Oncol. 2011, 50 (6): 745-756. 10.3109/0284186X.2011.590148.
Levy A, Malouf GG, Besse B, Massard C, Soria JC: Molecular targeted therapies in small-cell lung cancer. Bull Cancer. 2010, 97 (5): 535-545.
Savai R, Pullamsetti SS, Banat GA, Weissmann N, Ghofrani HA, Grimminger F, Schermuly RT: Targeting cancer with phosphodiesterase inhibitors. Expert Opin Investig Drugs. 2010, 19 (1): 117-131. 10.1517/13543780903485642.
Hahn O, Salgia R: Non-receptor tyrosine kinase inhibitors in lung cancer. Anticancer Agents Med Chem. 2007, 7 (6): 633-642. 10.2174/187152007784111322.
Salgia R, Skarin AT: Molecular abnormalities in lung cancer. J Clin Oncol. 1998, 16 (3): 1207-1217.
Handke W, Krause E, Brune W: Live or let die: manipulation of cellular suicide programs by murine cytomegalovirus. Med Microbiol Immunol. 2012, 201 (4): 475-486. 10.1007/s00430-012-0264-z.
Sanmartin C, Plano D, Sharma AK, Palop JA: Selenium compounds, apoptosis and other types of cell death: an overview for cancer therapy. Int J Mol Sci. 2012, 13 (8): 9649-9672.
Wen X, Lin ZQ, Liu B, Wei YQ: Caspase-mediated programmed cell death pathways as potential therapeutic targets in cancer. Cell Prolif. 2012, 45 (3): 217-224. 10.1111/j.1365-2184.2012.00814.x.
Kantari C, Walczak H: Caspase-8 and bid: caught in the act between death receptors and mitochondria. Biochim Biophys Acta. 2011, 1813 (4): 558-563. 10.1016/j.bbamcr.2011.01.026.
Tauzin S, Debure L, Moreau JF, Legembre P: CD95-Mediated cell signaling in cancer: mutations and post-translational modulations. Cell Mol Life Sci. 2012, 69 (8): 1261-1277. 10.1007/s00018-011-0866-4.
Vacher P, Khadra N, Vacher AM, Charles E, Bresson-Bepoldin L, Legembre P: Does calcium contribute to the CD95 signaling pathway?. Anticancer Drugs. 2011, 22 (6): 481-487. 10.1097/CAD.0b013e32834433ea.
Kaufmann T, Strasser A, Jost PJ: Fas death receptor signalling: roles of Bid and XIAP. Cell Death Differ. 2012, 19 (1): 42-50. 10.1038/cdd.2011.121.
Koncz G, Hueber AO: The Fas/CD95 receptor regulates the death of autoreactive B cells and the selection of antigen-specific B cells. Front Immunol. 2012, 3: 207-
Vilmont V, Tourneur L, Chiocchia G: Fas-associated death domain protein and adenosine partnership: fad in RA. Rheumatology (Oxford). 2012, 51 (6): 964-975. 10.1093/rheumatology/ker402.
Venderova K, Park DS: Programmed cell death in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012, 2: a009365-
Drel VR, Shymans’kyi IO, Sybirna NO, Velykyi MM: Role of PARP and protein poly-ADP-ribosylation process in regulation of cell functions. Ukr Biokhim Zh. 2011, 83 (6): 5-34.
Agarwal A, Mahfouz RZ, Sharma RK, Sarkar O, Mangrola D, Mathur PP: Potential biological role of poly (ADP-ribose) polymerase (PARP) in male gametes. Reprod Biol Endocrinol. 2009, 7: 143-10.1186/1477-7827-7-143.
Ame JC, Spenlehauer C, de Murcia G: The PARP superfamily. Bioessays. 2004, 26 (8): 882-893. 10.1002/bies.20085.
Heeres JT, Hergenrother PJ: Poly(ADP-ribose) makes a date with death. Curr Opin Chem Biol. 2007, 11 (6): 644-653. 10.1016/j.cbpa.2007.08.038.
Chang JS, Chiang LC, Chen CC, Liu LT, Wang KC, Lin CC: Antileukemic activity of Bidens pilosa L. var. minor (Blume) Sherff and Houttuynia cordata Thunb. Am J Chin Med. 2001, 29 (2): 303-312. 10.1142/S0192415X01000320.
Hu SH, Du AF: Treatment of bovine mastitis with houttuynin sodium bisulphate. Zentralbl Veterinarmed B. 1997, 44 (6): 365-370.
Hayashi K, Kamiya M, Hayashi T: Virucidal effects of the steam distillate from Houttuynia cordata and its components on HSV-1, influenza virus, and HIV. Planta Med. 1995, 61 (3): 237-241. 10.1055/s-2006-958063.
Park E, Kum S, Wang C, Park SY, Kim BS, Schuller-Levis G: Anti-inflammatory activity of herbal medicines: inhibition of nitric oxide production and tumor necrosis factor-alpha secretion in an activated macrophage-like cell line. Am J Chin Med. 2005, 33 (3): 415-424. 10.1142/S0192415X05003028.
Kusirisin W, Srichairatanakool S, Lerttrakarnnon P, Lailerd N, Suttajit M, Jaikang C, Chaiyasut C: Antioxidative activity, polyphenolic content and anti-glycation effect of some thai medicinal plants traditionally used in diabetic patients. Med Chem. 2009, 5 (2): 139-147. 10.2174/157340609787582918.
Ren X, Sui X, Yin J: The effect of Houttuynia cordata injection on pseudorabies herpesvirus (PrV) infection in vitro. Pharm Biol. 2011, 49 (2): 161-166. 10.3109/13880209.2010.505242.
Chen X, Wang Z, Yang Z, Wang J, Xu Y, Tan RX, Li E: Houttuynia cordata blocks HSV infection through inhibition of NF-kappaB activation. Antiviral Res. 2011, 92 (2): 341-345. 10.1016/j.antiviral.2011.09.005.
Lau KM, Lee KM, Koon CM, Cheung CS, Lau CP, Ho HM, Lee MY, Au SW, Cheng CH, Lau CB, Tsui SK, Wan DC, Waye MM, Wong KB, Wong CK, Lam CW, Leung PC, Fung KP: Immunomodulatory and anti-SARS activities of Houttuynia cordata. J Ethnopharmacol. 2008, 118 (1): 79-85. 10.1016/j.jep.2008.03.018.
Banjerdpongchai R, Kongtawelert P: Ethanolic extract of fermented Houttuynia cordata Thunb induces human leukemic HL-60 and Molt-4 cell apoptosis via oxidative stress and a mitochondrial pathway. Asian Pac J Cancer Prev. 2011, 12 (11): 2871-2874.
Prommaban A, Kodchakorn K, Kongtawelert P, Banjerdpongchai R: Houttuynia cordata Thunb fraction induces human leukemic Molt-4 cell apoptosis through the endoplasmic reticulum stress pathway. Asian Pac J Cancer Prev. 2012, 13 (5): 1977-1981. 10.7314/APJCP.2012.13.5.1977.
Tang YJ, Yang JS, Lin CF, Shyu WC, Tsuzuki M, Lu CC, Chen YF, Lai KC: Houttuynia cordata Thunb extract induces apoptosis through mitochondrial-dependent pathway in HT-29 human colon adenocarcinoma cells. Oncol Rep. 2009, 22 (5): 1051-1056.
Lai KC, Chiu YJ, Tang YJ, Lin KL, Chiang JH, Jiang YL, Jen HF, Kuo YH, Agamaya S, Chung JG, Yang JS: Houttuynia cordata Thunb extract inhibits cell growth and induces apoptosis in human primary colorectal cancer cells. Anticancer Res. 2010, 30 (9): 3549-3556.
Zhou NN, Tang J, Chen WD, Feng GK, Xie BF, Liu ZC, Yang D, Zhu XF: Houttuyninum, an active constituent of Chinese herbal medicine, inhibits phosphorylation of HER2/neu receptor tyrosine kinase and the tumor growth of HER2/neu-overexpressing cancer cells. Life Sci. 2012, 90 (19–20): 770-775.
Chen YF, Yang JS, Huang WW, Tsai HY: Novel anti-leukemia activities of pipoxolan operate via the mitochondria-related pathway in human leukemia U937 cells and attenuate U937 cell growth in an animal model. Molecular Medicine Reports. 2010, 3 (5): 851-856.
Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF, Li KH, Chen PY, Chung JG, Yang JS: Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol Nutr Food Res. 2010, 54 (11): 1585-1595. 10.1002/mnfr.201000005.
Huang WW, Ko SW, Tsai HY, Chugn JG, Chiang JH, Chen KT, Chen YC, Chen YF, Yang JS: Cantharidin induces G2/M phase arrest and apoptosis in human colorectal cancer colo 205 cells through inhibition of CDK1 activity and caspase-dependent signaling pathways. Int J Oncol. 2011, 38 (4): 1067-1073.
Lan YH, Wu YC, Wu KW, Chung JG, Lu CC, Chen YL, Wu TS, Yang JS: Death receptor 5-mediated TNFR family signaling pathways modulate gamma-humulene-induced apoptosis in human colorectal cancer HT29 cells. Oncol Rep. 2011, 25 (2): 419-424.
Guo L, Fan L, Ren J, Pang Z, Ren Y, Li J, Wen Z, Qian Y, Zhang L, Ma H, Jiang X: Combination of TRAIL and actinomycin D liposomes enhances antitumor effect in non-small cell lung cancer. Int J Nanomedicine. 2012, 7: 1449-1460.
Jin H, Yang R, Ross J, Fong S, Carano R, Totpal K, Lawrence D, Zheng Z, Koeppen H, Stern H, Schwall R, Ashkenazi A: Cooperation of the agonistic DR5 antibody apomab with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Clin Cancer Res. 2008, 14 (23): 7733-7740. 10.1158/1078-0432.CCR-08-0670.
Yang G, Li X, Wang L, Li J, Song X, Chen J, Guo Y, Sun X, Wang S, Zhang Z, Zhou X, Liu J: Traditional chinese medicine in cancer care: a review of case series published in the chinese literature. Evid Based Complement Alternat Med. 2012, 2012: 751046-
Ferreira AS, Lopes AJ: Chinese medicine pattern differentiation and its implications for clinical practice. Chin J Integr Med. 2011, 17 (11): 818-823. 10.1007/s11655-011-0892-y.
Tian G, Guo L, Gao W: Use of compound Chinese medicine in the treatment of lung cancer. Curr Drug Discov Technol. 2010, 7 (1): 32-36. 10.2174/157016310791162776.
The illustrated medicinal plants of taiwan. Edited by: Chiu NY CK. 1986
Xu X, Ye H, Wang W, Yu L, Chen G: Determination of flavonoids in Houttuynia cordata Thunb. and Saururus chinensis (Lour.) Bail. by capillary electrophoresis with electrochemical detection. Talanta. 2006, 68 (3): 759-764. 10.1016/j.talanta.2005.05.027.
Chou SC, Ku YC, Wu TS: The constituents and their bioactivities of Houttuynia cordata Thunb. Chem Pharm Bull. 2009, 57 (11): 1227-1230. 10.1248/cpb.57.1227.
Meng J, Dong XP, Zhou YS, Jiang ZH, Leung SY, Zhao ZZ: Studies on chemical constituents of phenols in fresh Houttuynia Cordata. Zhongguo Zhong Yao Za Zhi. 2007, 32 (10): 929-931.
Grant S, Roberts JD: The use of cyclin-dependent kinase inhibitors alone or in combination with established cytotoxic drugs in cancer chemotherapy. Drug Resist Updat. 2003, 6 (1): 15-26. 10.1016/S1368-7646(02)00141-3.
Morgan DO: Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997, 13: 261-291. 10.1146/annurev.cellbio.13.1.261.
Dai Y, Grant S: Cyclin-dependent kinase inhibitors. Curr Opin Pharmacol. 2003, 3 (4): 362-370. 10.1016/S1471-4892(03)00079-1.
King KL, Cidlowski JA: Cell cycle and apoptosis: common pathways to life and death. J Cell Biochem. 1995, 58 (2): 175-180. 10.1002/jcb.240580206.
St Croix B, Florenes VA, Rak JW, Bhattacharya N, Slingerland JM, Kerbel RS: Impact of the cyclin-dependent kinase inhibitors p27kip1 on resistance of tumor cells to anticancer agents. Nat Med. 1996, 2: 1204-1210. 10.1038/nm1196-1204.
Niu MY, Menard M, Reed JC, Krajewski S, Pratt MA: Ectopic expression of cyclin D1 amplifies a retinoic acid-induced mitochondrial death pathway in breast cancer cells. Oncogene. 2001, 20: 3506-3518. 10.1038/sj.onc.1204453.
Acknowledgements
Special acknowledgment is to Professor W. Gibson Wood, Department of Pharmacology, University of Minnesota, USA, for his helpful comments and proofreading this revised manuscript. The authors would like to thank Professor Tian-Shung Wu, Department of Chemistry, National Cheng-Kung University, Taiwan, for providing the detail information of chemical composition of HCT. The authors would also like to thank the National Science Council (Taipei, Taiwan) for supporting this work (NSC-101-2313-B-039-008, NSC-101-2320-B-039-026).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests related to this work.
Authors’ contributions
YFC conceived for the study, and participated in its design, performed the statistical analysis and figure drawing, coordination, draft and revise the manuscript. JSY helped to draw the figures and drafted the manuscript. WSC carried out the cell viability, cell cycle transition, G0/G1 relative protein levels assay and caspase activities assay. SCT helped to draft the manuscript. SFP carried out the DAPI staining and Comet assay. YRZ performed immunostaining assay for Fas/CD95 protein levels. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chen, YF., Yang, JS., Chang, WS. et al. Houttuynia cordata Thunb extract modulates G0/G1 arrest and Fas/CD95-mediated death receptor apoptotic cell death in human lung cancer A549 cells. J Biomed Sci 20, 18 (2013). https://doi.org/10.1186/1423-0127-20-18
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1423-0127-20-18