Abstract
Background
Advanced neuroimaging approaches have been employed to prove that migraine was a central nervous system disorder. This study aims to examine resting-state abnormalities in migraine without aura (MWoA) patients stratified by disease duration, and to explore the neuroimaging markers for reflecting the disease duration.
Methods
40 eligible MWoA patients and 20 matched healthy volunteers were included in the study. Regional homogeneity (ReHo) analysis was used to identify the local features of spontaneous brain activity in MWoA patients stratified by disease duration, and analysis was performed to investigate the correlation of overlapped brain dysfunction in MWoA patients with different disease duration (long-term and short-term) and course of disease.
Results
Compared with healthy controls, MWoA patients with long-term disease duration showed comprehensive neuronal dysfunction than patients with short-term disease duration. In addition, increased average ReHo values in the thalamus, brain stem, and temporal pole showed significantly positive correlations with the disease duration. On the contrary, ReHo values were negatively correlated with the duration of disease in the anterior cingulate cortex, insula, posterior cingulate cortex and superior occipital gyrus.
Conclusions
Our findings of progressive brain damage in relation to increasing disease duration suggest that migraine without aura is a progressive central nervous disease, and the length of the disease duration was one of the key reasons to cause brain dysfunction in MwoA patients. The repeated migraine attacks over time result in resting-state abnormalities of selective brain regions belonging to the pain processing and cognition. We predict that these brain regions are sensitive neuroimaging markers for reflecting the disease duration of migraine patients without aura.
Similar content being viewed by others
Background
Migraine headache is a common neurological disorder which causes significant individual and societal burden due to pain and environmental sensitivities [1]. It was ranked the seventh highest among specific causes of disability globally. Migraine has two subtypes, and two thirds of migraine patients suffer from MWoA which is typically characterized as a unilateral and pulsating headache, and an autonomic nervous system dysfunction [2]. The recurrent headache manifests in attacks lasting 4–72 hours and affects patients 1–14 times each month in the episodic form. It is aggravated by routine physical activity, and is accompanied by vomiting, nausea, photophobia or phonophobia. Migraine may result in substantial pain, a decreased overall quality of life, and cause higher risks for ischemic stroke, unstable angina, and affective disorders than people without migraine [3–6].
Advanced neuroimaging approaches have been employed to investigate structural and functional brain changes in migraineurs, and proved that migraine was a central nervous system disorder [1]. The insula, anterior cingulate cortex (ACC), thalamus, prefrontal cortex (PFC), orbitofrontal cortex (OFC), parahippocampal cortex, periaqueductal gray matter (PAG), inferior frontal gyrus (IFG), brainstem, precentral gyrus, and cerebellum have been reported to show structural and functional alterations [7–15]. Furthermore, gray matter reduction based on voxel-based morphometric (VBM) studies was correlated with attack frequency or headache duration in migraine patients [13, 16–18]. Moreover, task-related functional magnetic resonance imaging (fMRI) studies revealed abnormal activation of some brain regions associated with pain-related information processing in migraine patients, such as the ACC, the PFC, the OFC, insula and the supplementary motor area (SMA) [10, 11, 19]. Numerous findings have supported that migraine may have cumulative effects on brain structure and function, and repeated attacks over time would result in secondary damage on several brain regions involved in central pain processing [14, 17, 20–22]. Moreover, some preliminary neuroimaging studies provided some evidence about increased risk of brain abnormalities with increasing attack frequency [5, 17, 21, 23] and disease duration [15, 17, 24] in migraineurs. However, few studies have evaluated the characteristic in the resting-state in MWoA patients stratified by disease duration.
In the current study, we performed a ReHo approach [25] to compare the blood oxygen level-dependent (BOLD) signals of the brains in MWoA patients along with healthy subjects during the resting-state. The ReHo method focuses on the similarities or coherence of the intraregional spontaneous low-frequency (<0.08 Hz) BOLD signal, which enables a novel perspective to understand the functional deficits in particular brain regions. An important advantage of using the ReHo method over other methods is that it can examine the regional activity characteristics of each voxel. It can also detect changes or modulations that are induced by different conditions across the whole brain in a voxel-by-voxel manner, without requiring any prior knowledge. Previously, our group has employed the ReHo method only to find that MWoA patients showed a significant decrease in ReHo values in the right ACC, PFC, OFC and SMA [12]. In addition, the ReHo values were negatively correlated with the duration of disease in the right ACC and PFC [12]. In order to further assess and validate whether some brain abnormalities serve as markers for disease history in MWoA patients, we investigated the resting-state difference between MWoA patients with long-term (LT) disease duration and MWoA patients with short-term (ST) disease duration. We hypothesized that, as compared with healthy controls, (1) MWoA patients with LT disease duration would display more neuronal dysfunction than patients with ST disease duration; (2) the overlapped brain dysfunction in LT and ST patients group may be associated with the course of disease in migraineurs.
Methods
Study participants
40 eligible MWoA patients were recruited from the neurology department of the Teaching Hospital of Chengdu University of Traditional Chinese Medicine. The diagnosis of MWoA was established according to the classification criteria of the International Headache Society (IHS) [26]. The inclusion criteria were as follows:(1) all subjects were right-handed, and had 2 to 8 migraine attacks per month during the last 3 months and during the baseline period (4 weeks before enrolment); (2) all subjects were 18 to 55 years of age; in addition, start of headache should be before the age of 50 years; (3) had received education for more than 6 years and had completed a baseline headache diary; (4) MWoA patients were selected on the basis of disease duration >10 years (LT) or < 5 years (ST); (5) had no migraine 72 hours prior to the scan; (6) no habit of long-term analgesics consumption; (7) did not take any prophylactic migraine medication during the previous month; and (8) no contraindications for exposure to a high magnetic field. Healthy subjects were recruited from the local community and were screened by a neurologist specialized in headaches. 20 right-handed, age-matched and education-matched healthy subjects were enrolled. They either had no headache days per year or had family members who suffered regularly from a migraine or other headache.
Exclusion criteria for MWoA patients and healthy controls were: (1) existence of neurological diseases; (2) had hypertension, diabetes mellitus, hypercholesteremia, vascular/heart disease, and major systemic conditions; (3) pregnant or lactating women; (4) alcohol or drug abuse; (5) any neuroimaging research study participation during the last 6 months; and (6) inability to understand the doctor’s instructions.
This study was approved by the ethics committee at the Teaching Hospital of Chengdu University of Traditional Chinese Medicine. All subjects gave written, informed consent after the experimental procedures had been fully explained.
Study design
All patients should have recorded headache diaries for 4 weeks (baseline phase) before enrolment to assess disease activity (disease duration, headache degree, and attack frequency). Patients meeting the inclusion criteria were assigned to two groups based on different disease duration after the baseline period.
The headache diary documented the migraine attack frequency and severity of headache according to the guidelines of the IHS for clinical trials for migraine [27]. The VAS score 0–10 measured the intensity of headache. fMRI scans were scheduled 2 weeks after enrolment. In addition, records in the headache diary were checked to insure every patient did not suffer from a migraine attack at least 72 hours prior to the brain scan.
Imaging data acquisition
The imaging data were carried out in a 3 Tesla Siemens MRI system (Allegra, Siemens Medical System, Erlangen, Germany) at the Huaxi MR Research Center, West China Hospital of Sichuan University, Chengdu, China. A standard eight-channel phase-array head coil was used, along with restraining foam pads to minimize head motion and to diminish scanner noise. Prior to the functional run, a high-resolution T1structural image for each subject was acquired using a three-dimensional MRI sequence with a voxel size of 1 mm3 employing an axial fast spoiled gradient recalled sequence (TR = 1900 ms, TE = 2.26 ms, data matrix = 256 × 256, flip angle = 9°, FOV = 256 mm × 256 mm). The structural images were examined to exclude the possibility of clinically silent lesions for all of the participants by two expert radiologists. The resting-state functional images were obtained with echo-planar imaging (EPI) (30 continuous slices with a slice thickness = 5 mm, TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 240 mm × 240 mm, matrix = 64 × 64). During 6-min fMRI scanning, participants were instructed to keep their eyes closed, relax, move as little as possible, and stay awake. It needs to be emphasized that if there was an attack for migraine patients in the check reservation, they could not be scanned and the scan would be postponed to ensure they were scanned during the migraine interval.
Data preprocessing
In the functional image data preprocessing, the first five scans were discarded to eliminate nonequilibrium effects of magnetization and to allow participants to become familiar with the scanning circumstances. Data preprocessing was done using Statistical Parametric Mapping (SPM5, http://www.fil.ion.ucl.ac.uk/spm). The images were corrected for the acquisition delay between slices, aligned to the first image of each session for motion correction and spatially normalized to the standard Montreal Neurological Institute (MNI) template in SPM5. We calculated the maximum excursion movement values for each of the translation planes (x, y, and z) and each of the rotation planes (roll, pitch, and yaw) for every participant. None of them had head movements exceeding 1 mm on any axis and head rotation greater than 1° during the entire fMRI scan. Finally, a band-pass filter (0.01 Hz < f < 0.08 Hz) was applied to remove physiological and high-frequency noise.
Data analysis
Baseline and demographic data were analyzed by SPSS 14.0 statistical software (SPSS Inc., Chicago, IL, USA). Baseline characteristics were summarized by descriptive statistics for each group and in the total study population. Two independent-sample t-tests were used to examine differences between groups (95% CI, 2-sided).
Kendall’s coefficient of concordance (KCC) [28] was used to evaluate ReHo [25], which was performed using the Resting-State fMRI Data Analysis Toolkit (X.-W. Song et al., Beijing Normal University, Beijing, China, http://www.restfmri.net). Individual ReHo maps were generated by assigning each voxel a value corresponding to the KCC of its time series with its nearest 26 neighboring voxels [25]. Then, a mask (made from the MNI template to assure matching with the normalization step) was used to remove non-brain tissues and noise from the ReHo maps. Only the voxels within the mask were analyzed further. The individual ReHo maps were standardized by their own mean KCC within the mask. Then, a Gaussian kernel with a full-width at half-maximum of 4 mm was used to smooth the images in order to reduce noise and residual differences. Controlling for age, two independent-sample t-tests were used to compare the ReHo results between different groups. The false discovery rate (FDR) was used to correct the multiple comparisons. In addition, correlation analyses were performed in order to delineate possible correlations between average ReHo values of the overlapped brain dysfunctional regions in LT and ST groups and the disease duration.
Results
Participants
There were no significant differences in the demographics including age, gender, and education between MWoA patients and healthy subjects (p > 0.05) (Table 1). There were no significant differences in the demographics including sex, education, family history, migraine attack frequency, and visual analogue scale (VAS) score between ST group patients and LT group subjects (p > 0.05). Patients in the LT group were older and had longer disease duration compared with patients in the ST group (p < 0.05) (Table 2).
Neuroimaging results
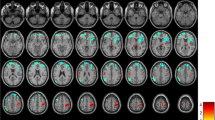
Compared with healthy subjects, MWoA patients with ST disease duration showed significantly higher ReHo values in the bilateral thalamus, IFG (Brodmman area (BA) 47), middle occipital gyrus (MOG) (BA19), left insula (BA13), caudate, middle frontal gyrus (MFG) (BA8), middle temporal gyrus (MTG) (BA37), inferior occipital gyrus (IOG) (BA19), right ACC (BA32), medial frontal gyrus (MeFG) (BA25), and superior temporal gyrus (STG) (BA42). The results revealed MWoA patients with ST disease duration showed a significant decrease in ReHo values in the bilateral MFG (BA8,BA10), MTG (BA21), left lingual gyrus (BA17), right MOG (BA19), cerebellum, and brain stem (controlling for age, p <0.01, FDR corrected) (Additional file 1, Figure 1a).
In this study, the MWoA patients with LT disease duration showed increased ReHo values in the bilateral ACC (BA24, BA32), amygdala, thalamus, caudate, lentiform nucleus, uncus, IFG (BA11, BA47), MFG (BA11), SFG (BA6, BA11), MTG (BA21), temporal pole (BA38), cerebellum, brain stem (including pons, medulla, and midbrain), and left hippocampus compared with healthy subjects. On the contrary, the results seemed decreased in the bilateral ACC (BA24), insula (BA13), IFG (BA45, BA47), MFG (BA6), MeFG (BA6, BA8), SFG (BA6), MTG (BA21, BA39), MOG (BA18, BA19), cuneus (BA18, BA19), lingual gyrus (BA18, BA19), inferior parietal lobule (IPL) (BA40), postcentral gyrus (BA6, BA43), and precuneus (BA19, BA31), left fusiform gyrus (BA19), and right posterior cingulate cortex (PCC) (BA31) (controlling for age, p < 0.01, FDR corrected) (Additional file 2, Figure 1b).
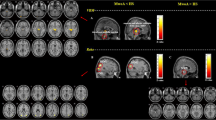
Correlation analysis results demonstrated that increased average ReHo values in the thalamus (r = 0.5269, p = 0.0014), brain stem (r = 0.4180, p = 0.0139), and temporal pole (r = 0.4939, p = 0.0030) showed significantly positive correlations with the disease duration (Figure 2). There were respectively significant negative correlations between the decreased average ReHo values of the ACC (r = -0.5452, p = 8.5452*e-4), insula (r = -0.5891, p = 2.4653*e-4), PCC (r = -0.5800, p = 3.2389*e-4), SOG (r = -0.36, p = 0.049) and the disease duration (Figure 2). The correlation between VAS score and attack frequency and resting-state properties were also checked, but no results exceeded the threshold.
The correlation of average ReHo values of the overlapped brain dysfunction in LT vs HC and LT vs ST with the disease duration. Warm colors indicate ReHo increases in MWoA patients; cool colors indicate ReHo decreases in MWoA patients; ACC, anterior cingulate cortex; THAL, thalamus; TP, temporal pole; PCC, posterior cingulate cortex; SOG, superior occipital gyrus; LT, MWoA patients with long-term disease duration; ST, MWoA patients with short-term disease duration; HC, healthy controls.
Discussion
To our knowledge, this study is the first one to investigate characteristic of regional homogeneity in patients with episodic migraine without aura stratified by disease duration. ReHo hypothesizes that a given voxel is temporally similar to that of its neighbors [25]. It is calculated by using Kendall’s coefficient of concordance, which could obtain reliable results in a resting-state fMRI data analysis [29]. Therefore, ReHo reflects the temporal homogeneity of the regional BOLD signal rather than its density. Compared with healthy controls, several common brain regions showed abnormalities in MWoA patients with ST and LT disease duration during the resting-state, including IFG, MFG, MTG, ACC, thalamus, and basal ganglia. These results were mainly involved in pain-related processing, and were similar to previous reports in migraineur studies which focused on structural [16, 17, 30, 31], task-related [11, 19, 21], and resting-state [12–15, 24] abnormalities. Furthermore, compared with healthy controls, MWoA patients with LT disease duration might display comprehensive neuronal dysfunction than patients with ST disease duration. PCC, lentiform nucleus, uncus, temporal pole, MOG, cuneus, fusiform gyrus, inferior parietal lobule, postcentral gyrus, precuneus, and brain stem were only found in MWoA patients with LT disease duration. In the current study, abnormal ReHo in MwoA patients was relevant to the changes of temporal aspects of neural activity in the brain regions. Increased or decreased ReHo suggests that neural function in local regions is more or less synchronous during resting-state. The results demonstrated that the long history of disease might contribute to accumulating brain damage due to the repetitive occurrence of pain-related processes.
We were interested in whether brain abnormalities would progressively influence individuals as the result of migraine attack history. To explore which brain regions might relate to the course of disease, a correlation analysis was performed. The results showed that the average ReHo value of the thalamus, brain stem, and temporal pole were positively related to the disease duration. The ReHo value of the ACC, insula, PCC and SOG were negatively correlated with the history of MWoA. Therefore, the ReHo increase in the thalamus, brain stem, and temporal pole in MwoA patients may reflect a dynamic compensation for the disorder signals from the brain, whereas the decreased hemodynamic synchronization in the ACC, insula, PCC, and SOG could be explained by MwoA -related dysfunction. Additionally, we speculated these ReHo changes might reflect not only as a consequence of repeated painful attacks in a pain disorder, but also as indicators specific to migraine without aura.
As we all know, the ACC, insula, and thalamus are the key regions composed of the “pain matrix”. Recent neuroimaging evidence supported that the ACC and insula were the common “brain signature” structures in chronic pain diseases, such as fibromyalgia [32], irritable bowel syndrome [33], chronic tension type headache [34], and migraine [16, 35, 36]. ACC has a close interconnection with the insula, thalamus, prefrontal cortex, and other subcortical structures, and is considered to be implicated in both affective and cognitive-attentional dimensions of pain and plays a deterministic role in pain modulation and analgesia [37]. In the current study, ACC demonstrated negative correlation with disease duration, which was consistent with previous correlation analysis reports separately on regional metabolism [35] and average ReHo values [12] of the ACC. The insula is a complex, multisensory integration area that is involved in processing many aspects involved with the conscious experience of pain such as affect, autonomic activity and interoception. A recent study strongly suggested that if the full pain experience involves the pain matrix network, a part of the insula seems to play a leading role in the triggering of this network and the resulting emergence of the subjective pain experience [38]. Functional imaging experiments have revealed that the insula is a major site for emotional processing, and it also processes sensory-discriminative aspects of pain perception [39]. Coghill et al., reported the insula cortex plays reciprocal role in pain, emotions and pain-related emotions, due to its anatomic connections [40]. We found that the progressive dysfunction of the insula showed a significant correlation with disease history, and did not detect a significant relationship between the insula and headache degree or attack frequency. The thalamus was also found to have a dysfunction in migraine patients in previous documents [41–43], but few studies have evaluated the correlation between the abnormality of the thalamus and clinical parameters. The thalamus is the “relay center” of the brain, and it is involved in the formation of the lateral and medial pain system. The lateral nuclei of the thalamus deal with discriminative sensory pain transmission, and the medial nuclei of the thalamus are involved in emotional and somatic responses to pain [44]. In the current study, increased ReHo values of the thalamus were positively correlated with disease duration, suggesting that this cumulative alteration was mainly due to migraine, and not only the secondary effect of having migraine headaches. Our results demonstrated that the ACC, insula, and thalamus were not only related to central pain processing for migraine without aura, but also involved in expressing the relationship between brain dysfunction and disease history.
Moreover, several independent functional imaging studies have reinforced the fact that the pathogenesis of migraine is related to the dysfunction of the brain stem. A series of positron emission tomographic (PET) studies consistently observed an increase in regional cerebral blood flow in the brain stem during migraine attacks [41, 45–47], and the brain stem was also found to be activated in migraine patients with some stimulus detected by fMRI [48–50]. Dysfunction of the brain stem is involved in antinociception, extracerebral and intracerebral vascular control and sensory gating provides an explanation for many of the facets of migraine. In this study, increased ReHo values in the brain stem were related with disease duration during the resting-state facilitation that the brain stem has a crucial role in migraine, and may serve as an indicator to reflect the progress of migraine.
PCC participates in the composition of the default mode network (DMN), and it seldom detected significant abnormal findings in migraine patients checked by neuroimaging. It is not the traditional pain-processing area, but recently, Loggia et al. reported that some DMN subregions (such as the PCC) respond in a perception-related manner to pain, suggesting closer linkage between the DMN and pain processing than previously thought [51]. Furthermore, the PCC is recognized that subjects with cognitive impairment showed reduced cerebral blood flow in the PCC, and some clinical evidence indicated that migraine patients had deficits in cognitive function relative to healthy controls [52–54]. Our findings of progressive ReHo changes in the PCC in relation to increasing disease duration suggest that repeated migraine attacks over time may lead to resting-state abnormalities of selective brain regions belonging to pain perception and cognitive control. The temporal pole was found to have an increase in the fMRI BOLD response during the interictal period in migraineurs in response to a thermal stimulus [11], and also showed significantly higher activation during odor stimulation by H2 15O-PET [55]. The role of the temporal pole in pain processing is not well understood, but it is an associative multisensory area and plays a role in assigning affective tone to short-term memories relating to pain, which may be related to reports of impaired memory in migraine patients during the interictal period [11]. We found the ReHo properties of the temporal pole were positively correlated with the duration of disease, which suggests that temporal pole excitability as sensitization during both the resting-state and stimulation may contribute to repeated migraine attack. Lesions in the occipital lobe result in visual disturbance, memory deficits and motion perception disorders. Occipital lobes had bilateral hypoperfusion in a patient with spontaneous migraine without aura as detected by PET [56, 57], and an fMRI study found that the occipital cortex showed structural deficits in MWoA patients [13]. In the current study, our results showed decreased ReHo values in the SOG which were negatively correlated with the disease duration. Therefore, we inferred that the observed PCC, temporal pole and SOG dysfunction in MWoA patients may provide a potential neurobiological mechanism for cognitive deficits in migraineurs.
There are some limitations in the present study. Firstly, disease duration was used to classify the MWoA patients with ST and LT, not including the MWoA patients with moderate-term disease duration (between 5 years and 10 years). Further studies need to recruit a large number of MWoA patients and stratify the detailed data, and give more evidence to strengthen our findings. Secondly, in order to test the reproducibility of our results and to verify the consequences of brain damage in migraineurs, further neuroimaging investigations have to quantify brain abnormalities in a longitudinal design. Lastly, we will plan to assess cerebral structural changes in MwoA patients by using DTI, VBM, or surface-based techniques in the future work, and help us to better understand the pathophysiology of migraine.
Conclusion
In conclusion, the current study employed the ReHo method to investigate the difference in resting-state properties between MWoA patients stratified with different disease duration and healthy controls, as well as the correlation of abnormal cerebral activity in MWoA patients and disease duration. Our findings of progressive abnormal ReHo values in relation to increasing disease duration suggest that migraine without aura is a progressive central nervous disease, and the length of the disease duration was one of the key reasons to cause brain dysfunction in MwoA patients. The repeated migraine attacks over time result in resting-state abnormalities of selective brain regions belonging to the pain processing and cognition. Our results provided more scientific and sensitive neuroimaging markers for reflecting the disease duration of migraine patients without aura, and helped to identify indicators of predilection sites for possible progressive brain damage in migraineurs. It is expected that these findings may be advance the understanding of the pathology of migraine without aura and helpful to the diagnosis and therapy for MwoA patients. For example, take the appropriate individual treatment program depending on the different length of duration of disease, and increase some special assessment in brain function for migraine patients with long disease duration.
Abbreviations
- ACC:
-
Anterior cingulate cortex
- PFC:
-
Prefrontal cortex
- OFC:
-
Orbitofrontal cortex
- PAG:
-
: Periaqueductal gray matter
- IFG:
-
Inferior frontal gyrus
- VBM:
-
Voxel-based morphometric
- fMRI:
-
Functional MRI
- SMA:
-
Supplementary motor area
- ReHo:
-
Regional homogeneity
- BOLD:
-
Blood oxygen level-dependent
- IHS:
-
International Headache Society
- VAS:
-
Visual analogue scale
- KCC:
-
Kendall’s coefficient of concordance
- FDR:
-
False discovery rate
- MOG:
-
Middle occipital gyrus
- MFG:
-
Middle frontal gyrus
- MTG:
-
Middle temporal gyrus
- IOG:
-
Inferior occipital gyrus
- SOG:
-
Superior occipital gyrus
- MeFG:
-
Medial frontal gyrus
- STG:
-
Superior temporal gyrus
- DMN:
-
Default mode network.
References
Schwedt TJ, Dodick DW: Advanced neuroimaging of migraine. Lancet Neurol 2009,8(6):560–568. doi:S1474–4422(09)70107–3 10.1016/S1474-4422(09)70107-3
Van DeVen RC, Kaja S, Plomp JJ, Frants RR, Van Den Maagdenberg AM: Ferrari MD (2007) Genetic models of migraine. Arch Neurol 2007,64(5):643–646. doi:64/5/643 10.1001/archneur.64.5.643
Raggi A, Leonardi M, Bussone G, D'Amico D: Value and utility of disease-specific and generic instruments for assessing disability in patients with migraine, and their relationships with health-related quality of life. Neurol Sci 2011,32(3):387–392. doi:10.1007/s10072–010–0466–3 10.1007/s10072-010-0466-3
Velentgas P, Cole JA, Mo J, Sikes CR, Walker AM: Severe vascular events in migraine patients. Headache 2004,44(7):642–651. doi:10.1111/j.1526–4610.2004.04122.x HED04122 10.1111/j.1526-4610.2004.04122.x
Kruit MC, van Buchem MA, Hofman PA, Bakkers JT, Terwindt GM, Ferrari MD, Launer LJ: Migraine as a risk factor for subclinical brain lesions. Jama 2004,291(4):427–434. doi:10.1001/jama.291.4.427 291/4/427 10.1001/jama.291.4.427
Hung CI, Liu CY, Cheng YT, Wang SJ: Migraine: a missing link between somatic symptoms and major depressive disorder. J Affect Disord 2009,117(1–2):108–115. doi:10.1016/j.jad.2008.12.015 S0165–0327(08)00490
Chiapparini L, Ferraro S, Grazzi L, Bussone G: Neuroimaging in chronic migraine. Neurol Sci 2010,31(Suppl 1):S19–22. doi:10.1007/s10072–010–0266–9
May A: New insights into headache: an update on functional and structural imaging findings. Nat Rev Neurol 2009,5(4):199–209. doi:10.1038/nrneurol.2009.28 nrneurol.2009.28 10.1038/nrneurol.2009.28
Sanchez Del Rio M, Alvarez Linera J: Functional neuroimaging of headaches. Lancet Neurol 2004,3(11):645–651. doi:S1474442204009044 10.1016/S1474–4422(04)00904–4 10.1016/S1474-4422(04)00904-4
Aderjan D, Stankewitz A, May A: Neuronal mechanisms during repetitive trigemino-nociceptive stimulation in migraine patients. Pain 2010,151(1):97–103. doi:S0304–3959(10)00386–6 10.1016/j.pain.2010.06.024 10.1016/j.pain.2010.06.024
Moulton EA, Becerra L, Maleki N, Pendse G, Tully S, Hargreaves R, Burstein R, Borsook D: Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex 2011,21(2):435–448. doi:bhq109 10.1093/cercor/bhq109 10.1093/cercor/bhq109
Yu D, Yuan K, Zhao L, Dong M, Liu P, Wang G, Liu J, Sun J, Zhou G, von Deneen KM, Liang F, Qin W, Tian J: Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR Biomed 2012,25(5):806–812. doi:10.1002/nbm.1796 10.1002/nbm.1796
Jin C, Yuan K, Zhao L, Yu D, von Deneen KM, Zhang M, Qin W, Sun W, Tian J: Structural and functional abnormalities in migraine patients without aura. NMR Biomed 2012. doi:10.1002/nbm.2819
Mainero C, Boshyan J, Hadjikhani N: Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 2011,70(5):838–845. doi:10.1002/ana.22537 10.1002/ana.22537
Liu J, Zhao L, Li G, Xiong S, Nan J, Li J, Yuan K, von Deneen KM, Liang F, Qin W, Tian J: Hierarchical alteration of brain structural and functional networks in female migraine sufferers. PLoS One 2012,7(12):e51250. doi:10.1371/journal.pone.0051250 PONE-D-12–16392 10.1371/journal.pone.0051250
Valfre W, Rainero I, Bergui M, Pinessi L: Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 2008,48(1):109–117. doi:HED723 10.1111/j.1526–4610.2007.00723.x
Schmitz N, Admiraal Behloul F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, Van Buchem MA: Attack frequency and disease duration as indicators for brain damage in migraine. Headache 2008,48(7):1044–1055. doi:HED1133 10.1111/j.1526–4610.2008.01133.x 10.1111/j.1526-4610.2008.01133.x
Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, Bernasconi L, Comi G, Scotti G, Filippi M: Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke 2006,37(7):1765–1770. doi:01.STR.0000226589.00599.4d 10.1161/01.STR.0000226589.00599.4d 10.1161/01.STR.0000226589.00599.4d
Eck J, Richter M, Straube T, Miltner WH, Weiss T: Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain 2011,152(5):1104–1113. doi:S0304–3959(11)00033–9 10.1016/j.pain.2011.01.026 10.1016/j.pain.2011.01.026
Peyron R, Laurent B, Garcia Larrea L: Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin 2000,30(5):263–288. doi:S0987–7053(00)00227–6 10.1016/S0987-7053(00)00227-6
Maleki N, Becerra L, Nutile L, Pendse G, Brawn J, Bigal M, Burstein R, Borsook D: Migraine attacks the Basal Ganglia. Mol Pain 2011, 7: 71. doi:1744–8069–7-71 10.1186/1744–8069–7-71 10.1186/1744-8069-7-71
Lipton RB, Pan J: Is migraine a progressive brain disease? Jama 2004,291(4):493–494. doi:10.1001/jama.291.4.493 291/4/493 10.1001/jama.291.4.493
Kruit MC, Van Buchem MA, Launer LJ, Terwindt GM, Ferrari MD: Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Cephalalgia 2010,30(2):129–136. doi:CHA1904 10.1111/j.1468–2982.2009.01904.x
Xue T, Yuan K, Zhao L, Yu D, Dong T, Cheng P, von Deneen KM, Qin W, Tian J: Intrinsic Brain Network Abnormalities in Migraines without Aura Revealed in Resting-State fMRI. PLoS One 2012,7(12):e52927. doi:10.1371/journal.pone.0052927 PONE-D-12–26054 10.1371/journal.pone.0052927
Zang Y, Jiang T, Lu Y, He Y, Tian L: Regional homogeneity approach to fMRI data analysis. Neuroimage 2004,22(1):394–400. doi:10.1016/j.neuroimage.2003.12.030 S1053811904000035 10.1016/j.neuroimage.2003.12.030
Headache Classification Subcommittee of the international Headache Society: The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004,1(24):9–160.
Tfelt Hansen P, Block G, Dahlof C, Diener HC, Ferrari MD, Goadsby PJ, Guidetti V, Jones B, Lipton RB, Massiou H, Meinert C, Sandrini G, Steiner T, Winter PB: Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000,20(9):765–786. doi:cha117
Kendall M, Gibbons J: Rank Correlation Methods. Oxford: Oxford University Press; 1990.
Zuo XN, Xu T, Jiang L, Yang Z, Cao XY, He Y, Zang YF, Castellanos FX, Milham MP: Toward reliable characterization of functional homogeneity in the human brain: preprocessing, scan duration, imaging resolution and computational space. Neuroimage 2013, 65: 374–386. doi:10.1016/j.neuroimage.2012.10.017 S1053–8119(12)01020–8
Kim JH, Suh SI, Seol HY, Oh K, Seo WK, Yu SW, Park KW, Koh SB: Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia 2008,28(6):598–604. doi:CHA1550 10.1111/j.1468–2982.2008.01550.x 10.1111/j.1468-2982.2008.01550.x
Schmidt Wilcke T, Ganssbauer S, Neuner T, Bogdahn U, May A: Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia 2008,28(1):1–4. doi:CHA1428 10.1111/j.1468–2982.2007.01428.x
Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC: Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain. J Neurosci 2007,27(15):4004–4007. doi:27/15/4004 10.1523/JNEUROSCI.0098–07.2007 10.1523/JNEUROSCI.0098-07.2007
Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE: Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology 2008,70(2):153–154. doi:01.wnl.0000295509.30630.10 10.1212/01.wnl.0000295509.30630.10
Schmidt Wilcke T, Leinisch E, Straube A, Kampfe N, Draganski B, Diener HC, Bogdahn U, May A: Gray matter decrease in patients with chronic tension type headache. Neurology 2005,65(9):1483–1486. doi:65/9/1483 10.1212/01.wnl.0000183067.94400.80 10.1212/01.wnl.0000183067.94400.80
Kim JH, Kim S, Suh SI, Koh SB, Park KW, Oh K: Interictal metabolic changes in episodic migraine: a voxel-based FDG-PET study. Cephalalgia 2010,30(1):53–61. doi:30/1/53 10.1111/j.1468–2982.2009.01890.x
Prescot A, Becerra L, Pendse G, Tully S, Jensen E, Hargreaves R, Renshaw P, Burstein R, Borsook D: Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol Pain 2009, 5: 34. doi:1744–8069–5-34 10.1186/1744–8069–5-34 10.1186/1744-8069-5-34
May A: Chronic pain may change the structure of the brain. Pain 2008,137(1):7–15. doi:S0304–3959(08)00128–0 10.1016/j.pain.2008.02.034 10.1016/j.pain.2008.02.034
Isnard J, Magnin M, Jung J, Mauguiere F, Garcia-Larrea L: Does the insula tell our brain that we are in pain? Pain 2011,152(4):946–951. doi:10.1016/j.pain.2010.12.025 S0304–3959(10)00775-X 10.1016/j.pain.2010.12.025
Duerden EG, Albanese MC: Localization of pain-related brain activation: A meta-analysis of neuroimaging data. Hum Brain Mapp 2011. doi:10.1002/hbm.21416
Coghill RC, Sang CN, Maisog JM, Iadarola MJ: Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol 1999,82(4):1934–1943.
Afridi SK, Giffin NJ, Kaube H, Friston KJ, Ward NS, Frackowiak RS, Goadsby PJ: A positron emission tomographic study in spontaneous migraine. Arch Neurol 2005,62(8):1270–1275. doi:62/8/1270 10.1001/archneur.62.8.1270 10.1001/archneur.62.8.1270
Kobari M, Meyer JS, Ichijo M, Imai A, Oravez WT: Hyperperfusion of cerebral cortex, thalamus and basal ganglia during spontaneously occurring migraine headaches. Headache 1989,29(5):282–289. 10.1111/j.1526-4610.1989.hed2905282.x
Gu T, Ma XX, Xu YH, Xiu JJ, Li CF: Metabolite concentration ratios in thalami of patients with migraine and trigeminal neuralgia measured with 1H-MRS. Neurol Res 2008,30(3):229–233. doi:10.1179/016164107X235473 10.1179/016164107X235473
May A: Neuroimaging: visualising the brain in pain. Neurol Sci 2007,28(Suppl 2):S101–107. doi:10.1007/s10072–007–0760-x
Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, Coenen HH, Diener HC: Brain stem activation in spontaneous human migraine attacks. Nat Med 1995,1(7):658–660. 10.1038/nm0795-658
Bahra A, Matharu MS, Buchel C, Frackowiak RS, Goadsby PJ: Brainstem activation specific to migraine headache. Lancet 2001,357(9261):1016–1017. doi:S0140673600042501 10.1016/S0140-6736(00)04250-1
Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ: A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 2005,128(Pt 4):932–939. doi:awh416 10.1093/brain/awh416
Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D: Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One 2008,3(11):e3799. doi:10.1371/journal.pone.0003799 10.1371/journal.pone.0003799
Cao Y, Aurora SK, Nagesh V, Patel SC, Welch KM: Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology 2002,59(1):72–78. 10.1212/WNL.59.1.72
Stankewitz A, May A: Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology 2011,77(5):476–482. doi:WNL.0b013e318227e4a8 10.1212/WNL.0b013e318227e4a8 10.1212/WNL.0b013e318227e4a8
Loggia ML, Edwards RR, Kim J, Vangel MG, Wasan AD, Gollub RL, Harris RE, Park K, Napadow V: Disentangling linear and nonlinear brain responses to evoked deep tissue pain. Pain 2012,153(10):2140–2151. doi:10.1016/j.pain.2012.07.014 S0304–3959(12)00425–3 10.1016/j.pain.2012.07.014
Calandre EP, Bembibre J, Arnedo ML, Becerra D: Cognitive disturbances and regional cerebral blood flow abnormalities in migraine patients: their relationship with the clinical manifestations of the illness. Cephalalgia 2002,22(4):291–302. doi:370 10.1046/j.1468-2982.2002.00370.x
Suhr JA, Seng EK: Neuropsychological functioning in migraine: clinical and research implications. Cephalalgia 2012,32(1):39–54. doi:0333102411430265 10.1177/0333102411430265 10.1177/0333102411430265
Koppen H, Palm Meinders I, Kruit M, Lim V, Nugroho A, Westhof I, Terwindt G, Van Buchem M, Ferrari M, Hommel B: The impact of a migraine attack and its after-effects on perceptual organization, attention, and working memory. Cephalalgia 2011,31(14):141–1427. doi:0333102411417900 10.1177/0333102411417900
Demarquay G, Royet JP, Mick G, Ryvlin P: Olfactory hypersensitivity in migraineurs: a H(2)(15)O-PET study. Cephalalgia 2008,28(10):1069. doi:CHA1672 10.1111/j.1468–2982.2008.01672.x 10.1111/j.1468-2982.2008.01672.x
Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G: Posterior cerebral hypoperfusion in migraine without aura. Cephalalgia 2008,28(8):856–862. doi:10.1111/j.1468–2982.2008.01623.x CHA1623 10.1111/j.1468-2982.2008.01623.x
Woods RP, Iacoboni M, Mazziotta JC: Brief report: bilateral spreading cerebral hypoperfusion during spontaneous migraine headache. N Engl J Med 1994,331(25):1689–1692. doi:10.1056/NEJM199412223312505 10.1056/NEJM199412223312505
Acknowledgements
This study was supported by the National Basic Research Program of China (973 Program, No. 2012CB518501), National Natural Science Foundation of China (Nos. 30930112, 30901900, 81001483), the Project of Administration of Traditional Chinese Medicine of Sichuan Province (No.2012-E-038).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no conflicts of interest or financial disclosures.
Authors’ contributions
LZ: conceived, designed, performed the experiment, and drafted the manuscript. JXL: analyzed the data and drafted the manuscript. XLD, YLP, and FMW: performed the experiments. KY and JBS: contributed to the acquisition of fMRI data and analyzed them. QYG: revised the manuscript. WQ and FRL: conceived, designed the experiments and revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript. Ling Zhao and Jixin Liu contributed equally to this article.
Ling Zhao, Jixin Liu contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zhao, L., Liu, J., Dong, X. et al. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J Headache Pain 14, 85 (2013). https://doi.org/10.1186/1129-2377-14-85
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1129-2377-14-85