Abstract
Objective
To examine biochemical differences in the anterior cingulate cortex (ACC) and insula during the interictal phase of migraine patients. We hypothesized that there may be differences in levels of excitatory amino acid neurotransmitters and/or their derivatives in migraine group based on their increased sensitivity to pain.
Methods
2D J-resolved proton magnetic resonance spectroscopy (1H-MRS) data were acquired at 4.0 Tesla (T) from the ACC and insula in 10 migraine patients (7 women, 3 men, age 43 ± 11 years) and 8 age gender matched controls (7 women, 3 men, age 41 ± 9 years).
Results
Standard statistical analyses including analysis of variance (ANOVA) showed no significant metabolite differences between the two subject cohorts in the ACC nor the insula. However, linear discriminant analysis (LDA) introduced a clear separation between subject cohorts based on N-acetyl aspartylglutamate (NAAG) and glutamine (Gln) in the ACC and insula.
Conclusion
These results are consistent with glutamatergic abnormalities in the ACC and insula in migraine patients during their interictal period compared to healthy controls. An alteration in excitatory amino acid neurotransmitters and their derivatives may be a contributing factor for migraineurs for a decrease in sensitivity for migraine or a consequence of the chronic migraine state. Such findings, if extrapolated to other regions of the brain would offer new opportunities to modulate central system as interictal or preemptive medications in these patients.
Similar content being viewed by others
Introduction
Migraine is a neurobiologic disorder that affects about 27 million women and 10 million men in the US [1]. Migraine attacks manifest themselves from childhood (usually 8–12 yrs.) to old age, with a decline among women during the postmenopausal years. Migraine is a unilateral throbbing headache that lasts 4–72 hours; it is idiopathic, episodic and recurrent [2]. Although the causes of migraine are unknown, it is generally thought that the pain originates from chemical activation of sensory nerves that supply intracranial blood vessels and the meninges [3]. However, the long-term consequences of repeated intermittent attacks of acute migraine on brain function, whatever the origin of the syndrome is, are not well defined.
Two major unanswered questions in the field of migraine relate to (1) Is there an underlying basis for the increased sensitivity to various stimuli of the migraine brain during [4, 5]and even between [6–8]acute attacks? and (2) What is the underlying basis for the recent evidence suggesting that migraine, may predispose to significant functional [9, 10]and structural changes [11–15]in the brain? One mechanism by which both of these changes may take place is through alterations in neurochemical systems in the brain that are augmented by the repeated acute attacks. Such changes may eventually drive the process on the evolution from acute migraine to chronic daily headaches [16] and also the resistance to drug therapy in the chronic daily headache group [17]. By using magnetic resonance spectroscopy, chemical changes in the brain can be measured in patients. Here we have begun to explore this issue by trying to define these changes during the interictal period in acute intermittent migraine patients for reasons discussed below. A definition of such chemical changes would provide a target for potential interictal therapies that may decrease the severity and/or frequency of migraine and provide a basis for evaluating changes that may take place in the transition to chronic migraine.
A number of recent reports suggest alterations in the interictal migraine brain based on changes in cerebral blood flow [18–20] as well as changes in interictal cognitive function in migraineurs with aura [21]. A wealth of evidence, including measurements demonstrating changes in physiological (i.e., evoked potentials) measures [22–24]), strongly supports the hypothesis of central neuronal hyperexcitability as playing a key role in the pathogenesis of migraine [25]. One potential mechanism for neuronal excitability includes an abnormality of the pre-synaptic release of excitatory amino acid neurotransmitters. Although increased platelet [26, 27] and plasma [27, 28] levels of neuroexcitatory amino acids including aspartate (Asp), glutamate (Glu), Gln and glycine (Gly) have been reported in migraine patients compared to healthy control subjects [29], these changes are not always good measures or indicators of changes of synaptic glutamate in the brain. In addition, cerebrospinal fluid (CSF) Gln, Gly and taurine (Tau) concentrations are elevated in migraineurs [30] suggesting glutamatergic systems are likely to be altered in the migraine brain. Indeed, given that glutamate is the main excitatory transmitter in the brain excess or under production of glutamate through injury or disease can have pathophysiological effects. The glutamate hypothesis for migraine has been discussed by Ramadan [31] and reviewed recently by Vikelis and Mitsokostkas [32]. Increased synaptic concentrations of excitatory amino acid neurotransmitters may lead to excessive activity at the N-methyl D-aspartate (NMDA) Glu receptor subtype, which may amplify and reinforce pain transmission in migraine and other types of headache. Indeed, low-affinity NMDA receptor (NMDAr) antagonists, such as memantine, have previously been shown to reduce frequency of migraine and tension-type headaches [33].A neuroimaging method capable of assessing potential glutamatergic imbalances in the migraine brain in vivo might provide key insights into the true nature of the neurochemical impairment and to monitor its modulation following pharmacotherapy.
1H-MRS is a potential candidate for investigating glutamate systems in vivo although its application to migraine is relatively sparse in the literature. Functional 1H-MRS studies have focused predominantly on changes in the visual cortex [34–36]. Other 1H-MRS studies have evaluated metabolite ratios in cluster headache in the hypothalamus and show that N-acetyl aspartate (NAA) to creatine (Cr) ratio is lower in patients with cluster headache vs. chronic migraine or controls [37]. In addition, single-voxel 1H-MRS studies have investigated potential cerebellar metabolite alterations demonstrating significantly decreased choline (Cho) levels in migraine patients compared to healthy controls [38]. A similar study detected decreased cerebellar NAA and Glu concentration and increased myo-inositol (mI) levels in familial hemiplegic migraine patients [39]. More recently, a 3.0 T 1H-MRS study reported differences in thalamic metabolite ratios in migraine patients compared to healthy controls [40].
Most of these earlier studies employed conventional 1H-MRS methodology at a low static magnetic field strength of 1.5 T and none reported changes in multiple brain regions. For the present study we evaluated brain chemistry using medium field 4.0 T 1H-MRS in two regions, the ACC and the insula. In addition, we employed a variant of single-voxel 2D J-resolved 1H-MRS method in an attempt to further enhance spectral resolution and sensitivity, and to provide access to the quantification of an increased number of metabolites [41–43]. The brain regions were selected as an initial focus on evaluating brain metabolites in migraine patients for a number of reasons. The ACC is involved in a number of behaviors [44–46] and is usually implicated in most pain studies [47, 48] including migraine [49] and related cognitive components [50]. With respect to the latter it is considered to be involved in reinforcement history [44] that may be relevant in repeated episodes of migraine. The region has been proposed as a model for understanding components of central sensitization of pain [51]. As for the insula, the region is involved in both pain [52] and emotional processing [53], including the unpleasantness of pain [54]. Given the nature of the regions in sensory and emotional processing, we hypothesized that differences in glutamatergic metabolism would be observed when comparing spectral data from patients and healthy controls.
Methods
Patients and Controls
The overall experimental approach is shown in Figure 1. The local Institutional Review Board of McLean Hospital approved this study (IRB), which met the criteria for investigations in human subjects based on the Helsinki Accord http://ohsr.od.nih.gov/guidelines/helsinki.html. Patients presenting with acute episodic migraine (AEM; n = 12) and healthy age (n = 8), gender matched controls (HC) were recruited for the study.
Data Acquisition
MRI Procedures
All measurements were performed at McLean Hospital on a Varian 4.0 T Varian Unity/INOVA whole body MRI scanner (Varian Inc., Palo Alto, CA, USA). A birdcage design radiofrequency (RF) head coil tuned to 170.3 MHz was used for RF transmission and signal reception. Three orthogonal gradient-recalled scout images were initially obtained to ensure optimal head positioning within the coil (TR/TE = 30/10 ms, field-of-view = 24 × 24 cm, matrix = 256 × 128, slice thickness = 5 mm). Manual global shimming subsequently was performed until the unsuppressed water resonance linewidth was ≤ 25 Hz. High-contrast, 3D mpFLASH (magnetization-prepared, fast, low-angle shot) T1-weighted axial, coronal and sagital MR images (TE/TR = 6.2/11.4 ms, FOV = 24 × 24 × 8 cm, in-plane resolution = 0.94 × 1.88 mm, slice thickness = 5 mm, readout points = 512, flip angle = 11°) then were acquired to more accurately delineate brain substructures and to enable more accurate positioning of the spectroscopy voxel within the region-of-interest (ROI).
MRS Procedures
For ACC 1H-MRS measurements, a cubic 8 cm3 voxel was positioned within the predominantly midline gray matter of the ACC (see Figure 2(a)). For insula measurements, a cubic 8 cm3 voxel was positioned contralateral to the side of the subjects headache (see Figure 2(b)). Automated routines initially were used to optimize spatial localization RF pulse flip angles for each ROI, and localized manual shimming subsequently was applied to until the full width at half maximum of the unsuppressed water-water peak was ≤ 12 Hz. Water-water suppression was achieved using a four-pulse WET module [55]; A modified PRESS sequence was used for 2D J-resolved 1H-MRS acquisitions, which utilized numerically optimized sinc RF waveforms for excitation (duration = 3.0 ms; BW = 2850 Hz) and refocusing (duration = 6.0 ms; BW = 1145 Hz). 2D J-resolved 1H-MRS spectra were acquired from each ROI using the following acquisition parameters (TR = 2000 ms, TE range = 30 – 260 ms, ΔTE = 10 ms, NEX = 16 per TE, dummy scans = 4, 1024 complex points). Data processing was performed offline with each of the 24 sub-TEs being stored separately for preprocessing purposes. The total measurement time for each 4.0 T study including MRI, shimming and 1H-MRS procedures was approximately one hour.
Data Processing
Spectral Analyses
The 2D J-resolved time-domain data were transferred to a personal computer and preliminary data pre-processing and formatting steps performed using home-written code written in C programming language. The processing specifics were as follows. A raw 2D data matrix was constructed and zero-filled to yield a 2048 (F2) × 64 (F1) matrix and a fast Fourier transformation subsequently was applied to the 2048 column vectors along the F1 dimension. The 64 row vectors then were extracted from the resulting interferrogram and each row was converted to Linear Combination Model (LCModel) format for spectral fitting and measurement of metabolite peak integrals [56]; version 6.0-1). For spectral fitting with LCModel, we utilized GAMMA-simulated [57] theoretical basis sets for alanine (Ala), aspartate (Asp), choline (Cho), creatine (Cr), N-acetyl-aspartate (NAA), N-acetyl-aspartyl-glutamate (NAAG), γ-aminobutyric-acid (GABA),, glutamine (Gln), glutamate (Glu), glutathione (GSH), glycerophosphocholine (GPC), glycine (Gly), lactate (Lac), myo-inositol (mI), phosphocholine (PC), phosphocreatine (PCr), scyllo-inositol (sI), serine (Ser), and taurine (Tau). We used GAMMA to generate 24 theoretical, TE-stepped spectra ranging from 30 ms to 260 ms in 10 ms increments, and each GAMMA spectrum was modeled with a 2 kHz spectral bandwidth, 1024 complex pairs and a 2 Hz Lorentzian lineshape. Each of the 24 spectra were zero and first-order phased with no baseline roll. A formate peak at 8.45 ppm and a 3-(trimethylsilyl)-1-propane-sulfonic acid (TSPS) reference peak at 0.0 ppm were also modeled in order to mimic the standard LCModel stock solution required for basis-set generation [58]. For each GAMMA-simulated metabolite TE-series, we zero-filled each complex time-point in each FID out to 128 TE points and apodized with an exponential filter to approximate the metabolite T2-decay at 4.0 T. These TE-specific metabolite FIDs were converted into separate LCModel basis sets for each metabolite thus providing a means to quantify the whole 2D surface of the in vivo datasets [59]. The LCModel/GAMMA-derived 2D surface metabolite integrals were normalized to the total Cr integral. 2D J-resolved 1H-MRS-derived ACC metabolite:Cr ratio reliability indices were taken from a previous study [59], which employed the identical sequence and similar parameters to that described for the present study. In brief, ten healthy adult subjects (5 males and 5 females; ages 18–35) were scanned three times in a one week period, except for two females whose third scan was 4 weeks later at the same phase of the menstrual cycle. Within-subject coefficients of variation (CV) were calculated as the standard deviation/mean of the three scans.
Image and Voxel Segmentation
All image analyses were performed using the freely-available FMRIB Software Library (FSL; for an overview see [60]). Tissue segmentation of the 3D T1-weighted FLASH MR images into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) used FSL's fast automated segmentation tool (FAST; [61]). The ACC and insula 8-cm3 voxels subsequently were extracted from segmented images using the FSL 'fslroi' tool, and voxel GM, WM and CSF fractions were determined from the generated image histogram.
Assessment of motion effects during 1H MRS
The duration of each TE-averaged 1H-MRS acquisition was 13 minutes for each brain region and subject/head motion throughout the measurement was a potential confound between datasets and subject cohorts. Motion effects would lead to modification of the local B0 field over the ROI potentially leading to modulation of peak linewidth throughout the 1H MRS scan. To evaluate motion effects we measured the NAA 2.01 ppm methyl proton signal linewidth for each of the 24 TE steps across all subjects for both brain regions. The intrasubject mean NAA peak linewidth then was calculated for both the ACC and insula and the group mean FWHM values were statistically compared (unpaired t-test). In addition, the intrasubject NAA peak linewidth CV was calculated using all 24 TE steps for both brain regions, and the group mean CV values were evaluated (unpaired t-test).
Statistical Analyses
Standard statistical analyses including ANOVA were performed using Origin version 7.5 (OriginLab Corp., Northampton, MA, USA). In addition LDA was used to evaluate separability of the two subject cohorts (migraine and control) using metabolite measurements across the two groups in each ROI. This method enables multivariate extraction of differences between groups. Home-written scripts were used for performing LDA analyses (MATLAB, The Mathworks, Natick, MA, USA). For each ROI a set of two metabolites were selected that had the best group discrimination ability via a stepwise forward search using the hotelling T2 statistic. We restricted our search to only two responses so as to enable robust estimation of the LDA covariance.
Results
Patients and Controls
As noted in Table 1, patients recruited all had migraine and were compared with age-gender matched controls. Of the 12 patients recruited, 1 dataset could not be used owing to poor spectral quality. A second patient had also been taking methylene sulfonyl methane (MSM) supplements and a large-amplitude MSM-specific resonance was observed at 3.1 ppm. Data from that subject was also excluded from the final analyses.
MRI/MRS
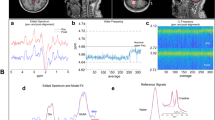
Figure 2 shows the high-resolution axial MR images recorded from a 32 year-old female migraine patient and displays positioning of the spectroscopy voxel within the (a) ACC and (b) left insula. The percentage (mean ± SD) of GM in ACC and insula was 69 ± 8% and 56 ± 5% (HC), and 72 ± 6% and 59 ± 7% (AEM), respectively. The percentage (mean ± SD) of GM in ACC and insula was 69 ± 8% and 56 ± 5% (HC), and 72 ± 6% and 59 ± 7% (AEM), respectively. Similarly, the percentage (mean ± SD) of WM in ACC and insula was 16 ± 6% and 37 ± 5% (HC), and 15 ± 6% and 33 ± 5% (AEM), respectively. For both brain regions, standard ANOVA showed that at the P < 0.05 level no significant differences in tissue content existed between the two groups. However, the WM fraction was significantly higher in the insula voxel compared to corresponding ACC voxel for both subject cohorts (ANOVA: P < 0.01).
For the control group, the FWHMs (mean ± SD) measured for the unsuppressed water resonance were 9.75 ± 1.6 and 9.25 ± 1.0 Hz for the ACC and insula voxels, respectively. For the migraine group, the FWHMs (mean ± SD) measured for the unsuppressed water resonance were 10.2 ± 2.0 and 10.0 ± 1.1 Hz for the ACC and insula voxels, respectively. Figure 3(a) shows a 2D J-resolved 1H-MRS representation of data acquired from the left insula in the same subject. Figure 3(b) then shows a 1H-MR spectrum (labeled 'Raw spectral data'), which was produced by extracting a single row from the 2D J-resolved spectrum at F1 = 0 HzThe scaled LCModel fits for thirteen individual metabolites are displayed at the bottom of Figure 3(b) and each metabolite fit is superimposed on a baseline calculated by the LCModel software. Lipid contamination is once again observed at and around 1.4 ppm although the broad peak has been treated as a macromolecular resonance and fitted by LCModel. The 1D and 2D 1H-MRS data presented in Figure 3 is representative of the spectra acquired from all brain regions in both subject cohorts. The above information indicates high spectral quality and therefore a basis for robust quantification via LCModel.
(a) Left Panel: 2D J -resolved 1 H-MR spectrum recorded from the voxel shown in figure 2b. Only the large singlet resonances are labeled at F1 = 0 Hz although information from J-coupled metabolites is spread over the 2D surface. The 2D spectrum is presented in magnitude mode for presentation purposes, and is characterized by two orthogonal frequency axes: chemical shift (F2) and J-coupling (F1) dimensions. Exponential apodization was applied along F2 (line broadening = 3 Hz) whereas a sinebell-squared apodization filter (30° phase-shifted) was applied along the F1 axis. The chemical shift axis has been expanded to show the 0.6 – 4.4 ppm region whereas the full J-coupling dimension (± 50 Hz) is presented. The dominating singlet resonances corresponding to the NAA, total Cr and total Cho methyl groups are labeled and reside perpendicular to F1 = 0 Hz (solid black line) at 2.0, 3.0 and 3.2 ppm, respectively. Lipid signals that probably arise due to chemical shift displacement are identified at and around 1.4 ppm (F1 = 0 Hz) and the total Cr methylene resonance is observed at 3.9 ppm. (b) Right Panel: LCModel analysis of a single row extracted from F1 = 0 Hz. The raw data, LCModel fit, residual and individual metabolite fits are displayed. It is important to note that the 1D 1H-MR spectrum is real data, which is the data type required for LCModel analysis. The LCModel fit (solid red line) for the F1 = 0 Hz extraction is overlaid on the raw data and the residual (LCModel fit minus raw data) is displayed at the top of the figure. The residual is free from large subtraction artifacts and clearly illustrates the high-quality spectral fit achieved using the described quantification methods. Data extracted from the center of the F1 dimension in this manner is entirely equivalent to signal averaging all 24 TE steps, a procedure often referred to as TE-averaging. TE-averaged 1H-MRS spectra typically show the large singlet methyl (CH3) resonances of NAA, total Cr and total Cho with significant attenuation of resonances J-coupled metabolite species. (see text for more details).
Assessment of Motion Effects during 1H MRS
Table 2 shows the group averaged NAA peak linewidth and CV data for the ACC and insula MRS voxels in the control and migraine patient populations. Note that the group mean FWHM values appear higher than those provided earlier for the unsuppressed water resonance, an observation that is likely due to signal contribution from other metabolite resonances including Gln, Glu, GABA and NAAG to the NAA 2.01 ppm resonance, which increases the 'raw' NAA signal linewidth particularly for the lower TE values. For each subject, the mean NAA peak FWHM and linewidth CV calculations were computed using all 24 TE steps (see methods section for more details). It is clear from Table 2 that no statistically significant differences in NAA signal FWHM or its CV were detected for both brain regions between the patient populations, inferring that any existing motion effects were comparable between groups.
Statistical Analyses
Standard statistical analyses showed no significant metabolite differences between the two subject cohorts. However, by using LDA, we show that we can separate patients vs. controls as shown for the ACC and insula in figures 4(a) and 4(b), respectively The classification is achieved using the metabolites NAAG and Gln for both brain regions, and the within-subject CV's for these particular metabolites were 23 and 21%, respectively. For each ROI, we also assessed the statistical accuracy of the estimated LDA coefficients using a bootstrap calculation [62]. The sampling unit for bootstrap included the set of metabolite measurements and a group label (migraine/control) sampled with replacement from the original data. We fitted the LDA model to each bootstrap data set and calculated a distribution of the LDA coefficient value for each metabolite. This distribution was then used to estimate the probability of LDA coefficient values being positive or negative. Small values of the coefficient being positive indicate a statistically significant negative coefficient and vice versa. Figures 4(c) and 4(d) show the bootstrap calculations for the ACC and insula, respectively. Since LDA allows for the simultaneous comparison of observables the approach allows us to utilize brain metabolite ratio's to establish differences between cohorts i.e., migraine and healthy controls.
Linear Discriminant Analysis (LDA). (a) The figure depicts 2 metabolite ratios (e.g., NAAG/Cr and Glu/Cr) that separate controls from migraineurs through the black line in the cingulate. (b) Similar data is shown for the insula. (c) and (d) show a bootstrap calculation for assessment of statistical accuracy of the estimated LDA coefficients for the ACC and insula, respectively.
Discussion
Here we report novel differences in 1H-MRS defined levels of metabolites in the ACC and insula measured in the interictal period of migraine patients. Although conventional descriptive statistics yielded no differences on the analysis of the spectra, a LDA demonstrated significant differences between migraine subjects and age-gender matched controls. This type of analysis allows for the determination of discrimination of two or more groups (e.g., migraine vs. healthy) based on Cr-normalized levels of specific metabolites. This analysis separated out a relationship between NAAG and Gln within the ACC and insula during their interictal period. NAAG is the most abundant peptide neurotransmitter in the mammalian CNS [63] being synthesized exclusively in neurons from NAA and Glu by NAAG synthetase. In addition to its role as a neurotransmitter, NAAG is a source of Glu [64] and like NAA is thought to play a role as a major osmolyte in the vertebrate brain [65, 66]. Glutamine on the other hand is synthesized exclusively in glial cells from Glu and ammonia by the enzyme glutamine synthetase. Subsequently, Gln is released back into the extracellular space, shuttled back into neurons and converted to Glu by glutaminase. The Glu that is regenerated may then go on to play a direct role in excitatory neurotransmission, packed and stored in vesicles or incorporated into NAAG. An intriguing observation in the present study is the LDA-detected classification of migraine patients and control subjects for two different brain regions based on NAAG and Gln, which are closely linked by this excitatory neurotransmitter system. Interestingly, the ACC and insula LDA plots show oppositely signed gradients, an observation that might be explained by (i) the significant tissue type differences within ACC and insula voxels detected by image segmentation and (ii) the known uneven distribution of Gln and NAAG throughout the brain and within brain tissue type [67]. The measured changes in these excitatory amino acid neurotransmitters (NAAG) and related species (Gln) provide some insights into altered central nervous system (CNS) mechanisms in migraine and may contribute to abnormal CNS processing including changes during the migraine state (e.g., process of central sensitization [68, 69], progressing from acute episodic to chronic/daily migraine [70] or abnormalities during the interictal period [71–74]. We did not detect direct differences in Glu levels between controls and migraine patients although preferential storage of excess synaptic Glu in the form of Gln and/or NAAG might explain comparable Glu levels within the two cohorts. Note that a previous 1H-MRS study showed decreased cerebellar Glu levels in migraine patients compared to healthy controls [39] yet similar cortical 1H-MRS findings have not been reported to date.
A growing body of preclinical and clinical data supports the notion of aminergic dysfunction in migraine headache including alterations in both the glutamatergic and glutaminergic systems [31, 32]. For example, NMDA receptor antagonists inhibit cortical spreading depression in the rat brain [75]. Cerebrospinal fluid [76] and plasma [27] Glu and Gln levels are increased in chronic migraine patients, although no such data is available for episodic migraine (i.e. our population). It has been postulated that increased brain Glu leads to cortical hyperexcitability typical of migraine [77, 78] and potential pharmacological targets for migraine therapy include the ionotropic (NMDA, AMPA and kainate) and metabotropic glutamate receptor antagonists [79]. The use of a tridimensional personality questionnaire in migraine and tension-type headache clinical sub-populations has shown that glutaminergic dysfunction might also be a specific feature associated with migraine headache [80]. The development of novel pharmaceutics that can modulate the glutaminergic system and block central and peripheral sensitization might be efficacious for treating migraine. It is also worth noting that, although little is known in the literature for a potential role of NAAG in migraine, there may be a potential role for NAAG antagonists (via mGluR3 receptor blockade) for the therapy of migraine.
A number of reports indicate that modulation of the glutamatergic system in the ACC takes place following pharmacological or sensory manipulation. Alterations in ACC neurons may be dependent on prior events that change or modulate neuronal activity. For example, drugs may decrease levels of glutamate in the ACC [81] and excitatory synapses into the ACC are in part NMDA mediated changes in this region [82]. In addition, amputation of a hind paw digit in rats results in a loss of activity-dependent long-term depression in the ACC [83] and potentiation of sensory responses [84]. NMDA receptors in the ACC mediates pain-related aversion [85]. Thus, in migraine patients either as a result of intermittent pain or medications, ACC glutamatergic impairment would account for an increase in activation in this region. In data from another report we observe increased sensitivity in the descending modulatory systems in the brainstem in interictal migraine patients vs. controls [86]. In functional imaging studies of pain, activation in the insula is observed and it has been suggested that the region has important contributions to both pain and emotional processing [87, 88]. However, 1H-MRS detected changes in this region in the interictal period have not been reported.
For the present study, we chose to use a 3D localized variant of J-resolved 1H-MRS, a method that has been shown to enhance spectral resolution at several field strengths including 1.5 T [42, 43], 3.0 T [89] and 4.0 T [90]. Increased spectral resolution is achieved as J-coupled metabolite resonances are effectively spread over a 2D surface whereas uncoupled peaks remain along F1 = 0 Hz. Glutamine contains a single methine (CH; 3.75 ppm) and two methylene (CH2; 2.1 and 2.4 ppm) groups and each proton resonance is split owing to J-coupling effects [91]. It follows that, for 2D J-resolved 1H-MRS data, glutamine shows multiple proton resonances across the 2D surface. In combination with LCModel fitting and GAMMA-simulated basis sets, we use information from the whole 2D datasets and this approach further improves multiple-metabolite quantification of 2D 1H-MRS data. Recently we applied these methods in vivo and demonstrated their utility for reliably measuring brain glutamate and glutamine levels [59]. NAAG, a dipeptide composed of NAA and Glu joined by a peptide bond, also benefits from the 2D 1H-MRS approach. The major resonance of NAAG is its CH3 resonance at 2.04 ppm that appears as a shoulder on the dominating NAA CH3 2.0 ppm peak. In conventional 1H-MR spectra, this chemical shift region is further complicated by underlying J-coupled resonances of Gln, Glu and GABA, and a major advantage of 2D J-resolved data is the fact these J- coupled metabolite resonances are shifted away from the F1 = 0 Hz axis. This yields a cleaner chemical shift region that is essentially comprised of NAA and NAAG CH3 singlet peaks, both of which are more reliably fitted by the described LCmodel template and fitting procedures.
It is certainly true that MRS studies are limited by the relatively low SNR of the spectra and some studies of chronic pain patients have noted larger between group differences. However, the methods that were employed in the present study were designed to allow the detection and quantitation of a larger number of lower concentration metabolites. This has to make observations that would not have been possible using more standard methods. In addition the interictal migraine group may differ from the chronic pain state in that it produces prolonged and continuous brain changes that manifest in profound structural [15] and functional changes [92, 93].
Conclusion
Our results have examined changes in biochemical concentrations in two brain regions in which we report some abnormal relation between a pair of peaks in only two brain regions. Nevertheless, the approach may be used for determining an underlying alteration in the biochemical dysfunction in this group of migraine patients would allow for specific therapeutic interventions that may normalize these changes in the interictal period rather than current approaches of treating migraine during the ictal period [94, 95].
References
Stewart WF, Lipton RB, Celentano DD, Reed ML: Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. Jama 1992, 267: 64–69. 10.1001/jama.267.1.64
Society IH: Headache Classification Comimittee of the International Headache Society: Classification and diagnosis criteria for headache disorders, cranial neuralgia and facial pain. Cephalagia 1997, 18: 43–110.
Moskowitz MA, Henrikson BM, Markowiz S, Saito K: Intra- and extracraniovascular nociceptive mechanisms and the pathogenesis of head pain. Amsterdam: Elsevier; 1988.
Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH: An association between migraine and cutaneous allodynia. Annals of neurology 2000, 47: 614–624. Publisher Full Text 10.1002/1531-8249(200005)47:5<614::AID-ANA9>3.0.CO;2-N
Irimia P, Cittadini E, Paemeleire K, Cohen AS, Goadsby PJ: Unilateral photophobia or phonophobia in migraine compared with trigeminal autonomic cephalalgias. Cephalalgia 2008, 28: 626–630. 10.1111/j.1468-2982.2008.01565.x
Amery WK, Waelkens J, Vandenbergh V: The sensorium of the migraineur. Italian journal of neurological sciences 1988, 9: 539–545.
Weissman-Fogel I, Sprecher E, Granovsky Y, Yarnitsky D: Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain 2003, 104: 693–700. 10.1016/S0304-3959(03)00159-3
Shibata K, Yamane K, Otuka K, Iwata M: Abnormal visual processing in migraine with aura: a study of steady-state visual evoked potentials. Journal of the neurological sciences 2008, 271: 119–126. 10.1016/j.jns.2008.04.004
Cohen AS, Goadsby PJ: Functional neuroimaging of primary headache disorders. Expert review of neurotherapeutics 2006, 6: 1159–1171. 10.1586/14737175.6.8.1159
Goadsby PJ: The migrainous brain: what you see is not all you get? PLoS medicine 2006, 3: e404. 10.1371/journal.pmed.0030404
May A, Ashburner J, Buchel C, McGonigle DJ, Friston KJ, Frackowiak RS, Goadsby PJ: Correlation between structural and functional changes in brain in an idiopathic headache syndrome. Nature medicine 1999, 5: 836–838. 10.1038/10561
Rocca MA, Colombo B, Pagani E, Falini A, Codella M, Scotti G, Comi G, Filippi M: Evidence for cortical functional changes in patients with migraine and white matter abnormalities on conventional and diffusion tensor magnetic resonance imaging. Stroke; a journal of cerebral circulation 2003, 34: 665–670.
Rocca MA, Ceccarelli A, Falini A, Tortorella P, Colombo B, Pagani E, Comi G, Scotti G, Filippi M: Diffusion tensor magnetic resonance imaging at 3.0 tesla shows subtle cerebral grey matter abnormalities in patients with migraine. Journal of neurology, neurosurgery, and psychiatry 2006, 77: 686–689. 10.1136/jnnp.2005.080002
DaSilva AF, Granziera C, Snyder J, Hadjikhani N: Thickening in the somatosensory cortex of patients with migraine. Neurology 2007, 69: 1990–1995. 10.1212/01.wnl.0000291618.32247.2d
DaSilva AF, Granziera C, Tuch DS, Snyder J, Vincent M, Hadjikhani N: Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport 2007, 18: 301–305. 10.1097/WNR.0b013e32801776bb
Cady RK, Schreiber CP, Farmer KU: Understanding the patient with migraine: the evolution from episodic headache to chronic neurologic disease. A proposed classification of patients with headache. Headache 2004, 44: 426–435. 10.1111/j.1526-4610.2004.04094.x
Biondi DM: Opioid resistance in chronic daily headache: a synthesis of ideas from the bench and bedside. Curr Pain Headache Rep. 2003,7(1):67–75. 10.1007/s11916-003-0013-5
Bartolini M, Baruffaldi R, Paolino I, Silvestrini M: Cerebral blood flow changes in the different phases of migraine. Funct Neurol 2005, 20: 209–211.
Shinoura N, Yamada R: Decreased vasoreactivity to right cerebral hemisphere pressure in migraine without aura: a near-infrared spectroscopy study. Clin Neurophysiol 2005, 116: 1280–1285. 10.1016/j.clinph.2005.01.016
Dora B, Balkan S: Exaggerated interictal cerebrovascular reactivity but normal blood flow velocities in migraine without aura. Cephalalgia 2002, 22: 288–290. 10.1046/j.1468-2982.2002.00365.x
Mulder EJ, Linssen WH, Passchier J, Orlebeke JF, de Geus EJ: Interictal and postictal cognitive changes in migraine. Cephalalgia 1999, 19: 557–565. 10.1046/j.1468-2982.1999.019006557.x
Ozkul Y, Uckardes A: Median nerve somatosensory evoked potentials in migraine. Eur J Neurol 2002, 9: 227–232. 10.1046/j.1468-1331.2002.00387.x
Schoenen J: Neurophysiological features of the migrainous brain. Neurol Sci 2006,27(Suppl 2):S77–81. 10.1007/s10072-006-0575-1
Afra J: Intensity dependence of auditory evoked cortical potentials in migraine. Changes in the peri-ictal period. Funct Neurol 2005, 20: 199–200.
Bussone G: Pathophysiology of migraine. Neurol Sci 2004,25(Suppl 3):S239–241. 10.1007/s10072-004-0295-3
D'Andrea G, Cananzi AR, Joseph R, Morra M, Zamberlan F, Ferro Milone F, Grunfeld S, Welch KM: Platelet glycine, glutamate and aspartate in primary headache. Cephalalgia 1991, 11: 197–200. 10.1046/j.1468-2982.1991.1104197.x
Cananzi AR, D'Andrea G, Perini F, Zamberlan F, Welch KM: Platelet and plasma levels of glutamate and glutamine in migraine with and without aura. Cephalalgia 1995, 15: 132–135. 10.1046/j.1468-2982.1995.015002132.x
Alam Z, Coombes N, Waring RH, Williams AC, Steventon GB: Plasma levels of neuroexcitatory amino acids in patients with migraine or tension headache. J Neurol Sci 1998, 156: 102–106. 10.1016/S0022-510X(98)00023-9
Alam SL, Volkman BF, Markley JL, Satterlee JD: Detailed NMR analysis of the heme-protein interactions in component IV Glycera dibranchiata monomeric hemoglobin-CO. J Biomol NMR 1998, 11: 119–133. 10.1023/A:1008202621725
Rothrock JF, Mar KR, Yaksh TL, Golbeck A, Moore AC: Cerebrospinal fluid analyses in migraine patients and controls. Cephalalgia 1995, 15: 489–493. 10.1046/j.1468-2982.1995.1506489.x
Ramadan NM: The link between glutamate and migraine. CNS Spectr 2003, 8: 446–449.
Vikelis M, Mitsikostas DD: The role of glutamate and its receptors in migraine. CNS & neurological disorders drug targets 2007, 6: T251–257. 10.2174/187152707781387279
Cammarata D, Krusz J: Memantine for Chronic Migraine Prophylaxis. J Pain Headache. 2006,7(4):S43. 10.1016/j.jpain.2006.01.169
Watanabe H, Kuwabara T, Ohkubo M, Tsuji S, Yuasa T: Elevation of cerebral lactate detected by localized 1H-magnetic resonance spectroscopy in migraine during the interictal period. Neurology 1996, 47: 1093–1095.
Sarchielli P, Tarducci R, Presciutti O, Gobbi G, Pelliccioli GP, Stipa G, Alberti A, Capocchi G: Functional 1H-MRS findings in migraine patients with and without aura assessed interictally. Neuroimage 2005, 24: 1025–1031. 10.1016/j.neuroimage.2004.11.005
Sandor PS, Dydak U, Schoenen J, Kollias SS, Hess K, Boesiger P, Agosti RM: MR-spectroscopic imaging during visual stimulation in subgroups of migraine with aura. Cephalalgia 2005, 25: 507–518. 10.1111/j.1468-2982.2005.00900.x
Wang SJ, Lirng JF, Fuh JL, Chen JJ: Reduction in hypothalamic 1H-MRS metabolite ratios in patients with cluster headache. J Neurol Neurosurg Psychiatry 2006, 77: 622–625. 10.1136/jnnp.2005.081836
Macri MA, Garreffa G, Giove F, Ambrosini A, Guardati M, Pierelli F, Schoenen J, Colonnese C, Maraviglia B: Cerebellar metabolite alterations detected in vivo by proton MR spectroscopy. Magn Reson Imaging 2003, 21: 1201–1206. 10.1016/j.mri.2003.08.021
Dichgans M, Herzog J, Freilinger T, Wilke M, Auer DP: 1H-MRS alterations in the cerebellum of patients with familial hemiplegic migraine type 1. Neurology 2005, 64: 608–613.
Gu T, Ma XX, Xu YH, Xiu JJ, Li CF: Metabolite concentration ratios in thalami of patients with migraine and trigeminal neuralgia measured with 1H-MRS. Neurol Res 2008, 30: 229–233. 10.1179/016164107X235473
Ryner LN, Sorenson JA, Thomas MA: Localized 2D J-resolved 1H MR spectroscopy: strong coupling effects in vitro and in vivo. Magn Reson Imaging 1995, 13: 853–869. 10.1016/0730-725X(95)00031-B
Ryner LN, Sorenson JA, Thomas MA: 3D localized 2D NMR spectroscopy on an MRI scanner. J Magn Reson B 1995, 107: 126–137. 10.1006/jmrb.1995.1068
Ke Y, Cohen BM, Bang JY, Yang M, Renshaw PF: Assessment of GABA concentration in human brain using two-dimensional proton magnetic resonance spectroscopy. Psychiatry Res 2000, 100: 169–178. 10.1016/S0925-4927(00)00075-5
Rushworth MF, Behrens TE, Rudebeck PH, Walton ME: Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci 2007, 11: 168–176. 10.1016/j.tics.2007.01.004
Posner MI, Rothbart MK, Sheese BE, Tang Y: The anterior cingulate gyrus and the mechanism of self-regulation. Cognitive, affective & behavioral neuroscience 2007, 7: 391–395. 10.3758/CABN.7.4.391
Drevets WC, Savitz J, Trimble M: The subgenual anterior cingulate cortex in mood disorders. CNS spectrums 2008, 13: 663–681.
Apkarian AV, Chialvo DR: The shadows of pain. Pain 2006, 123: 221–222. 10.1016/j.pain.2006.04.028
Vogt BA: Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 2005, 6: 533–544. 10.1038/nrn1704
Afridi SK, Goadsby PJ: Neuroimaging of migraine. Curr Pain Headache Rep 2006, 10: 221–224. 10.1007/s11916-006-0049-4
di Pellegrino G, Ciaramelli E, Ladavas E: The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. J Cogn Neurosci 2007, 19: 275–286. 10.1162/jocn.2007.19.2.275
Zhuo M: Molecular mechanisms of pain in the anterior cingulate cortex. J Neurosci Res 2006, 84: 927–933. 10.1002/jnr.21003
Henderson LA, Gandevia SC, Macefield VG: Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain 2007, 128: 20–30. 10.1016/j.pain.2006.08.013
Phan KL, Fitzgerald DA, Gao K, Moore GJ, Tancer ME, Posse S: Real-time fMRI of cortico-limbic brain activity during emotional processing. Neuroreport 2004, 15: 527–532. 10.1097/00001756-200403010-00029
Schreckenberger M, Siessmeier T, Viertmann A, Landvogt C, Buchholz HG, Rolke R, Treede RD, Bartenstein P, Birklein F: The unpleasantness of tonic pain is encoded by the insular cortex. Neurology 2005, 64: 1175–1183.
Ogg RJ, Kingsley PB, Taylor JS: WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B 1994, 104: 1–10. 10.1006/jmrb.1994.1048
Provencher SW: Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993, 30: 672–679. 10.1002/mrm.1910300604
Smith SA, Levante TO, Meier BH, Ernst RR: Computer Simulations in Magnetic Resonance. An Object-Oriented Programming Approach. Journal of Magnetic Resonance Series A 1994, 106: 75–105. 10.1006/jmra.1994.1008
Provencher SW: Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001, 14: 260–264. 10.1002/nbm.698
Ongur D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, Renshaw PF: Abnormal Glutamatergic Neurotransmission and Neuronal-Glial Interactions in Acute Mania. Biol Psychiatry 2008,64(8):718–726. 10.1016/j.biopsych.2008.05.014
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM: Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004,23(Suppl 1):S208–219. 10.1016/j.neuroimage.2004.07.051
Zhang Y, Brady M, Smith S: Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 2001, 20: 45–57. 10.1109/42.906424
Davison A, Hinkley : Bootstrap Methods and Their Application. Cambridge: Cambridge University; 1997.
Neale JH, Bzdega T, Wroblewska B: N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem 2000, 75: 443–452. 10.1046/j.1471-4159.2000.0750443.x
Coyle JT: The nagging question of the function of N-acetylaspartylglutamate. Neurobiol Dis 1997, 4: 231–238. 10.1006/nbdi.1997.0153
Baslow MH: Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J Neurochem 2000, 75: 453–459. 10.1046/j.1471-4159.2000.0750453.x
Gehl LM, Saab OH, Bzdega T, Wroblewska B, Neale JH: Biosynthesis of NAAG by an enzyme-mediated process in rat central nervous system neurons and glia. J Neurochem 2004, 90: 989–997. 10.1111/j.1471-4159.2004.02578.x
Pouwels PJ, Frahm J: Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR Biomed 1997, 10: 73–78. 10.1002/(SICI)1099-1492(199704)10:2<73::AID-NBM448>3.0.CO;2-4
Burstein R, Jakubowski M: Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol 2004, 55: 27–36. 10.1002/ana.10785
Sarchielli P, Di Filippo M, Nardi K, Calabresi P: Sensitization, glutamate, and the link between migraine and fibromyalgia. Curr Pain Headache Rep 2007, 11: 343–351. 10.1007/s11916-007-0216-2
Buchgreitz L, Lyngberg AC, Bendtsen L, Jensen R: Frequency of headache is related to sensitization: a population study. Pain 2006, 123: 19–27. 10.1016/j.pain.2006.01.040
Afra J, Proietti Cecchini A, Sandor PS, Schoenen J: Comparison of visual and auditory evoked cortical potentials in migraine patients between attacks. Clin Neurophysiol 2000, 111: 1124–1129. 10.1016/S1388-2457(00)00271-6
de Hoon JN, Willigers JM, Troost J, Struijker-Boudier HA, van Bortel LM: Cranial and peripheral interictal vascular changes in migraine patients. Cephalalgia 2003, 23: 96–104. 10.1046/j.1468-2982.2003.00465.x
Nedeltchev K, Arnold M, Schwerzmann M, Nirkko A, Lagger F, Mattle HP, Sturzenegger M: Cerebrovascular response to repetitive visual stimulation in interictal migraine with aura. Cephalalgia 2004, 24: 700–706. 10.1111/j.1468-2982.2004.00740.x
de Tommaso M, Losito L, Difruscolo O, Libro G, Guido M, Livrea P: Changes in cortical processing of pain in chronic migraine. Headache 2005, 45: 1208–1218. 10.1111/j.1526-4610.2005.00244.x
Peeters M, Gunthorpe MJ, Strijbos PJ, Goldsmith P, Upton N, James MF: Effects of pan- and subtype-selective N-methyl-D-aspartate receptor antagonists on cortical spreading depression in the rat: therapeutic potential for migraine. J Pharmacol Exp Ther 2007, 321: 564–572. 10.1124/jpet.106.117101
Peres MF, Zukerman E, Senne Soares CA, Alonso EO, Santos BF, Faulhaber MH: Cerebrospinal fluid glutamate levels in chronic migraine. Cephalalgia 2004, 24: 735–739. 10.1111/j.1468-2982.2004.00750.x
Goadsby PJ: Can we develop neurally acting drugs for the treatment of migraine? Nat Rev Drug Discov 2005, 4: 741–750. 10.1038/nrd1822
Goadsby PJ: Migraine, allodynia, sensitisation and all of that. Eur Neurol 2005,53(Suppl 1):10–16. 10.1159/000085060
Mehrotra S, Gupta S, Chan KY, Villalon CM, Centurion D, Saxena PR, Maassenvandenbrink A: Current and prospective pharmacological targets in relation to antimigraine action. Naunyn Schmiedebergs Arch Pharmacol 2008, 378: 371–394. 10.1007/s00210-008-0322-7
Di Piero V, Bruti G, Venturi P, Talamonti F, Biondi M, Di Legge S, Lenzi GL: Aminergic tone correlates of migraine and tension-type headache: a study using the tridimensional personality questionnaire. Headache 2001, 41: 63–71. 10.1046/j.1526-4610.2001.111006063.x
Hao Y, Yang JY, Guo M, Wu CF, Wu MF: Morphine decreases extracellular levels of glutamate in the anterior cingulate cortex: an in vivo microdialysis study in freely moving rats. Brain Res 2005, 1040: 191–196. 10.1016/j.brainres.2005.01.072
Sah P, Nicoll RA: Mechanisms underlying potentiation of synaptic transmission in rat anterior cingulate cortex in vitro. J Physiol 1991, 433: 615–630.
Wei F, Li P, Zhuo M: Loss of synaptic depression in mammalian anterior cingulate cortex after amputation. J Neurosci 1999, 19: 9346–9354.
Wei F, Zhuo M: Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol 2001, 532: 823–833. 10.1111/j.1469-7793.2001.0823e.x
Lei LG, Sun S, Gao YJ, Zhao ZQ, Zhang YQ: NMDA receptors in the anterior cingulate cortex mediate pain-related aversion. Exp Neurol 2004, 189: 413–421. 10.1016/j.expneurol.2004.06.012
Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D: Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS ONE 2008,3(11):e3799. 10.1371/journal.pone.0003799
Peyron R, Laurent B, Garcia-Larrea L: Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 2000, 30: 263–288. 10.1016/S0987-7053(00)00227-6
Apkarian AV, Bushnell MC, Treede RD, Zubieta JK: Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005, 9: 463–484. 10.1016/j.ejpain.2004.11.001
Thomas MA, Hattori N, Umeda M, Sawada T, Naruse S: Evaluation of two-dimensional L-COSY and JPRESS using a 3 T MRI scanner: from phantoms to human brain in vivo. NMR Biomed 2003, 16: 245–251. 10.1002/nbm.825
Jensen JE, Frederick BD, Wang L, Brown J, Renshaw PF: Two-dimensional, J-resolved spectroscopic imaging of GABA at 4 Tesla in the human brain. Magn Reson Med 2005, 54: 783–788. 10.1002/mrm.20644
Govindaraju V, Young K, Maudsley AA: Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed 2000, 13: 129–153. 10.1002/1099-1492(200005)13:3<129::AID-NBM619>3.0.CO;2-V
Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D: Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci 2006, 26: 10646–10657. 10.1523/JNEUROSCI.2305-06.2006
Maihofner C, Handwerker HO, Birklein F: Functional imaging of allodynia in complex regional pain syndrome. Neurology 2006,66(5):711–717. 10.1212/01.wnl.0000200961.49114.39
Jakubowski M, Levy D, Goor-Aryeh I, Collins B, Bajwa Z, Burstein R: Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache 2005, 45: 850–861. 10.1111/j.1526-4610.2005.05153.x
Burstein R, Collins B, Jakubowski M: Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol 2004, 55: 19–26. 10.1002/ana.10786
Acknowledgements
David Borsook NS056195; Perry Renshaw DA015116; Rami Burstein NS051484.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AP implemented MRS acquisition and data analysis methods, participated in organization of study design, carried out MRS acquisitions and drafted most parts of the manuscript. LB helped with overall study design and oversaw data analysis procedures. GP performed statistical data analyses. ST was entirely responsible for subject screening and enrollment. EJ provided and oversaw 2D MRS data analysis methods. RH was involved in the overall study design and oversaw data analysis procedures. PR was helped with the original study design and oversaw MRS data acquisition and analysis procedures. RB contributed to direction of the study and oversaw data analysis and interpretation. DB contributed to conceptual framework and overall direction of the study including overseeing data analysis, interpretation and manuscript preparation. All authors have read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Prescot, A., Becerra, L., Pendse, G. et al. Excitatory neurotransmitters in brain regions in interictal migraine patients. Mol Pain 5, 34 (2009). https://doi.org/10.1186/1744-8069-5-34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1744-8069-5-34