Abstract
Plant diseases are caused by various pathogenic microorganisms, leading to substantial economic losses and food insecurity worldwide. However, the extensive use of chemical-based nanopesticides has adverse effects on plants, soil, and environmental systems. There is increasing interest in developing eco-friendly and sustainable alternatives to manage plant diseases. Recently, microbe-mediated nanoparticles (NPs) as nanopesticides have attracted the interest of cultivators, specifically in plant disease management, compared to traditional physical and chemical approaches. This review focuses on the state-of-the-art formulations of nanopesticides by using microorganisms against bacterial and fungal phytopathogens. The article discusses the various mechanisms through which these microbes contribute to the enhanced effectiveness of NPs, including the production of bioactive compounds, improved nanoparticle synthesis, and the facilitation of targeted delivery. The review also highlights the advantages of using microbe-mediated nanopesticides, such as reduced environmental toxicity, increased biodegradability, and the potential to manage pesticide-resistant pathogens. Overall, the use of microbe-mediated NPs is an inexpensive, reliable, and eco-friendly approach for plant disease management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The continuous increase in food scarcity due to the emergence of outrageous plant diseases has become a global issue [105]. In addition, escalating food demands are experiencing exponential growth as a direct consequence of the population explosion, exacerbating the prevailing circumstances. Consequently, it is imperative that we augment global food production by up to 70% by 2050 to effectively meet the nutritional requirements of the burgeoning global population [22]. Various phyto-pathogens, such as fungi, bacteria, and viruses, pose a significant threat to global food security by reducing crop productivity and causing substantial economic losses [122, 124]. Recently, the scientific community has been trying to devise efficient alternatives to remote techniques to expand food production with minimum impacts on the environment and agricultural soil properties [4]. Traditionally, chemical pesticides have been widely used to combat plant diseases, but their indiscriminate use has led to the emergence of more harmful pathogens, environmental pollution, and negative effects on nontarget organisms [43, 54]. Hence, there is an urgent need for eco-friendly approaches to increase agricultural productivity and manage plant diseases that could substitute the previously available orthodox techniques.
Recently, nanotechnology has obtained considerable recognition because of its substantial applications in the agriculture sector, particularly the use of nanoparticles (NPs) (size < 100 nm) for soil conditioning and plant disease management [37]. Additionally, NPs are inexpensive, effective substitutes for parent materials that have a high reaction rate, increased efficiency, and a high surface-to-volume ratio [44]. Several studies have reported that the use of NPs significantly increases plant growth and development by modulating nutrient availability and plant defense responses as well as by directly interacting with environmental stresses (either biotic or abiotic) under greenhouse and field conditions [11, 34, 82]. The production of NPs relies on chemical and physical processes, which are associated with several drawbacks, including high production rates, limited biocompatibility, substantial energy requirements, and the utilization of various toxic chemicals [71, 77, 95]. In the literature, several studies have revealed that the chemical synthesis of NPs has toxic impacts on plant, soil and environmental systems [40, 46, 66]. The application of chemically synthesized silver NPs exhibits phytotoxic effects by permeating plant tissues, inducing oxidative stress, and adversely affecting cellular activities, leading to reduced photosynthesis, altered nutrient uptake, and impaired root development [29, 64]. Furthermore, chemically manufactured NPs can also have ecological consequences, potentially affecting beneficial soil microorganisms and changing ecosystem dynamics [8]. These risks highlight the significance of cautious assessment and management when using chemically produced NPs in plant disease control techniques.
Conversely, microbe-mediated biosynthesis of NPs has great ability with regards to nontoxicity, easy scaling-up, long-term stability and eco-friendliness in comparison with NPs synthesized by chemical and physical methods [73]. Various microorganisms belonging to different categories (e.g., bacteria, fungi, yeast and microalgae) have been considered potential nanofactories for synthesizing metallic NPs [110]. The synthesis of microbe-mediated NPs involves using microorganisms and various techniques, such as green synthesis, bioreduction, and extracellular synthesis, to produce NPs with desirable properties [102]. These NPs have shown promising results in controlling a wide range of plant diseases caused by various pathogens, including Fusarium oxysporum, Alternaria solani, and Xanthomonas oryzae pv. oryzae, among others [2, 59, 80]. In the past, several studies have demonstrated that microbe-mediated NPs are less toxic to nontarget organisms and have lower environmental persistence than chemical pesticides [67, 100]. These benefits have prompted researchers to investigate the potential of microbe-mediated NPs as a sustainable and eco-friendly alternative for managing plant diseases.
The present review provides the current advancements in the microbe-based synthesis of NPs for plant disease control and covers the role of microorganisms in the biosynthesis of NPs and their potential uses in agriculture. Additionally, the article will discuss the interaction between microbe-mediated NPs and plant pathogens, highlighting the different modes of action.
Microbe-mediated metallic nanoparticles
Nanoparticles have been manufactured using a variety of traditional chemical and physical techniques, such as the solvent evaporation process, vapor condensation, physical fragmentation, sol–gel process, precipitation from microemulsion, and interferometric lithography [5, 98]. These techniques involve using hazardous and toxic substances, which contribute to environmental pollutants. Furthermore, these toxic substances may bind with plants, causing NPs to accumulate in the food chain via food consumption, posing a risk to human health [60]. However, the microbe-mediated synthesis of NPs offers several benefits over traditionally used chemical processes, such as being eco-environmentally friendly, cost-effective, and biocompatible [107]. Microbe-mediated synthesis of NPs employing various microorganisms, such as bacteria, fungi, yeast, and microalgae, offers a promising approach due to its inherent ability to produce highly stable nanoparticles [18]. Furthermore, microbial synthesis allows for precise control over the size, shape, and composition of NPs, which can significantly impact their properties and potential applications [63]. The particle size, dispersion, and stability of biogenic NPs play crucial roles in determining their efficacy [69]. Numerous studies have highlighted the significance of these properties, showcasing the control and manipulation of particle size through microbial synthesis methods [106]. Additionally, the dispersion of microbially synthesized nanoparticles can be enhanced through surface modifications, allowing for improved interactions with target pathogens [92]. Furthermore, the stability of these nanoparticles, influenced by the capping agents produced by microorganisms, contributes to their sustained antimicrobial effect, as shown in Fig. 1.
Schematic illustration of microbe-mediated synthesis of nanoparticles (NPs). Microbe-mediated synthesis of NPs involves the reduction and stabilization of metal ions by microorganisms such as bacteria, fungi, and algae. The process starts with the exposure of the microorganisms to metal ions, followed by the reduction of the ions to form NPs. The NPs are then stabilized by various biomolecules present in the microorganisms, such as enzymes and proteins. The shape of the NPs depends on various factors, including the type of microorganism, the reaction conditions, and the metal ions used
Synthesis of nanoparticles using bacteria
The production of NPs by using bacteria is a promising and eco-friendly approach to produce NPs with various applications in the agriculture sector [47]. Bacteria have been used to synthesize NPs because they can produce extracellular enzymes that have the ability to reduce metal ions to their corresponding NPs [51]. The synthesis of NPs using bacteria can be achieved through various mechanisms, such as intracellular biosynthesis, extracellular biosynthesis, and bioaccumulation [17, 121]. In intracellular biosynthesis, bacteria synthesize NPs within their cells. The process involves the uptake of metal ions into the bacterial cells and the reduction of the ions to NPs through the action of intracellular enzymes. The NPs are then released into the extracellular environment after cell lysis [31, 88]. Bacteria are used to synthesize NPs outside their cells in extracellular biosynthesis. The process involves the secretion of extracellular enzymes, which can reduce metal ions to their corresponding NPs. The NPs are then released into the extracellular environment, where they can be harvested and purified [112, 113]. In the literature, various studies have reported the green synthesis of metallic NPs using microbes [12, 86, 87]. Ahmed et al. [11] described the biosynthesis of silver NPs via Bacillus cereus to control the rice bacterial pathogen. Similarly, Varshney et al. [117] showed the green synthesis of copper NPs by using Pseudomonas stutzeri, which was isolated from wastewater. Several bacterial strains, viz., B. amyloliquefaciens, Acinetobacter calcoaceticus, P. stutzeri, Escherichia coli and Lactobacillus sp. were previously used for microbe-mediated synthesis of NPs [49, 65, 104, 111]. The green synthesis of copper, silver and zinc NPs from Streptomyces sp. has revealed that the reductase enzyme plays a vital role in reducing metal ions [62]. Taken together, the biosynthesis of NPs from bacteria is a promising and eco-environmentally friendly technique to produce NPs.
Synthesis of nanoparticles using fungi
Fungi are a diverse group of organisms that have been widely used for the synthesis of NPs due to their ability to produce various extracellular enzymes and metabolites that can reduce metal ions to their corresponding NPs [38, 99, 119]. The biosynthesis of NPs from fungi can be attained through various mechanisms, including intracellular and extracellular biosynthesis and fungal biomass [78]. Intracellular biosynthesis involves the uptake of metal ions into fungal cells, which can be reduced to NPs through the action of different intracellular enzymes. The NPs are then released into the extracellular environment after cell lysis [101, 116]. Extracellular biosynthesis involves the secretion of extracellular enzymes that are able to reduce metallic ions into their corresponding NPs outside fungal cells [112, 123]. Many studies have reported that the fungal-based synthesis of NPs has several advantages, such as ease of cultivation, high production rate and low cost [96, 97]. For example, Tomah et al. [115] demonstrated the biosynthesis of silver NPs through the cell-free filtrate of Trichoderma virens HZA14 against the Sclerotinia sclerotiorum pathogen. Moreover, Singh et al. [109] showed that zinc oxide NPs were synthesized using Aspergillus niger against the Alternaria solani pathogen, which can cause early blight disease of potato (Solanum tuberosum L.) plants. Similarly, Jain et al. [56] reported the Aspergillus aeneus-mediated biosynthesis of zinc oxide NPs. Similar to other microbes, yeasts have also been generally applied for the biosynthesis of NPs on a large scale [19, 62, 79]. Taken together, the fungal-mediated synthesis of NPs offers a sustainable, cost-effective, and versatile approach for the green synthesis of NPs with unique properties and promising benefits in agricultural fields. Further research in this area could lead to the development of novel, eco-friendly technologies with a wide range of applications.
Synthesis of nanoparticles using microalgae
The biosynthesis of NPs from microalgae has several benefits, such as ease of cultivation, high production rate and low cost [26]. In addition, microalgae can be easily harvested and processed to synthesize NPs. Moreover, using microalgae for nanoparticle synthesis is environmentally friendly, as it reduces the use of hazardous chemicals and reduces waste production [32]. Microalgae are photosynthetic microorganisms that can reduce metal ions to their corresponding NPs through the action of intracellular or extracellular enzymes [3]. Many studies have previously tested the synthesis of biogenic NPs using microalgae through intra- and extracellular biosynthesis methods [24, 72]. For example, da Silva Ferreira et al. [30] synthesized silver chloride NPs through the microalgal species Chlorella vulgaris and observed their antibacterial potential against pathogenic bacteria. Likewise, Çalışkan et al. [23] showed the synthesis of zinc, iron and silver NPs using the microalga Galdieria sp. and characterize the NPs through standard material characterization techniques, such as UV‒Vis spectroscopy and Fourier transform infrared spectroscopy (FTIR). Similarly, Salas-Herrera et al. [103] revealed the synthesis of copper NPs using the microalgae Tetraselmis suecica, Dunaliella tertiolecta and Chlorella kessleri under different conditions. In conclusion, synthesizing NPs using microalgae is a promising and eco-friendly approach for producing NPs. Further research is required for the optimization of the synthesis process and for understanding the underlying mechanisms of nanoparticle synthesis using microalgae.
Mechanism of nanoparticles for controlling phytopathogenic diseases
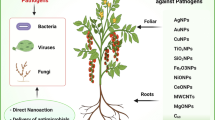
Nanopesticides can be termed any plant protectant that contains nanomaterials as active ingredients to enhance disease control efficacy and biocidal properties [50, 61]. Nanoparticles have demonstrated remarkable efficacy as antibacterial agents against plant pathogens, owing to their exceptional microcidal activity. Introducing microbe-oriented NPs represents a novel approach in the prevention of plant pathogenic diseases. Numerous studies have shown that microbe-based NPs have great potential to replace traditional pesticides [28, 33, 68]. The microorganisms used in these NPs have numerous mechanisms of action, such as antibiosis, competition for nutrients and space, and induction of systemic resistance in plants. Additionally, the size, shape and surface characteristics of biogenic NPs can be engineered to target specific phytopathogens, making these NPs highly efficient and selective. The most widely studied metallic NPs are silver, gold, manganese, zinc, copper, titanium, etc. [74]. Overall, nanoparticle-mediated disease control mechanisms are complex, involving a variety of direct and indirect impacts on the pathogen, the plant, and their interactions. Further research is needed to fully understand the underlying mechanisms and optimize the efficacy and safety of nanoparticle-based approaches for the management of plant diseases. A summary of various types of microbe-based NPs against plant diseases is shown in Table 1.
Direct interaction with pathogens
Microbe-mediated NPs have shown promising results in directly interacting with plant pathogens, inhibiting their growth, and reducing disease severity (Fig. 2). One of the mechanisms by which biogenic NPs interact with plant pathogens is through their physical and chemical properties [83, 118]. These NPs may contain compounds that disrupt the pathogen’s cell membrane, leading to cell lysis and reduced pathogen viability. Additionally, the production of reactive oxygen species (ROS) (e.g., hydrogen peroxide, superoxide anions and hydroxyl radicals) after exposure to NPs causes DNA damage, inhibiting mRNA and protein synthesis that ultimately leads to pathogen death [89]. Several studies have suggested that a large surface area, nanosize scale, easy cell penetration and other distinct characteristics of biogenic NPs can significantly increase their antimicrobial activities [18]. The controlled release of protein-capped metal ions such as Cu2+, Ag+, Ti4+ and Zn2+. from nanocrystals are proposed in antimicrobial mechanisms [13, 16]. Hence, the interaction between NPs and microbial cells disrupts the cellular membrane structure, depletes antioxidants and interferes with nutrient uptake by microbes [7].
Schematic illustration of the direct interaction of nanoparticles (NPs) with plant pathogens. NPs can directly interact with phytopathogens, causing an oxidative burst that can lead to a cascade of events including cell wall damage, membrane disruption, denaturation of enzymes, and disruption of cellular functions, ultimately resulting in the death of the pathogen
For example, Chen et al. [27] revealed strong antimicrobial activity produced by green MgO-NPs against Thielaviopsis basicola and Phytophthora nicotianae. They also observed that direct interactions between NPs and fungal cells triggered the production of ROS due to NP-associated damage to fungal cells. Furthermore, ultrastructural micrographs showed plasmalemma disappearance, partial cell wall injury and disorganized cytoplasm. Similarly, Ahmed et al. [9] demonstrated that green MgO-NPs have strong inhibitory effects on A. oryzae by using TEM analysis and showed a highly ruptured cell membrane structure, DNA damage and efflux of cytoplasmic materials that cause bacterial death. In a recent study, green-synthesized NPs were tested against the fungal pathogen F. graminearum, and the fungicidal effect causing alteration of hyphal and highly damaged cell wall structures was studied by electron microscopy analysis, e.g., SEM and TEM [52]. Similar effects of bioengineered chitosan-magnesium nanocomposites against the rice fungal pathogen R. solani and bacterial pathogen A. oryzae were also observed. Microscopic images showed extremely wounded structures of the cell membrane and cell wall of pathogens, cellular organelle damage and leakage of cytoplasmic materials after treatment with nanopesticides [7].
In a previous study, Hossain et al. [45] demonstrated the inhibitory ability of P. rhodesiae-mediated AgNPs to kill Dickeya dadantii (soft rot pathogen). In a recent study, Ibrahim et al. [51] found that green silver NPs synthesized using B. siamensis showed a proficient bactericidal effect against the rice pathogen Xanthomonas oryzae pv. oryzae that can cause bacterial leaf blight disease in rice. In another study, Kumari et al. [70] reported the in vitro fungicidal effect of biologically synthesized silver NPs against Alternaria solani by inhibiting spore germination and reducing biomass by 100% after 7 days. Similarly, another in vivo study revealed the inhibitory effect of three biologically synthesized metal oxide NPs (MgO, ZnO and MnO2) by Paenibacillus polymyxa against the rice bacterial leaf blight pathogen Xanthomonas oryzae pv. oryzae [90]. Recently, many studies have also determined the use of NPs for the control of viral diseases. For example, a study described the use of silver NPs synthesized from Pseudomonas fluorescens to control tobacco mosaic virus in tobacco plants [14]. However, extensive research is needed to enhance our understanding of the target specificity of biogenic NPs by assessing their effects on beneficial microorganisms.
Activation of plant defense responses
Microbe-mediated NPs have shown potential in indirectly controlling plant disease through their effects on plant growth and defense mechanisms (Fig. 3). For example, some NPs have been shown to increase the uptake and utilization of nutrients by plants, leading to improved growth and yield [25]. This can lead to the production of phytohormones, enzymes, and other defense molecules that inhibit pathogen growth and improve plant resistance to infection. Another mechanism by which biogenic NPs indirectly control plant disease is through their effects on plant defense mechanisms [58, 85]. Some NPs have been shown to induce the production of reactive oxygen species (ROS) in plants, which can trigger defense responses and reduce disease severity [120]. Additionally, NPs may stimulate the production of plant hormones such as salicylic acid and jasmonic acid, which play key roles in plant defense against pathogens [36, 75]. Previously, many studies have demonstrated the potential of biogenic NPs in controlling plant disease [81, 91].
Schematic illustration of indirect interaction of nanoparticles (NPs) with phytopathogens. NPs can interact with the plant host and modulate the plant defense response, which can ultimately affect the pathogen’s ability to infect and cause disease. NPs can stimulate the production of plant defense molecules, including antioxidative enzymes, metabolites, phytohormones and pathogenesis-related proteins, and reduce oxidative stress, which can enhance plant resistance to pathogen infection
For instance, biogenic copper NPs have demonstrated potential in suppressing bacterial fruit blotch in watermelon because they have direct antibacterial activity and can induce active immunity in watermelon [85]. Similarly, Cu nanoscale (250 mg L−1) amendments significantly suppressed soybean sudden death syndrome by activating plant immunity and enhancing the phytohormone content, photosynthetic endpoints, antioxidant enzymes and nutritional status [76]. In addition to their direct effects on plant growth and defense mechanisms, biogenic NPs can interact with soil and rhizosphere microorganisms to indirectly control plant disease [1, 93]. Furthermore, the use of chitosan-iron nanocomposites (BNCs) has shown promising results in controlling bacterial leaf blight disease in rice because it can inhibit Xanthomonas oryzae pv. oryzae growth and improve plant resistance through modulation of antioxidant enzymes, defense-related genes, and the plant’s microbiome [6]. Additionally, NPs may have antimicrobial properties that can inhibit the growth of pathogenic microbes in the soil, reducing the risk of disease [48]. Recently, Noman et al. [84] showed that biogenic manganese NPs (MnNPs) synthesized by Bacillus megaterium NOM14 have the potential to suppress watermelon Fusarium wilt through multiple mechanisms, including inhibition of pathogen growth, enhancement of the host defense response, and modulation of the soil microbial community. Overall, biogenic NPs offer a promising approach to the indirect control of plant disease by enhancing plant growth and defense mechanisms. However, further research is needed to fully understand their safety and environmental impacts and to optimize their use in agriculture.
Concluding remarks and future perspectives
In conclusion, the use of nanotechnology in agriculture, specifically in the development of NPs, is a promising approach to manage plant diseases. Microbe-mediated NPs have emerged as an innovative and effective approach to plant disease management due to their potential for targeted delivery and enhanced efficacy. The use of microorganisms in the production of NPs has the potential to address some of the limitations of traditional chemical-based pesticides, such as their nonspecificity and harmful effects on the environment. Microbial-based NPs can target specific pathogens and reduce the amount of pesticide needed, thereby minimizing environmental contamination. However, there are still significant limitations and knowledge gaps to be filled to guarantee the social acceptance of NPs under environmental conditions as well as their safe use. Optimization of synthesis, ensuring NP stability and bioavailability, and achieving efficient delivery to target sites are key challenges. Additionally, concerns regarding the ecological impact and safety of NPs need to be addressed.
Pioneering efforts are needed to optimize biological synthesis methods on an industrial scale with benefits, including an eco-friendly nature, ease of scaling up and cost-effectiveness. Although NPs have demonstrated potential applications in agriculture, new tools for smart delivery of nanopesticides should be designed and commercialized. One of the potential areas of research is the integration of these pesticides with other innovative technologies, such as precision agriculture and gene editing. Precision agriculture can help farmers optimize the use of these pesticides by providing real-time information on plant health and disease prevalence. One of the primary challenges is the lack of knowledge and awareness among farmers and researchers about the use and effectiveness of these pesticides. Additionally, the regulatory framework for these pesticides needs to be developed to ensure their safety and efficacy. Moreover, field experiments are required to govern the effectiveness, steadfastness, reproducibility, and fate of microbe-mediated NP effects under realistic agricultural conditions. Notably, it is important to confirm that nanoscale pesticides do not adversely impact the growth of plants, beneficial microbial communities, or environmental processes. Further research is needed to address these challenges and fully realize the potential of microbe-mediated NPs. We believe that our review would be a constructive addition to sustainable agricultural systems to develop novel NPs for effective and low-cost management of plant pathogens to achieve global food security.
Availability of data and materials
All data used in this study are included in this article.
References
Abd Alamer IS, Tomah AA, Ahmed T, Li B, Zhang J. Biosynthesis of silver chloride nanoparticles by rhizospheric bacteria and their antibacterial activity against phytopathogenic bacterium Ralstonia solanacearum. Molecules. 2021;27(1):224.
Abdel-Aziz MM, Emam TM, Elsherbiny EA. Bioactivity of magnesium oxide nanoparticles synthesized from cell filtrate of endobacterium Burkholderia rinojensis against Fusarium oxysporum. Mater Sci Eng C. 2020;109:110617.
Aboelfetoh EF, El-Shenody RA, Ghobara MM. Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): reaction optimization, catalytic and antibacterial activities. Environ Monit Assess. 2017;189:1–15.
Adisa IO, Pullagurala VLR, Peralta-Videa JR, Dimkpa CO, Elmer WH, Gardea-Torresdey JL, White JC. Recent advances in nanoenabled fertilizers and pesticides: a critical review of mechanisms of action. Environ Sci Nano. 2019;6(7):2002–30.
Agarwal H, Menon S, Kumar SV, Rajeshkumar S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem Biol Interact. 2018;286:60–70.
Ahmed T, Noman M, Jiang H, Shahid M, Ma C, Wu Z, Nazir MM, Ali MA, White JC, Chen J. Bioengineered chitosan-iron nanocomposite controls bacterial leaf blight disease by modulating plant defense response and nutritional status of rice (Oryza sativa L.). Nano Today. 2022;45:101547.
Ahmed T, Noman M, Luo J, Muhammad S, Shahid M, Ali MA, Zhang M, Li B. Bioengineered chitosan-magnesium nanocomposite: a novel agricultural antimicrobial agent against Acidovorax oryzae and Rhizoctonia solani for sustainable rice production. Int J Biol Macromol. 2021;168:834–45.
Ahmed T, Noman M, Manzoor N, Ali S, Rizwan M, Ijaz M, Allemailem KS, BinShaya AS, Alhumaydhi FA, Li B. Recent advances in nanoparticles associated ecological harms and their biodegradation: global environmental safety from nanoinvaders. J Environ Chem Eng. 2021;9(5):106093.
Ahmed T, Noman M, Shahid M, Shahid MS, Li B. Antibacterial potential of green magnesium oxide nanoparticles against rice pathogen Acidovorax oryzae. Mater Lett. 2021;282:128839.
Ahmed T, Ren H, Noman M, Shahid M, Liu M, Ali MA, Zhang J, Tian Y, Qi X, Li B. Green synthesis and characterization of zirconium oxide nanoparticles by using a native Enterobacter sp. and its antifungal activity against bayberry twig blight disease pathogen Pestalotiopsis versicolor. NanoImpact. 2021;21:100281.
Ahmed T, Shahid M, Noman M, Niazi MBK, Mahmood F, Manzoor I, Zhang Y, Li B, Yang Y, Yan C. Silver Nanoparticles synthesized by using Bacillus cereus SZT1 ameliorated the damage of bacterial leaf blight pathogen in rice. Pathogens. 2020;9(3):160.
Ahmed T, Shahid M, Noman M, Niazi MBK, Zubair M, Almatroudi A, Khurshid M, Tariq F, Mumtaz R, Li B. Bioprospecting a native silver-resistant Bacillus safensis strain for green synthesis and subsequent antibacterial and anticancer activities of silver nanoparticles. J Adv Res. 2020;24:475–83.
Ahmed T, Wu Z, Jiang H, Luo J, Noman M, Shahid M, Manzoor I, Allemailem KS, Alrumaihi F, Li B. Bioinspired green synthesis of zinc oxide nanoparticles from a native Bacillus cereus strain RNT6: characterization and antibacterial activity against rice panicle blight pathogens Burkholderia glumae and B. gladioli. Nanomaterials. 2021;11(4):884.
Ahsan T. Biofabrication of silver nanoparticles from Pseudomonas fluorescens to control tobacco mosaic virus. Egypt J Biol Pest Control. 2020;30(1):66.
Akther T, Hemalatha S. Mycosilver nanoparticles: synthesis, characterization and its efficacy against plant pathogenic fungi. Bionanoscience. 2019;9(2):296–301.
Al-Khattaf FS. Gold and silver nanoparticles: green synthesis, microbes, mechanism, factors, plant disease management and environmental risks. Saudi J Biol Sci. 2021;28(6):3624–31.
Alfryyan N, Kordy MG, Abdel-Gabbar M, Soliman HA, Shaban M. Characterization of the biosynthesized intracellular and extracellular plasmonic silver nanoparticles using Bacillus cereus and their catalytic reduction of methylene blue. Sci Rep. 2022;12(1):12495.
Ali M, Ahmed T, Wu W, Hossain A, Hafeez R, Islam Masum M, Wang Y, An Q, Sun G, Li B. Advancements in plant and microbe-based synthesis of metallic nanoparticles and their antimicrobial activity against plant pathogens. Nanomaterials. 2020;10(6):1146.
Apte M, Sambre D, Gaikawad S, Joshi S, Bankar A, Kumar AR, Zinjarde S. Psychrotrophic yeast Yarrowia lipolytica NCYC 789 mediates the synthesis of antimicrobial silver nanoparticles via cell-associated melanin. AMB Express. 2013;3(1):32.
Balakumaran M, Ramachandran R, Kalaichelvan P. Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol Res. 2015;178:9–17.
Baskar G, Chandhuru J, Fahad KS, Praveen A. Mycological synthesis, characterization and antifungal activity of zinc oxide nanoparticles. Asian J Pharm Technol. 2013;3(4):142–6.
Bindraban PS, Dimkpa CO, Angle S, Rabbinge R. Unlocking the multiple public good services from balanced fertilizers. Food Secur. 2018;10(2):273–85.
Çalışkan G, Mutaf T, Öncel SŞ, Elibol M. Green synthesis of metal nanoparticles using Microalga Galdieria sp. CMBEBIH 2019. In: Proceedings of the International Conference on Medical and Biological Engineering, 16 -- 18 May 2019, Banja Luka, Bosnia and Herzegovina. Switzerland: Springer Nature; 2020. pp. 219–24.
Castro L, Blázquez ML, Muñoz JA, González F, Ballester A. Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. 2013;7(3):109–16.
Chakraborty S, Singh A, Roychoudhury A. Biogenic nanoparticles and generation of abiotic stress-resilient plants: a new approach for sustainable agriculture. Plant Stress. 2022;6:100117.
Chan SS, Low SS, Chew KW, Ling TC, Rinklebe J, Juan JC, Ng EP, Show PL. Prospects and environmental sustainability of phyconanotechnology: a review on algae-mediated metal nanoparticles synthesis and mechanism. Environ Res. 2022;212:113140.
Chen J, Wu L, Lu M, Lu S, Li Z, Ding W. Comparative study on the fungicidal activity of metallic MgO nanoparticles and macroscale MgO against soilborne fungal phytopathogens. Front Microbiol. 2020;11:365.
Chhipa H. Nanofertilizers and nanopesticides for agriculture. Environ Chem Lett. 2017;15(1):15–22.
Cvjetko P, Zovko M, Štefanić PP, Biba R, Tkalec M, Domijan A-M, Vrček IV, Letofsky-Papst I, Šikić S, Balen B. Phytotoxic effects of silver nanoparticles in tobacco plants. Environ Sci Pollut Res. 2018;25:5590–602.
da Silva Ferreira V, ConzFerreira ME, Lima LMT, Frasés S, de Souza W, Sant’Anna C. Green production of microalgae-based silver chloride nanoparticles with antimicrobial activity against pathogenic bacteria. Enzyme Microb Technol. 2017;97:114–21.
Dağlıoğlu Y, Yılmaz Öztürk B. A novel intracellular synthesis of silver nanoparticles using Desmodesmus sp. (Scenedesmaceae): different methods of pigment change. Rend Lincei Sci Fis Nat. 2019;30:611–21.
Dahoumane SA, Mechouet M, Wijesekera K, Filipe CD, Sicard C, Bazylinski DA, Jeffryes C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology–a review. Green Chem. 2017;19(3):552–87.
Devi PV, Duraimurugan P, Chandrika K. Bacillus thuringiensis-based nanopesticides for crop protection. In: Nanobiopesticides today and future perspectives. Cambridge: Academic Press; 2019. pp. 249–260.
Dimkpa CO, Bindraban PS. Nanofertilizers: new products for the industry? J Agric Food Chem. 2017;66(26):6462–73.
El-Ashmony RM, Zaghloul NS, Milošević M, Mohany M, Al-Rejaie SS, Abdallah Y, Galal AA. The biogenically efficient synthesis of silver nanoparticles using the fungus Trichoderma harzianum and their antifungal efficacy against Sclerotinia sclerotiorum and Sclerotium rolfsii. J Fungi. 2022;8(6):597.
El-Shetehy M, Moradi A, Maceroni M, Reinhardt D, Petri-Fink A, Rothen-Rutishauser B, Mauch F, Schwab F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat Nanotechnol. 2021;16(3):344–53.
Elmer W, White JC. The future of nanotechnology in plant pathology. Annu Rev Phytopathol. 2018;56:111–33.
Guilger-Casagrande M, Lima RD. Synthesis of silver nanoparticles mediated by fungi: a review. Front Bioeng Biotechnol. 2019;7:287.
Gupta T, Saxena J. Biogenic synthesis of silver nanoparticles from aspergillus oryzae mtcc 3107 against plant pathogenic fungi sclerotinia sclerotiorum mtcc 8785. J Microbiol Biotechnol Food Sci. 2023;12(4):e9387–e9387.
Handy RD, Shaw BJ. Toxic effects of nanoparticles and nanomaterials: implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc. 2007;9(2):125–44.
Hassan SE-D, Fouda A, Radwan AA, Salem SS, Barghoth MG, Awad MA, Abdo AM, El-Gamal MS. Endophytic actinomycetes Streptomyces spp mediated biosynthesis of copper oxide nanoparticles as a promising tool for biotechnological applications. J Biol Inorg Chem. 2019;24(3):377–93.
Hassan SE-D, Salem SS, Fouda A, Awad MA, El-Gamal MS, Abdo AM. New approach for antimicrobial activity and biocontrol of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J Radiat Res Appl Sci. 2018;11(3):262–70.
Hatamleh AA, Danish M, Al-Dosary MA, El-Zaidy M, Ali S. Physiological and oxidative stress responses of Solanum lycopersicum (L.)(tomato) when exposed to different chemical pesticides. RSC Adv. 2022;12(12):7237–52.
Hofmann T, Lowry GV, Ghoshal S, Tufenkji N, Brambilla D, Dutcher JR, Gilbertson LM, Giraldo JP, Kinsella JM, Landry MP. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat Food. 2020;1(7):416–25.
Hossain A, Hong X, Ibrahim E, Li B, Sun G, Meng Y, Wang Y, An Q. Green synthesis of silver nanoparticles with culture supernatant of a bacterium Pseudomonas rhodesiae and their antibacterial activity against soft rot pathogen Dickeya dadantii. Molecules. 2019;24(12):2303.
Hu C, Li M, Cui Y, Li D, Chen J, Yang L. Toxicological effects of TiO2 and ZnO nanoparticles in soil on earthworm Eisenia fetida. Soil Biol Biochem. 2010;42(4):586–91.
Hulkoti NI, Taranath T. Biosynthesis of nanoparticles using microbes—a review. Colloids Surf B Biointerfaces. 2014;121:474–83.
Hussain M, Shakoor N, Adeel M, Ahmad MA, Zhou H, Zhang Z, Xu M, Rui Y, White JC. Nanoenabled plant microbiome engineering for disease resistance. Nano Today. 2023;48:101752.
Husseiny M, Abd El-Aziz M, Badr Y, Mahmoud M. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim Acta A Mol Biomol Spectrosc. 2007;67(3–4):1003–6.
Iavicoli I, Leso V, Beezhold DH, Shvedova AA. Nanotechnology in agriculture: opportunities, toxicological implications, and occupational risks. Toxicol Appl Pharmacol. 2017;329:96–111.
Ibrahim E, Fouad H, Zhang M, Zhang Y, Qiu W, Yan C, Li B, Mo J, Chen J. Biosynthesis of silver nanoparticles using endophytic bacteria and their role in inhibition of rice pathogenic bacteria and plant growth promotion. RSC Adv. 2019;9(50):29293–9.
Ibrahim E, Luo J, Ahmed T, Wu W, Yan C, Li B. Biosynthesis of silver nanoparticles using onion endophytic bacterium and its antifungal activity against rice pathogen Magnaporthe oryzae. J Fungi. 2020;6(4):294.
Ibrahim E, Zhang M, Zhang Y, Hossain A, Qiu W, Chen Y, Wang Y, Wu W, Sun G, Li B. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat Fusarium head blight pathogen Fusarium graminearum. Nanomaterials. 2020;10(2):219.
Intisar A, Ramzan A, Sawaira T, Kareem AT, Hussain N, Din MI, Bilal M, Iqbal HM. Occurrence, toxic effects, and mitigation of pesticides as emerging environmental pollutants using robust nanomaterials–a review. Chemosphere. 2022;293:133538.
Jain D, Kothari S. Green synthesis of silver nanoparticles and their application in plant virus inhibition. J Mycol Plant Pathol. 2014;44(1):21–4.
Jain N, Bhargava A, Tarafdar JC, Singh SK, Panwar J. A biomimetic approach toward synthesis of zinc oxide nanoparticles. Appl Microbiol Biotechnol. 2013;97(2):859–69.
Jayaseelan C, Rahuman AA, Kirthi AV, Marimuthu S, Santhoshkumar T, Bagavan A, Gaurav K, Karthik L, Rao KB. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta A Mol Biomol Spectrosc. 2012;90:78–84.
Jiang L, Xiang S, Lv X, Wang X, Li F, Liu W, Liu C, Ran M, Huang J, Xu X. Biosynthesized silver nanoparticles inhibit Pseudomonas syringae pv. tabaci by directly destroying bacteria and inducing plant resistance in Nicotiana benthamiana. Phytopathol Res. 2022;4(1):43.
Joshi SM, De Britto S, Jogaiah S, Ito S-I. Mycogenic selenium nanoparticles as potential new generation broad spectrum antifungal molecules. Biomolecules. 2019;9(9):419.
Jovanović B, Bezirci G, Çağan AS, Coppens J, Levi EE, Oluz Z, Tuncel E, Duran H, Beklioğlu M. Food web effects of titanium dioxide nanoparticles in an outdoor freshwater mesocosm experiment. Nanotoxicology. 2016;10(7):902–12.
Kah M, Hofmann T. Nanopesticide research: current trends and future priorities. Environ Int. 2014;63:224–35.
Karthik L, Kumar G, Kirthi AV, Rahuman A, Rao KB. Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess Biosyst Eng. 2014;37(2):261–7.
Kaur M, Gautam A, Guleria P, Singh K, Kumar V. Green synthesis of metal nanoparticles and their environmental applications. Curr Opin Environ Sci Health. 2022;29:100390.
Ke M, Qu Q, Peijnenburg W, Li X, Zhang M, Zhang Z, Lu T, Pan X, Qian H. Phytotoxic effects of silver nanoparticles and silver ions to Arabidopsis thaliana as revealed by analysis of molecular responses and of metabolic pathways. Sci Total Environ. 2018;644:1070–9.
Khan R, Fulekar M. Biosynthesis of titanium dioxide nanoparticles using Bacillus amyloliquefaciens culture and enhancement of its photocatalytic activity for the degradation of a sulfonated textile dye Reactive Red 31. J Colloid Interface Sci. 2016;475:184–91.
Korani M, Ghazizadeh E, Korani S, Hami Z, Mohammadi-Bardbori A. Effects of silver nanoparticles on human health. Eur J Nanomed. 2015;7(1):51–62.
Koul B, Poonia AK, Yadav D, Jin J-O. Microbe-mediated biosynthesis of nanoparticles: applications and future prospects. Biomolecules. 2021;11(6):886.
Kovendan K, Chandramohan B, Govindarajan M, Jebanesan A, Kamalakannan S, Vincent S, Benelli G. Orchids as sources of novel nanoinsecticides? Efficacy of Bacillus sphaericus and Zeuxine gracilis-fabricated silver nanoparticles against dengue, malaria and filariasis mosquito vectors. J Cluster Sci. 2018;29(2):345–57.
Kumar P, Pandhi S, Mahato DK, Kamle M, Mishra A. Bacillus-based nanobioformulations for phytopathogens and insect–pest management. Egypt J Biol Pest Control. 2021;31:1–11.
Kumari M, Pandey S, Bhattacharya A, Mishra A, Nautiyal C. Protective role of biosynthesized silver nanoparticles against early blight disease in Solanum lycopersicum. Plant Physiol Biochem. 2017;121:216–25.
Lassoued A, Dkhil B, Gadri A, Ammar S. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 2017;7:3007–15.
Li L, Zhang Z. Biosynthesis of gold nanoparticles using green alga pithophora oedogoniawith their electrochemical performance for determining carbendazim in soil. Int J Electrochem Sci. 2016;11:4550–9.
Li X, Xu H, Chen ZS, Chen G. Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater. 2011;2011:1–16.
Li Y, Zhang P, Li M, Shakoor N, Adeel M, Zhou P, Guo M, Jiang Y, Zhao W, Lou B. Application and mechanisms of metal-based nanoparticles in the control of bacterial and fungal crop diseases. Pest Manag Sci. 2023;79(1):21–36.
Luo X, Wang Z, Wang C, Yue L, Tao M, Elmer WH, White JC, Cao X, Xing B. Nanomaterial size and surface modification mediate disease resistance activation in cucumber (Cucumis sativus). ACS Nano. 2023;17(5):4871–85.
Ma C, Borgatta J, Hudson BG, Tamijani AA, La Torre-Roche D, Zuverza-Mena N, Shen Y, Elmer W, Xing B, Mason SE. Advanced material modulation of nutritional and phytohormone status alleviates damage from soybean sudden death syndrome. Nat Nanotechnol. 2020;15(12):1033–42.
Menazea A, Ismail A, Awwad NS, Ibrahium HA. Physical characterization and antibacterial activity of PVA/Chitosan matrix doped by selenium nanoparticles prepared via one-pot laser ablation route. J Market Res. 2020;9(5):9598–606.
Mistry H, Thakor R, Patil C, Trivedi J, Bariya H. Biogenically proficient synthesis and characterization of silver nanoparticles employing marine procured fungi Aspergillus brunneoviolaceus along with their antibacterial and antioxidative potency. Biotech Lett. 2021;43:307–16.
Mourato A, Gadanho M, Lino AR, Tenreiro R. Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorg Chem Appl. 2011;2011:546074.
Namburi KR, Kora AJ, Chetukuri A, Kota VSMK. Biogenic silver nanoparticles as an antibacterial agent against bacterial leaf blight causing rice phytopathogen Xanthomonas oryzae pv. oryzae. Bioprocess Biosyst Eng. 2021;44(9):1975–88.
Nandini B, Krishna L, Jogigowda SC, Nagaraja G, Hadimani S, Ali D, Sasaki K, Jogaiah S. Significance of Bryophyllum pinnatum (Lam.) for green synthesis of antibacterial copper and selenium nanoparticles and their influence on soil microflora. Appl Nanosci. 2023;13:1–15.
Noman M, Ahmed T, Hussain S, Niazi MBK, Shahid M, Song F. Biogenic copper nanoparticles synthesized by using a copper-resistant strain Shigella flexneri SNT22 reduced the translocation of cadmium from soil to wheat plants. J Hazard Mater. 2020a;398:123175.
Noman M, Ahmed T, Ijaz U, Hameed A, Shahid M, Azizullah, Li D, Song F. Microbe-oriented nanoparticles as phytomedicines for plant health management: An emerging paradigm to achieve global food security. Crit Rev Food Sci Nutr. 2022:1–21.
Noman M, Ahmed T, Ijaz U, Shahid M, Nazir MM, White JC, Li D, Song F. Bio-functionalized manganese nanoparticles suppress Fusarium wilt in watermelon (Citrullus lanatus L.) by infection disruption, host defense response potentiation, and soil microbial community modulation. Small. 2023a;19(2):2205687.
Noman M, Ahmed T, White JC, Nazir MM, Li D, Song F. Bacillus altitudinis-stabilized multifarious copper nanoparticles prevent bacterial fruit blotch in watermelon (Citrullus lanatus L.): direct pathogen inhibition, in planta particles accumulation, and host stomatal immunity modulation. Small. 2023b;19(15):2207136.
Noman M, Shahid M, Ahmed T, Niazi MBK, Hussain S, Song F, Manzoor I. Use of biogenic copper nanoparticles synthesized from a native Escherichia sp. as photocatalysts for azo dye degradation and treatment of textile effluents. Environ Pollut. 2020b;257:113514.
Noman M, Shahid M, Ahmed T, Tahir M, Naqqash T, Muhammad S, Song F, Abid HMA, Aslam Z. Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol Environ Saf. 2020;192:110303.
Öcal N, Ceylan A, Duman F. Intracellular biosynthesis of PbS quantum dots using Pseudomonas aeruginosa ATCC 27853: evaluation of antibacterial effects and DNA cleavage activities. World J Microbiol Biotechnol. 2020;36(10):147.
Ogunsona EO, Muthuraj R, Ojogbo E, Valerio O, Mekonnen TH. Engineered nanomaterials for antimicrobial applications: a review. Appl Mater Today. 2020;18:100473.
Ogunyemi SO, Zhang M, Abdallah Y, Qiu W, Ahmed T, Ali MA, Yan C, Yang Y, Chen J, Li B. The biosynthesis of three metal oxide nanoparticles (ZnO, MnO2, and MgO) and their antibacterial activity against the bacterial leaf blight pathogen. Front Microbiol. 2020;11:3099.
Omran BA, Baek K-H. Control of phytopathogens using sustainable biogenic nanomaterials: recent perspectives, ecological safety, and challenging gaps. J Clean Prod. 2022;372:133729.
Osonga FJ, Akgul A, Yazgan I, Akgul A, Eshun GB, Sakhaee L, Sadik OA. Size and shape-dependent antimicrobial activities of silver and gold nanoparticles: a model study as potential fungicides. Molecules. 2020;25(11):2682.
Panichikkal J, Thomas R, John JC, Radhakrishnan E. Biogenic gold nanoparticle supplementation to plant beneficial Pseudomonas monteilii was found to enhance its plant probiotic effect. Curr Microbiol. 2019;76:503–9.
Ponmurugan P, Manjukarunambika K, Elango V, Gnanamangai BM. Antifungal activity of biosynthesized copper nanoparticles evaluated against red root-rot disease in tea plants. J Exp Nanosci. 2016;11(13):1019–31.
Pooyandeh S, Shahidi S, Khajehnezhad A, Ghoranneviss Z. Synthesizing and deposition of nickel oxide nanoparticles on glass mat using sol–gel method (morphological and magnetic properties). J Text Inst. 2020;112:1–9.
Qamar SUR, Ahmad JN. Nanoparticles: Mechanism of biosynthesis using plant extracts, bacteria, fungi, and their applications. J Mol Liq. 2021;334:116040.
Qu Y, Li X, Lian S, Dai C, Jv Z, Zhao B, Zhou H. Biosynthesis of gold nanoparticles using fungus Trichoderma sp. WL-Go and their catalysis in degradation of aromatic pollutants. IET Nanobiotechnol. 2019;13(1):12–7.
Radzimska AK, Jesionowski T. Zinc oxide-from synthesis to application: a review. Materials. 2014;7(4):2833–81.
Rai M, Bonde S, Golinska P, Trzcińska-Wencel J, Gade A, Abd-Elsalam KA, Shende S, Gaikwad S, Ingle AP. Fusarium as a novel fungus for the synthesis of nanoparticles: mechanism and applications. J Fungi. 2021;7(2):139.
Raj N, Swamy M, Purushotham B, Sukrutha S. Applications of microbe-based nanoparticles in agriculture: present state and future challenges. Microb Nanobiotechnol Princ Appl. 2021:343–382.
Rajeshkumar S, Sivapriya D. Fungus-mediated nanoparticles: characterization and biomedical advances. Nanoparticles Med. 2020:185–199.
Reddy KV, Sree NRS, Kumar PS, Ranjit P. Microbial enzymes in the biosynthesis of metal nanoparticles. In: Ecological interplays in microbial enzymology. Singapore: Springer Nature; 2022. pp. 329–350.
Salas-Herrera G, González-Morales S, Benavides-Mendoza A, Castañeda-Facio AO, Fernández-Luqueño F, Robledo-Olivo A. Impact of microalgae culture conditions over the capacity of copper nanoparticle biosynthesis. J Appl Phycol. 2019;31(4):2437–47.
Selvarajan E, Mohanasrinivasan V. Biosynthesis and characterization of ZnO nanoparticles using Lactobacillus plantarum VITES07. Mater Lett. 2013;112:180–2.
Seppelt R, Klotz S, Peiter E, Volk M. Agriculture and food security under a changing climate: an underestimated challenge. Iscience. 2022;25:105551.
Sharma B, Tiwari S, Kumawat KC, Cardinale M. Nanobiofertilizers as bioemerging strategies for sustainable agriculture development: potentiality and their limitations. Sci Total Environ. 2023;860:160476.
Shinde BH, Inamdar SN, Nalawade SA, Chaudhari SB. A systematic review on antifungal and insecticidal applications of biosynthesized metal nanoparticles. Mater Today Proc. 2022;73:412–7.
Shobha B, Lakshmeesha TR, Ansari MA, Almatroudi A, Alzohairy MA, Basavaraju S, Alurappa R, Niranjana SR, Chowdappa S. Mycosynthesis of ZnO nanoparticles using Trichoderma spp. isolated from Rhizosphere soils and its synergistic antibacterial effect against Xanthomonas oryzae pv oryzae. J Fungi. 2020;6(3):181.
Singh A, Gaurav SS, Shukla G, Rani P. Assessment of mycogenic zinc nanofungicides against pathogenic early blight (Alternaria solani) of potato (Solanum tuberosum L.). Mater Today Proc. 2022;49:3528–37.
Singh P, Kim Y-J, Zhang D, Yang D-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34(7):588–99.
Singh R, Wagh P, Wadhwani S, Gaidhani S, Kumbhar A, Bellare J, Chopade BA. Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int J Nanomed. 2013;8:4277.
Spagnoletti FN, Spedalieri C, Kronberg F, Giacometti R. Extracellular biosynthesis of bactericidal Ag/AgCl nanoparticles for crop protection using the fungus Macrophomina phaseolina. J Environ Manage. 2019;231:457–66.
Taha ZK, Hawar SN, Sulaiman GM. Extracellular biosynthesis of silver nanoparticles from Penicillium italicum and its antioxidant, antimicrobial and cytotoxicity activities. Biotech Lett. 2019;41:899–914.
Thakker JN, Dalwadi P, Dhandhukia PC. Biosynthesis of gold nanoparticles using Fusarium oxysporum f. sp. cubense JT1, a plant pathogenic fungus. ISRN Biotechnol. 2012;2013:1–5.
Tomah AA, Alamer ISA, Li B, Zhang J-Z. Mycosynthesis of silver nanoparticles using screened trichoderma isolates and their antifungal activity against Sclerotinia sclerotiorum. Nanomaterials. 2020;10(10):1955.
Tyagi S, Tyagi PK, Gola D, Chauhan N, Bharti RK. Extracellular synthesis of silver nanoparticles using entomopathogenic fungus: characterization and antibacterial potential. SN Appl Sci. 2019;1:1–9.
Varshney R, Bhadauria S, Gaur MS, Pasricha R. Characterization of copper nanoparticles synthesized by a novel microbiological method. Jom. 2010;62(12):102–4.
Xu L, Zhu Z, Sun D-W. Bioinspired nanomodification strategies: moving from chemical-Based agrosystems to sustainable agriculture. ACS Nano. 2021;15(8):12655–86.
Yadav SA, Suvathika G, Alghuthaymi MA, Abd-Elsalam KA. Fungal-derived nanoparticles for the control of plant pathogens and pests. In: Fungal cell factories for sustainable nanomaterials productions and agricultural applications. 2023. pp. 755–784.
Yan X, Chen S, Pan Z, Zhao W, Rui Y, Zhao L. AgNPs-triggered seed metabolic and transcriptional reprogramming enhanced rice salt tolerance and blast resistance. ACS Nano. 2022;17:492–504.
Yang Y, Waterhouse GI, Chen Y, Sun-Waterhouse D, Li D. Microbial-enabled green biosynthesis of nanomaterials: current status and future prospects. Biotechnol Adv. 2022;55:107914.
Zeng H, Bai Y, Wei Y, Reiter RJ, Shi H. Phytomelatonin as a central molecule in plant disease resistance. J Exp Bot. 2022;73(17):5874–85.
Zhang H, Zhou H, Bai J, Li Y, Yang J, Ma Q, Qu Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf, A. 2019;571:9–16.
Zhang W. Global pesticide use: profile, trend, cost/benefit and more. Proc Int Acad Ecol Environ Sci. 2018;8(1):1.
Acknowledgements
The work is partially supported by Shanghai Agriculture Applied Technology Development Program (2021-02-08-00-12-F00771), National Key Research and Development Program of Ningbo (2022Z175), Zhejiang Provincial Key R&D Program of China (2019C02006, 2020C02001, 2023C04005), National Natural Science Foundation of China (32072472, 31872017), Hangzhou Science and Technology Development Plan Project (202003A05), Agricultural and social development project of Jiangbei District, Ningbo in 2021 (2021B01), State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agro-products (grant number 2010DS700124-ZZ2014;-KF202101;-KF202205).
Author information
Authors and Affiliations
Contributions
“Temoor Ahmed: Conceptualization, Investigation, Validation, Resources and Writing – original draft. Jinyan Luo and Muhammad Noman: Validation, Investigation, Writing – review & editing. Munazza Ijaz, Xiao Wang, Hafiza Ayesha Masood and Natasha Manzoor: Investigation, Writing – original draft. Yanli Wang and Bin Li: Supervision, Writing – review & editing, Resource, Validation”.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
“The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper”.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, T., Luo, J., Noman, M. et al. Microbe-mediated nanoparticle intervention for the management of plant diseases. Crop Health 1, 3 (2023). https://doi.org/10.1007/s44297-023-00006-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44297-023-00006-9