Abstract
Trigeminal neuralgia is a common disorder caused mainly by compression of the trigeminal nerve root by an overlying blood vessel. Pharmacotherapy and surgery are ineffective or unsuitable in many patients. Therefore, other therapeutic modalities have been tried, including injection of botulinum toxin type A (BTX-A). This study aims to systematically review the therapeutic efficacy and safety of BTX-A in trigeminal neuralgia. PubMed, EMBASE, Cochrane Library Clinical Trials and Web of Science from January 1966 to March 2013 were searched with the terms of “botulinum toxin” AND “trigeminal neuralgia”, and references of related articles were traced. Data on the efficacy and safety of BTX-A in this disorder were extracted and analyzed by at least 2 reviewers. Data for individual studies were reported, and pooled data were analyzed if appropriate. Five prospective studies and one double-blind, randomized, placebo-controlled study were identified. Response was achieved in approximately 70-100% of patients, and the mean pain intensity and frequency were reduced by approximately 60-100% at 4 weeks after treatment in most studies. Major adverse events were not reported. Available studies show BTX-A may be effective in treatment of trigeminal neuralgia. However, well-designed randomized, controlled, double-blinded trial is still lacking. Future BTX-A treatment studies on optimal dose, duration of the therapeutic efficacy, common AEs, and the time and indications for repeat injection would be promising.
Similar content being viewed by others
Review
Introduction
Trigeminal neuralgia is a unilateral disorder characterized by brief electric shock-like pains, abrupt in onset and termination, limited to the distribution of one or more divisions of the trigeminal nerve [1]. Epidemiological studies reveal that approximately 4-28.9/100,000 individuals worldwide experience TN [2–5]. It is the most widely recognized neuropathic pain of the face and has been shown to be profoundly distressing the patient’s well-being [6]. TN frequently occurs in subjects aged 50-70 years and is more common in women [2, 7]. Compression of the trigeminal nerve near the dorsal root entry zone [8–12] by an overlaying blood vessel is a major causative or contributing factor [8]. In addition, it can also be caused by tumor, multiple sclerosis [13, 14], infiltration, amyloid [15–18], small infarcts or angiomas in the pons or medulla [19–21]. In a small fraction of patients, the cause of TN cannot be identified [22].
The treatment of TN continues to be a major challenge due to the complexity of TN’s causes and the trigeminal nerve. The antiepileptic drugs, such as carbamazepine [23, 24] oxcarbazepine [25, 26] and phenytoin [27, 28], are commonly used in the treatment of TN, but a substantial proportion of patients have poor response to this treatment, predominantly because of their side effects related to the central nervous system [6]. Eventually, many TN patients become refractory to antiepileptic drugs and other drugs [29–32]. The quality of evidence on the efficacy of neurosurgical procedures (such as percutaneous interventions of the Gasserian ganglion, stereotactic radiosurgery or microvascular decompression [33]) is very low. Although these procedures may relieve the pain to different extents, many may result in sensory side effects.
Botulinum toxin type A (BTX-A), one of the seven antigenically different botulinum neurotoxins derived from Clostridium botulinum, appears to be the most potent subtype [34]. It can cleave the synaptosome-associated protein of 25 kDa (SNAP-25) in the motor nerve terminals [35, 36]. BTX-A is reported to be effective in the treatment of migraine and myofacial pain syndrome [37–40]. The mechanism of potential analgesic effect of BTX-A is still unclear. In vitro studies have shown that BTX-A can inhibit the release of pro-inflammatory neuropeptides. Animal experiments also reveal the antinociceptive effect of BTX-A in both inflammatory and neuropathic pain models [41–47]. In 2002, Micheli et al reported the successful treatment of a patient with hemifacial spasm associated with TN with onabotulinumtoxin A, which opens up new possibilities for its use [48]. After that, several other open-label trials have examined the preventive effects of BTX-A on TN [49–51].
The current review is to systematically review the therapeutic efficacy of BTX-A in TN. The secondary goal of this review was to address the safety and tolerability of BTX-A in the treatment of TN.
Methods
The methodology utilized in this review followed the review process derived from evidence-based systematic reviews and meta-analyses [52–55] of clinic trials and semi-trials.
Literature search
A comprehensive search was conducted from 1966 to 2012 using databases including PubMed, EMBASE (OVID), Cochrane Library Clinical Trials and Web of Science. The PubMed, search was conducted by using combinations of Medical Subject Heading (MeSH) search terms and keywords according to the following algorithm: (((“Trigeminal Neuralgia”[Mesh]) OR ((trigeminal [All Fields]) AND neuralgia [All Fields]))) AND (((“Botulinum Toxins, Type A”[Mesh] OR “Botulinum Toxins”[Mesh])) OR botuli* [All Fields]). Other databases were queried by using identical terms for keyword searching. The cross-referencing of bibliographies from notable primary and review articles, and abstracts from scientific meetings and peer-reviewed non-indexed journals were also searched. Only English articles were collected.
At least 2 authors independently, in an unblinded standardized manner, performed searching. Any disagreements were resolved by a third author.
Criteria for inclusion of studies for review
All studies were reviewed by at least 2 reviewers for inclusion. Any disagreements were resolved by a third author. If there was a conflict of interest with the reviewed manuscripts with authorship, the involved authors did not review the manuscripts.
Types of studies
Randomized controlled trials, semi-trials (case–control studies, open-label studies and case series studies) were selected for evaluation the efficacy and/or safety of BTX in the treatment of TN. When the selected articles reported the same trial, only the latest study with the largest sample size or longest follow-up period was included.
Articles having no original data (such as letters, editorials, commentaries and reviews) and those without adequate information regarding the outcome were excluded. Nonhuman studies were also excluded.
Types of participants
Patients with TN of all ages, sex, and degrees of severity were included. TN was diagnosed according to the criteria developed by the International Headache Society (IHS) or other criteria that conformed in general to the IHS diagnostic criteria [1].
Types of interventions
Included studies had to use either a single dose of BTX-A to treat TN, or investigate different dosing strategies. There was no restriction on source of BTX-A, dose of administration, injection sites or number of injections.
Types of outcome measures
The primary outcome measure for this review was proportion of responders, defined as patients with at least 50% reduction in frequency and/or intensity of pain. For the secondary outcomes of interests, we focused on the mean scores of pain, mean attacks per day and treatment-related AEs.
Data extraction and management
A standardized form was used to extract the relevant data on the patients’ and studies’ characteristics, injection protocol, clinical variables, and adverse events by 2 reviewers. Disagreements were resolved by discussion among 3 reviewers.
Data interpretation
The extracted data were reviewed, interpreted, and discussed to compile into level data according to “Oxford Center for Evidence-based Medicine” criteria (http://www.cebm.net/index.aspx?o=1025; updated March 2009) for use in clinical practice. The outcome is integrated in the Results and Discussion sections.
Results
Literature search
Figure 1 gives a flow diagram illustrating the results of the literature search for BTX-A therapy in TN. After a comprehensive search, the references of several review articles were checked, the available studies were evaluated, and then 6 trials [51, 56–60] were identified. Two studies of Gazerani et al concerning BTX-A in the treatment of capsaicin-evoked TN were not included in this review [61, 62].
Study characteristics
Table 1 illustrates the characteristics of studies on the treatment of TN with BTX-A in this review. The number of patients ranged from 8 to 42; and a total of 101 patients were included in 6 selected studies. The majority of studies were open-label studies, except for Wu’s study [56], enrolled 42 patients, in double-blind, randomized and placebo-controlled. Follow-up period ranged from 8 wk to 24 wk, except for a study evaluating the impact of repeated injections which lasted 16-80 wk [60].
Injection protocol
In most of the studies, the amount of BTX-A injected subcutaneously was 20-50 U in the trigger zones (Table 2). In Wu’s study [56], 75 U of BTX-A (Lanzhou Biological Products Institute) was used in each patient. In addition, 6-9 U and 100 U were used in two independent studies.
Efficacy
Primary outcome
The proportion of responders, defined as patients with at least 50% reduction in frequency and/or intensity of pain, was all above 60% and the mean proportion was 80% (Figure 2). In Bohluli’s study [57], patients with complete eradication of the pain were also reported: the pain was completely eradicated in 7 patients and there was no need for further medication.
Secondary outcomes
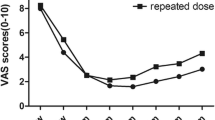
In studies reporting the effect of BTX-A on the pain intensity, the mean scores measured by VAS were between 7 and 10 at baseline (Table 3). A controlled study demonstrated that the therapeutic efficacy of BTX-A was significantly superior to that of placebo in pain intensity [56]. Open-label trials confirmed this trend [51, 57–60]. After BTX-A injection, the reduction in the mean pain intensity from baseline was 41-81% at 1 wk, 66-98% at 4 wk, about 80% at 8 wk and 12 wk.
A controlled study and open-label trials also demonstrated that the therapeutic efficacy of BTX-A was significantly superior to that of placebo in reducing daily pain frequency (Table 4). The mean daily attacks were 21-33 at baseline, but 3.6-8.4 at 1 wk, 4.1-4.7 at 4 wk and 1.8-2.3 at 8-12 wk after BTX-A injection. In Piovesan’s study [51], the average pain area also significantly reduced.
BTX-A was well tolerated in all 6 studies. Although the local or systemic adverse events (AEs) were not very well reported in all studies, most frequent AEs were transient facial asymmetry (Table 5). Facial asymmetry was not severe and resolved within 2 weeks in most studies, except for one patient developing severe side effects which required physiotherapy and took 3 months to resolve in Bohluli’s trial [57]. Other reported AEs of BTX-A injection included transient edema (2.2%), eyelid ptosis (1.1%), dysesthesia (1.1%) and difficulty in chewing (1.1%). Dysphagia and systemic side effects were not reported in all the 5 trials [51, 56–60].
Analysis of evidence
The evidence for BTX-A in the treatment of TN was quantified as Level 1b on the basis of one properly randomized controlled trial and multiple open-label studies.
Discussion
From this systematic review, we can conclude that subcutaneous or mucosal injection of BTX-A is effective for adult TN patients.
Response was achieved in approximately 70-100% of patients. In most studies, the mean pain intensity and frequency were reduced by approximately 60-100% at 4 wk after injection. In Bohluli’s study [57], 47% of patients didn’t need further treatment,; nonsteroidal antiinflammatory drugs were enough to alleviate pain in 33% of patients, and 20% of patients again responded to anticonvulsive drugs after BTX-A injection. In Piovesan’s study [51], the pain area was reduced after injection. However, in the majority of studies, changes in medications and pain area throughout the study were not clearly described. A better understanding of this field requires more studies In the future.
BTX-A has a faster onset of action with its significant effect reaching within 1-2 wk and maximum effect within 4-6 wk. Two studies suggest that the effect of a single BTX-A injection could last for 6 mo or approximately 24 wk [57, 59], whereas a few studies show the efficacy reduced at 4-8 wk after treatment. The duration that the therapeutic effect continues should be studied in future well designed trials.
Before injection, physicians should adequately inform TN patients about the potential risk of BTX-A-related AEs. Although BTX-A was well tolerated in TN patients, transient facial asymmetry, transient edema, eyelid ptosis, dysesthesia and difficulty in chewing were still reported in 6 studies. To adequately assess the incidence of specific AEs and prevent the underestimate, future studies should adequately document and report the local and systemic AEs.
An important issue is, based on the currently available evidence and physician experience, how BTX-A can be best applied in clinical practice?
The first question is the dosage of BTX-A. The most commonly used dose of BTX-A is 20-75 U. However, Piovesan et al [51] found that 6-9 U of BTX-A induced significant decreases in the pain area and intensity, suggesting that lower doses are also feasible. Türk et al [59] also reported the effectiveness after treatment with 100 U of BTX-A. Because no study was designed to compare the therapeutic efficacy of BTX-A at different doses, the optimal dose cannot be concluded. Also, no study was undertaken to compare of the efficacy or tolerance of BTX-A from different manufacturers.
Another variable is the number of injection sites. In Wu’s study [56], injection was done at 15 sites. However, injection was done at only 2 sites in Türk’s study [59]. It is still unclear if the same efficacy with a less painful and faster injection can be achieved by reducing the number of injections with the same dose of BTX-A.
The optimal indications for re-injection are also important, but they weren’t clarified in these studies. In our opinion, re-injection should be performed only when the worsening of symptoms is present. Patients should not receive repeated injections once the symptomatic improvement occurs after two injections, or severe AEs are present.
Conclusions
We speculate that BTX-A treatment may provide a clinically significant benefit to TN adults. The effect is rapidly achieved, usually within 1-2 wk. Of importance, BTX-A treatment seems to be well tolerated with minimal injections and to result in limited systemic adverse events. Therefore, it represents a promising treatment of TN with favorable risk-to-benefit ratio. However, well-designed randomized, controlled, double-blinded trial is still lacking. Future adequately powered studies are needed to investigate the optimal dose of BTX-A treatment, the duration of therapeutic efficacy, common AEs, and the time and indications for repeat injection.
References
Headache Classification Subcommittee of the International Headache S: The international classification of headache disorders: 2nd edition. Cephalalgia 2004,24(Suppl 1):9–160.
Katusic S, Beard CM, Bergstralh E, et al.: Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945–1984. Ann Neurol 1990, 27: 89–95. 10.1002/ana.410270114
Hall GC, Carroll D, Parry D, et al.: Epidemiology and treatment of neuropathic pain: the UK primary care perspective. Pain 2006, 122: 156–162. 10.1016/j.pain.2006.01.030
Dieleman JP, Kerklaan J, Huygen FJ, et al.: Incidence rates and treatment of neuropathic pain conditions in the general population. Pain 2008, 137: 681–688. 10.1016/j.pain.2008.03.002
Koopman JS, Dieleman JP, Huygen FJ, et al.: Incidence of facial pain in the general population. Pain 2009, 147: 122–127. 10.1016/j.pain.2009.08.023
Zakrzewska JM: Medical management of trigeminal neuropathic pains. Expert Opin Pharmacother 2010, 11: 1239–1254. 10.1517/14656561003767449
Yoshimasu F, Kurland LT, Elveback LR: Tic douloureux in Rochester, Minnesota, 1945–1969. Neurology 1972, 22: 952–956. 10.1212/WNL.22.9.952
Devor M, Amir R, Rappaport ZH: Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain 2002, 18: 4–13. 10.1097/00002508-200201000-00002
Cheshire WP: Trigeminal neuralgia: for one nerve a multitude of treatments. Expert Rev Neurother 2007, 7: 1565–1579. 10.1586/14737175.7.11.1565
Tomasello F, Alafaci C, Angileri FF, et al.: Clinical presentation of trigeminal neuralgia and the rationale of microvascular decompression. Neurol Sci 2008,29(Suppl 1):S191-S195.
Gnanalingham K, Joshi SM, Lopez B, et al.: Trigeminal neuralgia secondary to Chiari’s malformation–treatment with ventriculoperitoneal shunt. Surg Neurol 2005, 63: 586–588. discussion 588–589 10.1016/j.surneu.2004.06.021
Jo KW, Kong DS, Hong KS, et al.: Long-term prognostic factors for microvascular decompression for trigeminal neuralgia. J Clin Neurosci 2013, 20: 440–445. 10.1016/j.jocn.2012.03.037
De Santi L, Annunziata P: Symptomatic cranial neuralgias in multiple sclerosis: clinical features and treatment. Clin Neurol Neurosurg 2012, 114: 101–107. 10.1016/j.clineuro.2011.10.044
Nurmikko TJ, Gupta S, Maclver K: Multiple sclerosis-related central pain disorders. Curr Pain Headache Rep 2010, 14: 189–195. 10.1007/s11916-010-0108-8
Benoliel R, Epstein J, Eliav E, et al.: Orofacial pain in cancer: part I–mechanisms. J Dent Res 2007, 86: 491–505. 10.1177/154405910708600604
Viviano M, Donati D, Lorenzini G: Metastatic carcinoma presenting as neuralgia involving the trigeminal nerve. J Can Dent Assoc 2012, 77: c32.
Shulev Y, Trashin A, Gordienko K: Secondary trigeminal neuralgia in cerebellopontine angle tumors. Skull Base 2011, 21: 287–294. 10.1055/s-0031-1284218
Bornemann A, Bohl J, Hey O, et al.: Amyloidoma of the gasserian ganglion as a cause of symptomatic neuralgia of the trigeminal nerve: report of three cases. J Neurol 1993, 241: 10–14. 10.1007/BF00870665
Cheng TM, Cascino TL, Onofrio BM: Comprehensive study of diagnosis and treatment of trigeminal neuralgia secondary to tumors. Neurology 1993, 43: 2298–2302. 10.1212/WNL.43.11.2298
Singh D, Jagetia A, Sinha S: Brain stem infarction: a complication of microvascular decompression for trigeminal neuralgia. Neurol India 2006, 54: 325–326. 10.4103/0028-3886.27177
Deshmukh VR, Hott JS, Tabrizi P, et al.: Cavernous malformation of the trigeminal nerve manifesting with trigeminal neuralgia: case report. Neurosurgery 2005, 56: E623. discussion E623 10.1227/01.NEU.0000154063.05728.7E
Nurmikko TJ, Eldridge PR: Trigeminal neuralgia–pathophysiology, diagnosis and current treatment. Br J Anaesth 2001, 87: 117–132. 10.1093/bja/87.1.117
Wiffen PJ, Derry S, Moore RA, et al.: Carbamazepine for acute and chronic pain in adults. Cochrane Database Syst Rev 2011. 10.1002/14651858.CD005451
Wang QP, Bai M: Topiramate versus carbamazepine for the treatment of classical trigeminal neuralgia: a meta-analysis. CNS Drugs 2011, 25: 847–857. 10.2165/11595590-000000000-00000
Nasreddine W, Beydoun A: Oxcarbazepine in neuropathic pain. Expert Opin Investig Drugs 2007, 16: 1615–1625. 10.1517/13543784.16.10.1615
Gomez-Arguelles JM, Dorado R, Sepulveda JM, et al.: Oxcarbazepine monotherapy in carbamazepine-unresponsive trigeminal neuralgia. J Clin Neurosci 2008, 15: 516–519. 10.1016/j.jocn.2007.04.010
Tate R, Rubin LM, Krajewski KC: Treatment of refractory trigeminal neuralgia with intravenous phenytoin. Am J Health Syst Pharm 2011, 68: 2059–2061. 10.2146/ajhp100636
Lu DP, Lu WI, Lu GP: Phenytoin (Dilantin) and acupuncture therapy in the treatment of intractable oral and facial pain. Acupunct Electrother Res 2011, 36: 65–84.
Jorns TP, Zakrzewska JM: Evidence-based approach to the medical management of trigeminal neuralgia. Br J Neurosurg 2007, 21: 253–261. 10.1080/02688690701219175
Canavero S, Bonicalzi V: Drug therapy of trigeminal neuralgia. Expert Rev Neurother 2006, 6: 429–440. 10.1586/14737175.6.3.429
Yang M, Zhou M, He L, et al.: Non-antiepileptic drugs for trigeminal neuralgia. Cochrane Database Syst Rev 2011. 10.1002/14651858.CD004029
Lenchig S, Cohen J, Patin D: A minimally invasive surgical technique for the treatment of posttraumatic trigeminal neuropathic pain with peripheral nerve stimulation. Pain physician 2012, 15: E725-E732.
Zakrzewska JM, Akram H: Neurosurgical interventions for the treatment of classical trigeminal neuralgia. Cochrane Database Syst Rev 2011. 10.1002/14651858.CD007312
Trindade De Almeida AR, Secco LC, Carruthers A: Handling botulinum toxins: an updated literature review. Dermatol Surg 2011, 37: 1553–1565. 10.1111/j.1524-4725.2011.02087.x
Humeau Y, Doussau F, Grant NJ, et al.: How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie 2000, 82: 427–446. 10.1016/S0300-9084(00)00216-9
Pearce LB, First ER, MacCallum RD, et al.: Pharmacologic characterization of botulinum toxin for basic science and medicine. Toxicon 1997, 35: 1373–1412. 10.1016/S0041-0101(96)00180-8
Frampton JE: OnabotulinumtoxinA (BOTOX(R)): a review of its use in the prophylaxis of headaches in adults with chronic migraine. Drugs 2012, 72: 825–845. 10.2165/11208880-000000000-00000
Schulte-Mattler WJ, Martinez-Castrillo JC: Botulinum toxin therapy of migraine and tension-type headache: comparing different botulinum toxin preparations. Eur J Neurol 2006,13(Suppl 1):51–54.
Casale R, Tugnoli V: Botulinum toxin for pain. Drugs R D 2008, 9: 11–27.
Porta M, Camerlingo M: Headache and botulinum toxin. J Headache Pain 2005, 6: 325–327. 10.1007/s10194-005-0222-x
McMahon HT, Foran P, Dolly JO, et al.: Tetanus toxin and botulinum toxins type A and B inhibit glutamate, gamma-aminobutyric acid, aspartate, and met-enkephalin release from synaptosomes. Clues to the locus of action. J Biol Chem 1992, 267: 21338–21343.
Purkiss JR, Welch MJ, Doward S, et al.: Capsaicin stimulates release of substance P from dorsal root ganglion neurons via two distinct mechanisms. Biochem Soc Trans 1997, 25: 542S.
Welch MJ, Purkiss JR, Foster KA: Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 2000, 38: 245–258. 10.1016/S0041-0101(99)00153-1
Bach-Rojecky L, Lackovic Z: Antinociceptive effect of botulinum toxin type a in rat model of carrageenan and capsaicin induced pain. Croat Med J 2005, 46: 201–208.
Cui M, Khanijou S, Rubino J, et al.: Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004, 107: 125–133. 10.1016/j.pain.2003.10.008
Luvisetto S, Marinelli S, Cobianchi S, et al.: Anti-allodynic efficacy of botulinum neurotoxin A in a model of neuropathic pain. Neurosci 2007, 145: 1–4. 10.1016/j.neuroscience.2006.12.004
Park HJ, Lee Y, Lee J, et al.: The effects of botulinum toxin A on mechanical and cold allodynia in a rat model of neuropathic pain. Canadian journal of anaesthesia =. J canadien d’anesthesie 2006, 53: 470–477. 10.1007/BF03022619
Micheli F, Scorticati MC, Raina G: Beneficial effects of botulinum toxin type a for patients with painful tic convulsif. Clin Neuropharmacol 2002, 25: 260–262. 10.1097/00002826-200209000-00006
Ngeow WC, Nair R: Injection of botulinum toxin type A (BOTOX) into trigger zone of trigeminal neuralgia as a means to control pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010, 109: e47-e50.
Allam N, Brasil-Neto JP, Brown G, et al.: Injections of botulinum toxin type a produce pain alleviation in intractable trigeminal neuralgia. Clin J Pain 2005, 21: 182–184. 10.1097/00002508-200503000-00010
Piovesan EJ, Teive HG, Kowacs PA, et al.: An open study of botulinum-A toxin treatment of trigeminal neuralgia. Neurology 2005, 65: 1306–1308. 10.1212/01.wnl.0000180940.98815.74
Karsenty G, Denys P, Amarenco G, et al.: Botulinum toxin A (Botox) intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: a systematic literature review. Eur Urol 2008, 53: 275–287. 10.1016/j.eururo.2007.10.013
Singh JA, Fitzgerald PM: Botulinum toxin for shoulder pain: a cochrane systematic review. J Rheumatol 2011, 38: 409–418. 10.3899/jrheum.101081
Mangera A, Andersson KE, Apostolidis A, et al.: Contemporary management of lower urinary tract disease with botulinum toxin A: a systematic review of botox (onabotulinumtoxinA) and dysport (abobotulinumtoxinA). Eur Urol 2011, 60: 784–795. 10.1016/j.eururo.2011.07.001
Hansen H, Manchikanti L, Simopoulos TT, et al.: A systematic evaluation of the therapeutic effectiveness of sacroiliac joint interventions. Pain Physician 2012, 15: E247-E278.
Wu CJ, Lian YJ, Zheng YK, et al.: Botulinum toxin type A for the treatment of trigeminal neuralgia: results from a randomized, double-blind, placebo-controlled trial. Cephalalgia 2012, 32: 443–450. 10.1177/0333102412441721
Bohluli B, Motamedi MH, Bagheri SC, et al.: Use of botulinum toxin A for drug-refractory trigeminal neuralgia: preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011, 111: 47–50. 10.1016/j.tripleo.2010.04.043
Zuniga C, Diaz S, Piedimonte F, et al.: Beneficial effects of botulinum toxin type A in trigeminal neuralgia. Arq Neuropsiquiatr 2008, 66: 500–503. 10.1590/S0004-282X2008000400012
Turk U, Ilhan S, Alp R, et al.: Botulinum toxin and intractable trigeminal neuralgia. Clin Neuropharmacol 2005, 28: 161–162. 10.1097/01.wnf.0000172497.24770.b0
Borodic GE, Acquadro MA: The use of botulinum toxin for the treatment of chronic facial pain. J Pain 2002, 3: 21–27. 10.1054/jpai.2002.27142
Gazerani P, Staahl C, Drewes AM, et al.: The effects of Botulinum Toxin type A on capsaicin-evoked pain, flare, and secondary hyperalgesia in an experimental human model of trigeminal sensitization. Pain 2006, 122: 315–325. 10.1016/j.pain.2006.04.014
Gazerani P, Pedersen NS, Staahl C, et al.: Subcutaneous Botulinum toxin type A reduces capsaicin-induced trigeminal pain and vasomotor reactions in human skin. Pain 2009, 141: 60–69. 10.1016/j.pain.2008.10.005
Acknowledgements
The work was supported by National Natural Science Foundation (No: 81000481) and “Fundamental Research Funds for Central Universities” (No: 1508219048). We thank Dr. Qianglin Duan for critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interest
The authors declare that they have no competing interest.
Authors’ contributions
LJ and YH designed this study. YH, YL and XG carried out the searches, identified studies for inclusion and extracted relevant data. ML, ZN and LF were involved in analysis. LJ acted as arbitrator. All authors read and approved the final version.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Hu, Y., Guan, X., Fan, L. et al. Therapeutic efficacy and safety of botulinum toxin type A in trigeminal neuralgia: a systematic review. J Headache Pain 14, 72 (2013). https://doi.org/10.1186/1129-2377-14-72
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1129-2377-14-72