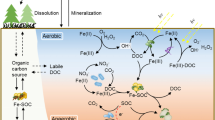
Abstract
The significant impact of vegetation restoration on the turnover of organic carbon (OC) is mainly attributed to biological processes. However, the contribution of abiotic processes associated with reactive oxygen species (ROS) during vegetation restoration has remained largely overlooked. Therefore, we systematically explored ROS distribution and production mechanisms in sandy soil and their corresponding effects on OC oxidation through field monitoring and incubation experiments. The cumulative concentrations of hydroxyl radicals (•OH) produced within 24 h varied in different soils undergoing vegetation restoration, ranging from 2.36 to 22.05 μmol kg‒1, which were derived from the Fenton-like reaction of hydrogen peroxide (H2O2) and biotic-induced Fe(II). In addition, during the oxidation of reductive SOC, H2O2 can be formed and react with Fe(II) to generate •OH. Further, •OH significantly induced the abiotic transformation of OC, accounting for 15.93%‒25.80% of carbon dioxide (CO2) efflux. Specifically, 1 mol •OH can induce the production of 0.01–0.02 mol CO2 for particulate organic carbon (POC) and 0.03‒0.23 mol CO2 for mineral-associated organic carbon (MOC), as demonstrated by chemical quenching and sterilisation experiments. This indicated that MOC was more sensitive to •OH attacks. Collectively, our findings provide insights into the accumulation of ROS in sandy soils during vegetation restoration, with •OH playing a key role in SOC oxidation, as well as the patterns of SOC turnover and its response to changes in the soil environment.
Graphical Abstract

Highlights
• Vegetation restoration exerts a regulatory influence on ROS production in sandy soil.
• ROS accumulation in tree-covered soil is more than that in shrub-covered soil.
• 15.93%‒25.80% of total CO2 efflux in sandy soils is from •OH mediated OC mineralisation.
• Mineral-associated OC exhibits a higher susceptibility to •OH attack than particulate OC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Soils store at least three times as much organic carbon (OC) as the atmosphere or living plants (Schmidt et al. 2011). Notably, the carbon sequestration potential of different soil types can be significantly influenced by variations in soil texture, structure, active components, and microbial communities (Lehmann et al. 2020). Sandy soil, which is characterised by sparse vegetation and low soil organic carbon (SOC) content, is considered one of the soil types with the highest C sequestration potential (Dong et al. 2022). Vegetation restoration is widely used to enhance the carbon sequestration capacity of sandy soil (Feng et al. 2016; Wang et al. 2020). During vegetation restoration, the decomposition and transformation of plant inputs into stable SOC play a crucial role in carbon sequestration (Cotrufo et al. 2015; Craig et al. 2022). Particularly, the size of the carbon pool is determined by the equilibrium between the production of SOC through the decomposition of plant litter and its mineralisation to inorganic carbon (Cotrufo et al. 2015). The mineralisation of SOC has long been thought to be primarily driven by biotic processes (Liang et al. 2017; Jansson and Hofmockel 2020). However, recent studies have revealed the significant contribution of abiotic processes such as photodegradation, hydrolysis, desorption from the solid phase, and reactive oxygen species (ROS)-mediated chemical oxidation (Brookes et al. 2009; Chen et al. 2021; Yu and Kuzyakov 2021).
ROS, including superoxide (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH), are commonly found in natural environments and play significant roles in element cycling, nutrient dynamics, and biogeochemical cycles (Liu et al. 2022; Zhao et al. 2022). Furthermore, ROS production has important implications for SOC transformation (Hall and Silver 2013; Trusiak et al. 2018; Kleber et al. 2021). Among them, the •OH radical is widely known as a non-selective and strong oxidant (Vaughan and Blough 1998; Page et al. 2013; Kleber et al. 2021) It has high reaction rates with OC (108 M C−1 s−1), which is difficult to biodegrade or photodegrade. Therefore, OH radical could mediate a wide range of chemical reactions, including the CO2 emissions and the cycling of nutrient elements (Goldstone et al. 2002; Tong et al. 2016; Trusiak et al. 2018). For instance, Trusiak et al. (2018) reported that the oxidation of dissolved organic matter to CO2 was attributed to •OH generated by the oxidation of Fe(II) in Arctic soil waters. Likewise, the increased stimulation of C mineralisation observed in aerobic incubations of humid and tropical soils following the addition of Fe(II) is ascribed to the effects of ROS (Hall and Silver 2013; Chen et al. 2020). However, very little is known regarding the effect of ROS on carbon transformation in sandy soils during vegetation restoration. Additionally, the physical separation of SOC can be classified into particulate organic carbon (POC) and mineral-associated organic carbon (MOC) based on size and density (Cotrufo et al. 2019; Witzgall et al. 2021; Heckman et al. 2022). POC and MOC have distinct biogeochemical properties and turnover rates due to the inherent differences in their sources and degrees of physicochemical protection (Cotrufo et al. 2019; Yu et al. 2022). These SOC forms may thus exhibit different reactivities toward ROS, and therefore exploring their susceptibility to ROS attack is of great significance for assessing carbon turnover during vegetation restoration.
ROS production is generally associated with photochemical processes occurring in surface water, atmospheric particles, and the upper layers of soil (Burns et al. 2010; Page et al. 2013; Tong et al. 2016). However, in intermittent redox turnover, the extracellular electrons generated through anaerobic microbial respiration are exposed to oxygen, which can also produce ROS (Zhang et al. 2016; Han et al. 2020). Recent studies have also indicated that the oxygenation of the reduced species (e.g., Fe and S-associated minerals and organic matter) can produce abundant ROS (Hall and Silver 2013; Tong et al. 2016; Yu and Kuzyakov 2021; Liu et al. 2022). Vegetation restoration can induce the accumulation of the reduced species in the soil (Cui et al. 2020), which play key roles in storing and shuttling electrons that induce ROS production. Meanwhile, the diversity of microbial communities can also be influenced by vegetation restoration (Cui et al. 2020). For instance, vegetation litter or root exudates provide an important source of nutrients and carbon substrates to microbes, thus promoting the increase of microbial abundance (bacteria and fungi) in soil (Craig et al. 2022). These microbes can directly produce extracellular ROS, such as O2•− and H2O2 (Diaz et al. 2013; Liu et al. 2022). In addition, ROS can also be triggered by the interaction between oxygen released by roots and extracellular electrons released by microbial respiration (Dai et al. 2022). Moreover, different vegetation types such as trees and shrubs can lead to variations in these phenomena (Jilling et al. 2021; Craig et al. 2022; Huang et al. 2022). Compared to shrubs, trees typically introduce more litter and root exudates into soil ecosystems (Huang et al. 2022). This leads to the accumulation of active species and the restructuring of microbial communities, which likely result in variations in ROS production. Therefore, assessing ROS production in various vegetation restoration scenarios is crucial for gaining a comprehensive understanding of the biogeochemical processes in sandy soil.
The Mu Us sandy land, located in central north China, is among the most representative sandy regions worldwide (Liu et al. 2021). Long-term desertification management has significantly improved the local surface vegetation composition, transforming bare sandy land into areas covered by various shrubs and trees, which have important impacts on multiple biogeochemical processes (Heimann et al. 2008; Zeng et al. 2009). Hence, soil samples were collected from the Mu Us Sandy Land to investigate the generation of ROS in response to different types of vegetation restoration. The main objectives of this study were to: (i) explore the variations in ROS generation among various vegetation restoration soils and identify potential influencing factors, (ii) examine the fluctuation of ROS during soil respiration and quantify its contribution to OC mineralisation, and (iii) investigate the susceptibility of POC and MOC to abiotic oxidation driven by •OH. These results will provide new insights into the ROS-driven abiotic mechanisms for SOC decomposition in vegetation-restored soils and are of great significance for the evaluation of carbon biogeochemical cycles.
2 Materials and methods
2.1 Study site and sampling
The study was conducted at Hongshixia Sandy Botanical Garden of Mu Us Sandy Land, Yulin City, Shaanxi Province, China (38°19’–22’ N; 109°37’–49’ E). The site covers an area of approximately 300 hm2 and an altitude of 1080 m. The region has a semi-arid continental monsoon climate that falls within the temperate zone, with an annual rainfall of 250‒450 mm and annual temperature of 6‒8.5 °C, and the predominant soil type is aeolian sand soil (Chao et al. 2018). Soil samples were collected from six distinct vegetation types, including three types of trees (Pinus sylvestris, Pinus tabuliformis, and Populus alba) and three shrubs (Artemisia desertorum, Hedysarum multijugum Maxim., and Salix cheilophila) at depths ranging from 0 to 20 cm. Additionally, bare sand samples were collected as a control. PVC pipes with a 15 cm diameter and a 30 cm height were inserted into the soil to collect the soil samples, which were quickly transferred to the laboratory and were stored in an environment-controlled incubation room to preserve their original state. The samples were then divided into two subsamples. One for ROS determination where the samples were immediately moved from the interior of the soil column of PVC pipes into an anaerobic glovebox (Mikrouna China) filled with ultrapure N2 gas to prevent interference from oxygen. Subsequently, 0.5 g of soil was mixed with the molecular probe for quantifying the production of ROS. Then, it was air-dried for physicochemical parameter analysis, whereas the other subsample was stored at ‒80 °C for the microbial analyses.
2.2 Incubation experiment
2.2.1 Soil respiration experiment
An incubation experiment was performed to better understand the variations in ROS levels during soil respiration and their impact on SOC mineralisation. Here, 50 g of representative soil samples (tree: Populus alba, shrub: Hedysarum multijugum Maxim., control: bare sand) were selected, and the field water holding capacity was maintained at 40% (This moisture level provides enough water for both microbial activity and nutrient availability without causing water stress or over-saturation). To ensure the recovery of microbial activity, the soils were pre-incubated for 14 d at 25 °C prior to the experiment (Singh et al. 2017). Three 500-mL jars were prepared for each of the aforementioned samples, nine jars in total, and the atmosphere inside the jars was air. The jars were sealed with a rubber stopper and incubated in the dark for 36 d at 25 °C. Soil samples and jars were both oxygenated during sample collection to maintain aerobic conditions. At 1, 3, 6, 10, 15, 21, 28, and 36 d, the initial and final top atmosphere of the jars were sampled into a 25 mL injector and CO2 concentration analysis was performed using Agilent Technologies 7890B gas chromatography system. Additionally, 5 g of soil samples were collected for other analyses including ROS, dissolved Fe(II), β-1,4-glucosidase (BG), SOC, POC, MOC, and dissolved organic carbon (DOC).
2.2.2 Microbial inactivation and chemical quenching experiments
To investigate the role of abiotic processes in the production of ROS and CO2, the soils were sterilised using γ-irradiation at 50 kGy (Co source, BFT-IV, China). Additionally, a separate incubation experiment was carried out to further determine the contribution of •OH to CO2 efflux. Briefly, 20 g soil samples of Populus alba, Hedysarum multijugum Maxim., and bare sand were treated with γ-irradiation and 10% tertbutyl alcohol (TBA), and then incubated in a jar in the dark for 24 h. The native soil was used as the control group, and the accumulated •OH and CO2 production were monitored during this period.
2.2.3 POC and MOC incubation experiment
POC and MOC were physically isolated using a particle size-based technique to explore their susceptibility to •OH (Lavallee et al. 2020). More details are provided in the Text S1 (Supporting Information (SI)). Similar to the above-described incubation experiment, chemical quenching and sterilisation experiments were also conducted for these two fractions of OC. The incubation experiment was conducted for 24 h and the cumulative concentrations of •OH and CO2 were measured. Additionally, to further investigate the effects of •OH on CO2 emission, incubation experiments were performed by introducing Fe(II) and H2O2 (Fenton reagents) into the sterilised POC and MOC pre-treated Populus alba samples. Briefly, 400 μL of 100 mM FeSO4·7H2O and 400 μL of deionised water (without H2O2) or a designated content of H2O2 were added into the jars containing 1 g (dry weight) of soil. Based on the detected concentration of H2O2 in the monitoring experiment, the concentration gradient of H2O2 in this incubation experiment was set as follows: 0, 10, 50, 100, and 250 μmol kg‒1. The entire incubation experiment was conducted under anaerobic conditions in an anaerobic glovebox. All samples were analysed in triplicate and incubated in the dark at 25 °C for 8 h, after which the cumulative CO2 production was measured.
2.3 Chemical analysis
Reagents and chemicals are provided in Text S2. The soil physicochemical properties in the experiment, including pH, dissolved Fe(II), SOC, POC, and MOC and DOC concentrations, were analyzed systemically. A detailed description of measurements is provided in Text S3. β-1,4-glucosidase was measured as described by Li et al. (2018).
2.4 ROS measurement
2.4.1 Qualification of ROS by electron paramagnetic resonance
Electron paramagnetic resonance (EPR) analyses were applied and qualitative detection of ROS production was performed. Specifically, 1 mL of 5,5-dimethylpyrroline-N-oxide (DMPO) solution (100 mM) was added to 200 mg soil for •OH analysis. For O2•− analysis, 200 mg of soil was mixed with 1 mL of DMPO (100 mM) prepared in dimethyl sulfoxide (DMSO) as O2•− trap. More details are provided in the Text S4.
2.4.2 Detection production of ROS by chemical probe
To assess the ROS production ability of soil, the mixed samples after the addition of molecular probes were thoroughly reacted in glovebox. At different time intervals, 1 mL samples were taken out for determination of ROS concentration until the reaction reached an equilibrium state. The cumulative concentration of •OH was measured using benzoic acid (BA) as a chemical probe, which reacts with •OH to form p-hydroxybenzoic acid (p-HBA) (Mopper and Zhou 1990). H2O2 was analysed using the titanium sulphate colourimetric method at 415 nm using a UV–vis spectrophotometer (Gokdere et al. 2019). O2•− was analysed using a modified hydroethidine-based assay (Georgiou et al. 2015). The detailed operating steps for •OH, H2O2 and O2•− detection are summarized in Text S5.
2.5 Statistical analysis
Data analysis was performed using the SPSS 20 software (IBM Corp., Armonk, NY, USA). After testing for homogeneity of variance and normality, one-way ANOVA was performed at a p < 0.05 significance level. Pearson correlation coefficients were used to examine the correlation between ROS production and the following variables: dissolved Fe(II), SOC, POC, MOC, DOC, and pH. After elucidating the correlation between response and explanatory variables, redundancy analysis (RDA) was employed to identify the primary contributing factors affecting OC transformation during vegetation restoration.
3 Results
3.1 pH, Fe(II), and organic carbon contents in various soils
To explore the influence of vegetation restoration on soil physicochemical properties, the pH, dissolved Fe(II), and OC contents of soil were measured. The pH of vegetation-covered soil varied from 8.61 to 8.74, which was slightly higher than that of bare sand (8.30, Fig. S1). The contents of Fe(II) in tree-covered and shrub-covered soils ranged from 8.96 to 49.65 mg kg‒1 and 9.05 to 43.34 mg kg‒1 (Fig. S2a), respectively. Among them, the Fe(II) contents in Populus alba (tree) and Hedysarum multijugum Maxim. (shrub) were the highest, reaching 33.27 and 29.19 mg kg‒1, respectively, which were remarkably higher than the levels in bare sand (6.98 mg kg‒1, p < 0.05, Fig. 1a). The content of SOC in vegetated soils ranged from 2.25 to 8.31 g kg‒1, and was significantly higher than that in bare sand (0.89 g kg‒1, p < 0.05, Fig. 1b). Moreover, a higher SOC content with an average of 5.50 g kg‒1 was observed in tree-covered soils compared with shrub-covered soils (3.71 g kg‒1 Fig. S2b). The POC contents in tree-covered and shrub-covered soils accounted for 64%‒67% and 65%‒76% of the SOC content, respectively, which represented a 23%‒35% increase compared to bare sand (Fig. S3a). In contrast, the proportion of MOC decreased by 23%‒26% and 24%‒35% in tree-covered and shrub-covered soils, respectively. Furthermore, tree-covered soils had a significantly higher average DOC concentration of 95.69 mg kg‒1 compared to shrub-covered soils with 79.95 mg kg‒1 and bare sand with 23.79 mg kg‒1 (Fig. S3b).
3.2 Evolution of the microbial community in various soils
To characterise the effect of vegetation restoration on bacterial community abundance, 16S rDNA amplicon libraries were constructed and sequenced via Illumina sequencing. The Shannon diversity indexes in vegetation restoration soil ranged from 10.14 to 10.52 (Table S1), whereas that in bare sand was only 9.80, indicating that vegetation restoration significantly increased the diversity of bacterial communities. To further explore the differences in the bacterial community structure in different vegetation restoration soils, the relative abundance of the 20 most-abundant phyla was analysed (Fig. 2a). The dominant bacterial phyla in vegetated soils were Proteobacteria, Acidobacteria, Actinobacteria, Planctomycetes, and Bacteroidetes. The tree-covered soils had a higher relative abundance of the aforementioned dominant bacterial phyla compared to the shrub-covered soils. Moreover, our findings indicated that the relative abundance of Deinococcus, a dominant Fe-reducing bacterial genus (Bao and Li 2017), increased significantly (p < 0.05) in vegetated soils (Fig. 2b).
3.3 ROS concentrations in various soils
As shown in Fig S4a, the EPR spectrum was deconvoluted and found to be fitted with DMPO-OOH (hyperfine coupling constants of aN = 14.3 G, aH = 8.1 G), which were characteristic for the O2•− signals (Zhang et al. 2021a, b). A four-line signal with relative intensities of 1:2:2:1 was detected in the aqueous suspension containing soil (Fig S4b), and this signal pattern is recognized as a typical characteristic signals of •OH (Liu et al. 2022).
To investigate the effects of vegetation restoration on ROS production, we quantified the concentrations of three typical ROS (O2•−, H2O2, and •OH). We chose •OH as an example, which is a potent ROS species in the environment (Tong et al. 2016). Notably, •OH is produced through reactions of O2•− and H2O2, and productions of •OH also indicate the formations of H2O2 and O2•− (Zhao et al. 2022). The •OH concentration reached equilibrium after 24 h of reaction (Fig. S5). Hence, the accumulated concentrations of ROS after 24 h were used as the production flux for in situ ROS. As illustrated in Fig. 3a, the average concentration of O2•− was 228.41 μmol kg‒1 in bare sand, but increased to 341.43‒636.11 μmol kg‒1 in vegetation-amended soils, suggesting that vegetation restoration promoted the accumulation of O2•−. For tree-covered soils, the average concentration of O2•− was 574.50 μmol kg‒1, which was significantly higher than that in soils covered by shrubs, with an average concentration of 399.25 μmol kg‒1 (Fig. S6a). Similarly, the average concentration of H2O2 in tree-covered soils with 256.92 μmol kg‒1 was higher than that in shrub-covered soils (94.24 μmol kg‒1). However, the H2O2 yield in vegetated soils was one order of magnitude lower than that in bare sand (Fig. S6b). Conversely, the •OH concentrations in soils with vegetation coverage were much higher than that of bare sand (Fig. 4a). Specifically, in tree-covered soils, the average concentration of •OH was 16.34 μmol kg‒1, whereas the shrub-covered soils had an average concentration of 9.37 μmol kg‒1, which were significantly higher than that in bare sand with 2.36 μmol kg‒1 (Fig. 4b). Therefore, in terms of the type of vegetation restoration, tree planting was more beneficial for the accumulation of •OH.
Concentrations of O2•− (a) and H2O2 (b) at different soils under vegetation restoration and bare sand. O2•− and H2O2 were the cumulative concentrations after 24 h of reaction, which were considered as the production flux for in situ O2.•− and H2O2. The bars indicate the standard error of the mean and different letters indicate significant differences (p < 0.05)
Cumulative concentrations of •OH production within 24 h at different soils under vegetation restoration and bare sand (a). Difference of cumulative concentrations of •OH production within 24 h in soils covered by trees and shrubs, and bare sand (b). The cumulative •OH concentrations after 24 h of reaction was considered as the production flux for in situ •OH. The bars indicate the standard error of the mean and different letters indicate significant differences (p < 0.05). Correlation between the cumulative •OH concentration and Fe(II) (c) and SOC (d). The lines indicate significant regressions (p < 0.05)
Previous studies have reported that the production of ROS in soil can be influenced by microbial activity (Diaz et al. 2013; Han et al. 2020). After sterilisation, almost no microorganisms were detected in the soil solution (Fig. S7a), meaning that the majority of microbial cells or colonies had been inactivated. Furthermore, the concentrations of H2O2 in Populus alba, Hedysarum multijugum Maxim., and bare sand decreased by 46.61%, 26.40%, and 27.90% after sterilisation (Fig. S7b), respectively. Similarly, the •OH concentrations before and after sterilisation were 2.18‒59.57 μmol kg−1 and 1.72‒55.80 μmol kg‒1, respectively (Fig. S8).
3.4 Simulation of soil respiration in the laboratory
To better understand the variations in ROS levels during soil respiration and its influence on SOC mineralisation, incubation experiments were conducted.
3.4.1 Physicochemical properties of soil during incubation
The pH value of soils did not change significantly after 36 d of incubation, with fluctuations ranging from 8.40 to 8.33, and 8.59 to 8.66, and 8.32 to 8.35, respectively, in Populus alba, Hedysarum multijugum Maxim., and bare sand samples (Table S2). The concentrations of dissolved Fe(II) in Populus alba, Hedysarum multijugum Maxim., and bare sand decreased from 66.49, 48.92, and 10.46 mg kg‒1 to 55.05, 43.74, and 6.85 mg kg‒1, respectively, in the first 15 d. However, in the last 21 d, they increased to 79.50, 61.47, and 10.67 mg kg‒1 respectively. Meanwhile, DOC concentrations in Populus alba, Hedysarum multijugum Maxim., and bare sand decreased dramatically from 143.15, 136.52, and 34.59 mg kg‒1 to 103.03, 89.38 and 20.64 mg kg‒1, respectively (Table S2). Likewise, the contents of SOC and POC in Populus alba, Hedysarum multijugum Maxim., and bare sand were also decreased. In contrast, MOC contents did not change significantly.
3.4.2 ROS production and CO2 efflux during incubation
During the entire incubation, the trend of •OH variation was similar for the different vegetation types, showing an upward and then downward trend. Specifically, the concentration of •OH increased rapidly on the 3 d of the incubation, reaching a maximum of 164.20, 133 and 48.92 μmol kg‒1 at 10 d for Populus alba, Hedysarum multijugum Maxim., and bare sand, respectively, after which it gradually decreased (Fig. 5a). The concentration of •OH in Populus alba samples was the highest during incubation, and was higher than those in Hedysarum multijugum Maxim. and bare sand samples. In contrast, in the first 10 d of incubation, the concentration of H2O2 in Populus alba, Hedysarum multijugum Maxim., and bare sand samples gradually decreased from 477.80, 428.52, and 229.37 μmol kg‒1 to 448.93, 261.70, and 94.21 μmol kg‒1, respectively (Fig. S9a). Similar to the •OH results, the CO2 efflux was also largely affected by vegetation type. Among the three types of soil, the CO2 efflux in Populus alba samples was the highest (Fig. 5b) and the CO2 efflux gradually decreased and stabilised as the incubation experiment progressed.
Production of •OH (a) and CO2 (b) during 36 days of incubation in the soil samples (Populus alba, Hedysarum multijugum Maxim., and bare sand). Effects of γ-irradiation sterilisation and addition of TBA on •OH (c) and CO2 production (d) of Populus alba, Hedysarum multijugum Maxim., and bare sand soil samples at 24 h. Sterilisation + TBA refers to sterilised soil with 10% tertbutyl alcohol. The grey-shaded region represents the time period prior to sampling during the incubation experiment
3.4.3 Effect of •OH on OC mineralisation
Estimation of the relative contributions of biotic processes and •OH to OC mineralisation was achieved by γ-irradiation sterilisation and chemical quenching experiments. After sterilisation, the •OH concentration in Populus alba, Hedysarum multijugum Maxim., and bare sand samples decreased slightly from 32.19, 15.05, and 5.41 μmol kg‒1 to 31.20, 12.88, and 4.37 μmol kg‒1 (Fig. 5c), respectively, suggesting that the production of •OH was not significantly affected by biotic processes. However, the CO2 efflux in the sterilised Populus alba, Hedysarum multijugum Maxim., and bare sand soils decreased from 64.19, 43.74, and 20.88 μg C g‒1 soil d‒1 to 25.96, 20.64, and 10.01 μg C g‒1 soil d‒1, respectively, meaning that the inhibition of CO2 efflux ranged between 52.82% and 64.04% after sterilisation (Fig. 5d). After the addition of 10% TBA in the sterilised soil, •OH production was inhibited by 70.68%‒85.59% compared with the sterilised soil (Fig. 5c). As expected, CO2 efflux was also significantly reduced by 39.40%‒56.64%. Therefore, after sterilisation and quenching experiments, •OH was estimated to contribute 15.93%‒25.80% to CO2 efflux in sandy soils, which was calculated by Eq. 1:
where C represents the contribution of •OH to CO2 efflux, C1 represents the contribution of •OH to CO2 efflux in abiotic processes, C2 represents the contribution of abiotic processes to the efflux of CO2, and C3 represents the total of CO2 efflux.
Additionally, to explore the susceptibility of POC and MOC for •OH attack, a 24 h incubation experiment was conducted with two OC fractions. Sterilisation had an insignificant effect on •OH generation, whereas the addition of TBA quenched the generated •OH in POC and MOC samples, which decreased from 41.14‒149.72 and 20.38‒24.79 μmol kg‒1 to 3.53‒11.88 and 1.31‒2.20 μmol kg‒1, respectively (Fig. 6a, b). After sterilisation, the CO2 efflux of POC and MOC decreased substantially, accounting for 63.91%‒74.45% and 32.96%‒58.63% of that in native soils, respectively. When TBA was added, CO2 production in POC and MOC was 2.60%‒19.80% and 1.52%‒9.23% of that in sterilised soil, respectively (Fig. 6c, d). These results were once again verified by adding Fenton reagents into sterile soils (Fig. 7). The simultaneous addition of Fe(II) and H2O2 resulted in significantly higher CO2 efflux compared to Fe(II) or H2O2 alone (Fig. 7a). When H2O2 contents were greater than 10 μmol kg‒1 in soil, CO2 efflux exhibited a clear upward trend as H2O2 concentration increased. However, the reactivity of POC and MOC towards •OH was different, and the CO2 efflux released by POC after •OH oxidation was 57.66%‒89.68% of that of MOC under the same condition (Fig. 7b).
4 Discussion
4.1 Vegetation restoration influences ROS production
The concentrations of three representative types of ROS in vegetation-restored soil were detected. Our findings demonstrated the eminent role of vegetation in regulating ROS production. Particularly, tree planting was more conducive to ROS accumulation than shrub planting. For instance, the O2•− concentration in tree-covered soils was higher than that in shrub-covered soils, which might be due to the higher content of Fe(II), reduced OC, and microbial abundance (Figs. 1 and 2). Both of them can donate electron donors to molecular oxygen, inducing the production of O2•− (Murphy et al. 2016; Tong et al. 2016). Specifically, vegetation restoration promoted the accumulation of Fe(II) (Fig. 1a), especially in tree-covered soils, which may be related to the high abundance of Fe-reducing bacteria (Deinococcus) in the soil (Fig. 2b). When Fe(II) is exposed to O2, the high redox potential of O2 readily initiates the exergonic abiotic oxidation of Fe(II) to produce O2•− (Eq. 2) (Melton et al. 2014). In addition to the effect of Fe(II), OC can also promote the production of O2•−. Vegetation restoration greatly promoted the accumulation of OC, including SOC, POC, and DOC, and this phenomenon was more significant in tree-planted soil (Figs. 1b and S3). This might be because trees possess a stronger ability to introduce litter and root exudates into soil ecosystems, which can effectively control soil erosion and improve soil conditions, thus strengthening the C sequestration capacity of terrestrial ecosystems (Zhang et al. 2017; Huang et al. 2022). Regarding the natural evolution of OC, its major reduced components (fulvic acid and humic acid) can react with O2 to form O2•− by one- and two-electron transfer processes (Han et al. 2022). Furthermore, Diaz et al. (2013) detected O2•− production in 27 out of 30 environmentally common strains, including Proteobacteria, Actinobacteria, Bacteroidetes, Planctomycetes, and Firmicutes. These microbes also dominated the bacterial community in our study. Among them, Planctomycetes and Actinobacteriota had a positive correlation with O2•− production (Fig. S10, p < 0.05), suggesting that the metabolism of the aforementioned bacteria promoted O2•− production through extracellular single electron transfer (Han et al. 2020).
Similarly, H2O2 was produced by several pathways in sandy soil. The sterilisation experiment demonstrated that biotic processes dominate the production of H2O2 (Fig. S7b), and this phenomenon could be explained by the significant positive correlation (p < 0.05) between the abundance of Firmicutes and the concentration of H2O2 (Fig. S10). As reported previously, some of the bacterial genera and species in Firmicutes are able to generate H2O2 (Michlits et al. 2020; Redanz et al. 2018). Moreover, the reaction of O2•− produced by microbes and Fe(II) can further generate H2O2 (Eq. 3). Here, we could not completely define the reaction described by Eq. 3 as abiotic process. O2•− and Fe(II) produced by abiotic mediation were also important contributors to H2O2 production. However, H2O2 concentration in vegetated soils was significantly lower than in bare sand (Fig. 3b). The higher abundance of Firmicutes in bare sand (Fig. 2a), might contribute to the higher H2O2 content in bare sand compared to vegetated soil. Conversely, the content of OC and Fe in vegetated soil was higher. Previous studies have reported that H2O2 tends to decay rapidly in surface soils with higher OC or Fe content, decomposing into water and oxygen via disproportionation reactions (Petigara et al. 2002). The higher abundance of Deinococcus in vegetated soil can also mediate the decomposition of H2O2 through the secretion of catalase (Fig. 2b) (Zhang et al. 2021a, b). In contrast, the decomposition of H2O2 was much slower in bare sand soils with lower OC and iron-reducing bacteria than vegetated soil. The production of •OH through the Haber–Weiss (Eq. 4) mechanism and Fenton-like reaction (Eqs. 5 and 6) in this environment were also the primary pathways for the decomposition of H2O2:
Unlike O2•− and H2O2, vegetation restoration effectively promoted the production of •OH. In this study, the sterilised controls exhibited no significant change in •OH production (Fig. S8), suggesting that abiotic processes were primarily responsible for •OH production. This might be due to the evolution of reduced species in vegetated soils, which controlled the generation of •OH. As mentioned above, vegetation restoration promoted the accumulation of Fe(II) in sandy soils (Fig. 1a), which is the basis for Fenton (Eq. 5) or Fenton-like reaction (Eq. 6):
Here, ≡Fe represents surface-adsorbed Fe, which serves as either a catalyst or nanocatalyst in Fenton-like reactions (Duesterberg et al. 2008; Garrido-Ramirez et al. 2010). Correlation analysis indicated that Fe(II) had a positive (p < 0.05) correlation with various C compositions (SOC, POC, MOC and DOC) (Fig. 8a), among which the correlation with SOC, POC and MOC was stronger than that with DOC. This suggested that the accumulation of different fractions OC was coupled with the production of Fe(II), and most Fe(II) existed in the form of surface-adsorbed Fe (Zhang et al. 2022), which could effectively promote the production of •OH. Additionally, •OH production was significantly and positively correlated with the concentrations of Fe(II) (R2 = 0.78, p < 0.05) and SOC (R2 = 0.80, p < 0.05) (Fig. 4c, d). This further confirmed that a large amount of •OH was formed via reductive SOC and Fe(II), suggesting that the high content of SOC and Fe(II) was also responsible for the high level of •OH in tree-covered soils. Concretely, under anaerobic conditions, Fe-reducing bacteria promote the reduction of Fe(III) in the presence of OC (Lovley et al. 2004; Zhao et al. 2021), and the variations in Fe(II) and •OH remained consistent during incubation, further confirming the contribution of Fe to the production of •OH. In this process, H2O2 produced in vegetation restoration can react with Fe(II) to generate •OH through Fenton-like reactions (Page et al. 2012; Tong et al. 2016). Meanwhile, the quinones of SOC undergo an organic reduction reaction, converting H2O2 into •OH (Zhu et al. 2002; Yu et al. 2021). During incubation, the concentration of H2O2 in the Populus alba, Hedysarum multijugum Maxim., and bare sand soil samples decreased significantly in the early stage (Fig. S9a), which accelerated the production of •OH with Fe(II) through Fenton-like reactions or organic reduction reactions (Trusiak et al. 2018). This could partially explain why •OH production increased during the first 10 d. Additionally, DOC concentration decreased significantly during incubation (Table S2), the reduced component (e.g., sugars, quinones, and amino acids) of which can be oxidised and react with Fe(II) to form H2O2 and promote •OH production via Fenton-like reactions (Page et al. 2012; Yu et al. 2021). Ultimately, •OH may initiate additional degradation of OC, and induce the formation of aliphatic compounds, low molecular weight organic acids, or CO2 (Goldstone et al. 2002; Waggoner et al. 2015), thus accelerating the transformation and cycle of carbon. Moreover, our findings further demonstrated that •OH production was significantly influenced by vegetation species (Fig. 5a), which was mainly due to the difference in Fe(II) and OC contents in soils with different vegetation covers.
Correlation heat maps between soil physicochemical properties and contents of ROS and OC (a). The relationship between Fe(II), ROS, bacterial community beta diversity, and carbon fraction in soils was analysed using RDA (b). The results were presented as a visualisation of the relationship between the variables
4.2 OC mineralisation associated with ROS
Redundancy analysis (RDA) was performed to assess the influence of ROS and other soil parameters on OC components (Fig. 8b). The RDA results showed that Fe(II) (71.8%, p < 0.005) and •OH (6.8%, p < 0.05) were the two most important variables determining soil carbon composition. The first axis of RDA explained 84.9% of the variation, and Fe(II) and •OH were well aligned with this first axis, further indicating that these two variables played important roles in C turnover.
SOC serves as an electron donor, which can promote •OH production while also contributing to organic carbon mineralisation (Zhao et al. 2021). Among Populus alba, Hedysarum multijugum Maxim., and bare sand samples, the CO2 efflux in Populus alba samples was the highest (Fig. 5b), which was attributed to its higher SOC, Fe(II), and •OH concentration (Figs. 1 and 4a). Except for •OH, compared with bare sand, the enzyme activity in the Populus alba and Hedysarum multijugum Maxim. samples increased gradually from 488.72 and 623.67 nmol g‒1 h‒1 to 683.16 and 1416.62 g‒1 h‒1 during incubation, respectively (Fig. S9b). This phenomenon demonstrated that the SOC decomposition was also regulated by the release of extracellular enzymes (β-1,4-glucosidase) driven by microbial activity (Kuzyakov et al. 2009).
After sterilisation, the inhibition of CO2 production was 52.82%‒64.04% compared with the native soil, indicating that the contribution of microbial activity to OC mineralisation was higher than that of abiotic processes. However, •OH can effectively promote the emission of CO2 due to its role as an electron acceptor, which is consistent with our results. Specifically, the decreased •OH production was found to be positively correlated (R2 = 0.87, p < 0.05) with decreased CO2 production in TBA-treated soils (Fig. S11), which contributed roughly 15.93%‒25.80% to CO2 production in sandy soils. This likely occurred through the hydroxylation process, in which a hydroxyl group is added to the aromatic ring by •OH, which attacks the aromatic structure, resulting in the abiotic oxidation of CO2 by •OH (Qiu et al. 2018; Rodrigues et al. 2018).
As two important fractions of SOC, POC, and MOC exhibited different reactivities towards •OH. By calculating the difference between •OH and CO2 production after sterilisation and TBA quenching, we estimated that 1 mol •OH produced approximately 0.01‒0.02 mol and 0.03‒0.23 mol CO2 by oxidising POC and MOC, respectively. Moreover, after the addition of Fenton reagents (Fe(II) and H2O2) in the incubation experiment, our findings demonstrated that the introduction of more •OH stimulated CO2 efflux (Fig. 7). Notably, within different ranges of H2O2 concentrations, compared to POC, MOC exhibited a greater susceptibility to •OH attacks. This phenomenon is closely linked to the general properties of POC and MOC. POC encompasses highly complex compounds with high activation energies (e.g., phenols, celluloses, and hemicelluloses). In contrast, MOC generally consists of relatively simple compounds with lower activation energies, such as microbial polysaccharides, amino sugars, and muramic acid (Lavallee et al. 2020; Yu et al. 2022), which are more easily attacked by •OH. Therefore, •OH can destroy the molecular structure of SOC (Kuzyakov et al. 2009), resulting in the production of low-molecular-weight compounds and CO2 (Goldstone et al. 2002). However, these findings only reflect the short-term effects of •OH on CO2, and further research is thus required to determine its long-term effects on CO2 efflux.
5 Conclusions
We investigated the production of ROS in sandy soils covered by various vegetation types and found that trees planting promoted higher ROS accumulation than shrubs planting. The production of O2•− and H2O2 were affected by the interplay of microbially mediated reactions and abiotic processes. In contrast, the majority of •OH production was likely driven by abiotic processes and was also affected by the vegetation type, with tree-covered soil being more conducive to •OH generation. The Fe(II) species and reductive SOC were the main factors contributing to •OH production. Moreover, the results of the incubation experiment further demonstrated the crucial role of Fe(II) and OC in •OH production. The generated •OH could then efficiently oxidise OC to produce CO2. As an important fraction of SOC, MOC was more vulnerable to the attack by •OH. In turn, •OH promoted the mineralisation of OC and played an important role in the soil carbon cycle due to its nature as a strong oxidant. Therefore, future studies should explore the production of •OH in soil biogeochemical cycles, which will be instrumental in fully assessing its implications for carbon budgets.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- OC:
-
Organic carbon
- ROS:
-
Reactive oxygen species
- •OH:
-
Hydroxyl radicals
- SOC:
-
Soil organic carbon
- CO2 :
-
Carbon dioxide
- POC:
-
Particulate organic carbon
- MOC:
-
Mineral-associated organic carbon
- O2 •− :
-
Superoxide
- H2O2 :
-
Hydrogen peroxide
- BG:
-
β-1,4-Glucosidase
- DOC:
-
Dissolved organic carbon
- TBA:
-
Tertbutyl alcohol
- EPR:
-
Electron paramagnetic resonance
- DMPO:
-
5,5-Dimethylpyrroline-N-oxide
- DMSO:
-
Dimethyl sulfoxide
- BA:
-
Benzoic acid
- p-HBA:
-
p-Hydroxybenzoic acid
- RDA:
-
Redundancy analysis
References
Bao P, Li GX (2017) Sulfur-driven iron reduction coupled to anaerobic ammonium oxidation. Environ Sci Technol 51:6691–6698. https://doi.org/10.1021/acs.est.6b05971
Brookes PC, Kemmitt SJ, Addiscott TM, Bird N (2009) Reply to Kuzyakov et al’.s comments on our paper: ’Kemmitt, S. Lanyon, C. V., Waite, I.S., Wen, Q., O’Donnell, A.G., Brookes, P.C., 2008. Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbil biomass—a new perspective. Soil Biol Biochem 41:440–443. https://doi.org/10.1016/j.soilbio.2008.09.002
Burns JM, Craig PS, Shaw TJ, Ferry JL (2010) Multivariate examination of Fe(II)/Fe(III) cycling and consequent hydroxyl radical generation. Environ Sci Technol 44:7226–7231. https://doi.org/10.1021/es903519m
Chao J, Liu YU, He H, Miao HT, Wu GL (2018) Formation of litter crusts and its multifunctional ecological effects in a desert ecosystem. Ecosphere 9:e02196. https://doi.org/10.1002/ecs2.2196
Chen C, Hall SJ, Coward E, Thompson A (2020) Iron-mediated organic matter decomposition in humid soils can counteract protection. Nat Commun 11:2255. https://doi.org/10.1038/s41467-020-16071-5
Chen N, Fu Q, Wu T, Cui P, Fang G, Liu C, Chen C, Liu G, Wang W, Wang D (2021) Active iron phases regulate the abiotic transformation of organic carbon during redox fluctuation cycles of paddy soil. Environ Sci Technol 55:14281–14293. https://doi.org/10.1021/acs.est.1c04073
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, Wall DH, Parton AJ (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8:776–779. https://doi.org/10.1038/NGEO2520
Cotrufo MF, Ranalli MG, Haddix ML, Six J, Lugato E (2019) Soil carbon storage informed by particulate and mineral-associated organic matter. Nat Geosci 12:989–994. https://doi.org/10.1038/s41561-019-0484-6
Craig ME, Geyer KM, Beidler KV, Brzostek ER, Frey SD, Stuart Grandy A, Liang C, Phillips RP (2022) Fast-decaying plant litter enhances soil carbon in temperate forests but not through microbial physiological traits. Nat Commun 13:1229. https://doi.org/10.1038/s41467-022-28715-9
Cui YX, Wang X, Zhang XC, Ju WL, Duan CJ, Guo XB, Wang YQ, Fang LC (2020) Soil moisture mediates microbial carbon and phosphorus metabolism during vegetation succession in a semiarid region. Soil Biol Biochem 147:107814. https://doi.org/10.1016/j.soilbio.2020.107814
Dai HY, Wu BB, Chen BL, Ma B, Chu CH (2022) Diel Fluctuation of Extracellular Reactive Oxygen Species Production in the Rhizosphere of Rice. Environ Sci Technol 56:9075–9082. https://doi.org/10.1021/acs.est.2c00005
Diaz JM, Hansel CM, Voelker BM, Mendes CM, Zhang T (2013) Widespread production of extracellular superoxide by heterotrophic bacteria. Science 340:1223–1226. https://doi.org/10.1126/science.1237331
Dong, L., Li, J., Zhang, Y., Liu, Y., Li, A., Shangguan, Z., Deng, L., 2022. Forests have a higher soil C sequestration benefit due to lower C mineralization efficiency: Evidence from the central loess plateau case. Agric., Ecosyst. Environ. 339, 108144. https://doi.org/10.1016/j.agee.2022.108144.
Duesterberg CK, Mylon SE, Waite TD (2008) pH effects on iron-catalyzed oxidation using Fenton’s reagent. Environ Sci Technol 42:8522–8527. https://doi.org/10.1021/es801720d
Feng X, Fu B, Piao S, Wang S, Ciais P, Zeng Z, Lu Y, Zeng Y, Li Y, Jiang X, Wu B (2016) Revegetation in China’s Loess Plateau is approaching sustainable water resource limits. Nat Clim Change 6:1019–1022. https://doi.org/10.1038/NCLIMATE3092
Garrido-Ramirez EG, Theng B, Mora ML (2010) Clays and oxide minerals as catalysts and nanocatalysts in Fenton-like reactions — A review. Appl Clay Sci 47:182–192. https://doi.org/10.1016/j.clay.2009.11.044
Georgiou CD, Sun HJ, Mckay CP, Grintzalis K, Papapostolou I, Zisimopoulos D, Panagiotidis K, Zhang G, Koutsopoulou E, Christidis GE (2015) Evidence for photochemical production of reactive oxygen species in desert soils. Nat Commun 6:7100. https://doi.org/10.1038/ncomms8100
Gokdere B, Uzer A, Durmazel S, Ercag E, Apak R (2019) Titanium dioxide nanoparticles-based colorimetric sensors for determination of hydrogen peroxide and triacetone triperoxide (TATP). Talanta 202:402–410. https://doi.org/10.1016/j.talanta.2019.04.071
Goldstone JV, Pullin MJ, Bertilsson S, Voelker BM (2002) Reactions of hydroxyl radical with humic substances: Bleaching, mineralization, and production of bioavailable carbon substrates. Environ Sci Technol 36:364–372. https://doi.org/10.1021/es0109646
Hall SJ, Silver WL (2013) Iron oxidation stimulates organic matter decomposition in humid tropical forest soils. Global Change Biol 19:2804–2813. https://doi.org/10.1111/gcb.12229
Han RX, Lv JT, Huang ZQ, Zhang SH, Zhang SZ (2020) Pathway for the production of hydroxyl radicals during the microbially mediated redox transformation of iron (oxyhydr) oxides. Environ Sci Technol 54:902–910. https://doi.org/10.1021/acs.est.9b06220
Han RX, Wang Z, Lv J, Zhu Z, Yu GH, Li G, Zhu YG (2022) Multiple Effects of Humic Components on Microbially Mediated Iron Redox Processes and Production of Hydroxyl Radicals. Environ Sci Technol 56:16419–16427. https://doi.org/10.1021/acs.est.2c03799
Heckman K, Pries CEH, Lawrence CR, Rasmussen C, Crow SE, Hoyt AM, von Fromm SF, Shi Z, Stoner S, McGrath C, Beem-Miller J, Berhe AA, Blankinship JC, Keiluweit M, Marin-Spiotta E, Monroe JG, Plante AF, Schimel J, Sierra CA, Thompson A, Wagai R (2022) Beyond bulk: Density fractions explain heterogeneity in global soil carbon abundance and persistence. Global Change Biol 28:1178–1196. https://doi.org/10.1111/gcb.16023
Heimann M, Reichstein M (2008) Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451:289–292. https://doi.org/10.1038/nature06591
Huang Y, Xin Z, Ran L, Qin Y, Cai M (2022) Topsoil carbon sequestration of vegetation restoration on the Loess Plateau. Ecol Eng 177:106570. https://doi.org/10.1016/j.ecoleng.2022.106570
Jansson JK, Hofmockel KS (2020) Soil microbiomes and climate change. Nat Rev Microbiol 18:35–46. https://doi.org/10.1038/s41579-019-0265-7
Jilling A, Keiluweit M, Gutknecht JLM, Grandy AS (2021) Priming mechanisms providing plants and microbes access to mineral-associated organic matter. Soil Biol Biochem 158:108256. https://doi.org/10.1016/j.soilbio.2021.108265
Kleber M, Bourg IC, Coward EK, Hansel CM, Myneni SCB, Nunan N (2021) Dynamic interactions at the mineral-organic matter interface. Nat Rev Earth Environ 2:402–421. https://doi.org/10.1038/s43017-021-00162-y
Kuzyakov Y, Blagodatskaya E, Blagodatsky, S.,Comments on the paper by Kemmitt, et al (2009) (2008) ’Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass - a new perspective. Soil Biol Biochem 41:435–439. https://doi.org/10.1016/j.soilbio.2008.07.023
Lavallee, J.M., Soong, J.L., Cotrufo, M.F., 2020. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. 26, 261–273. https://doi.org/10.1111/gcb.14859.
Lehmann J, Hansel CM, Kaiser C, Kleber M, Maher K, Manzoni S, Nunan N, Reichstein M, Schimel JP, Torn MS, Wieder WR, Koegel-Knabner I (2020) Persistence of soil organic carbon caused by functional complexity. Nat Geosci 13:529–534. https://doi.org/10.1038/s41561-020-0612-3
Li G, Kim S, Han SH, Chang H, Du D, Son Y (2018) Precipitation affects soil microbial and extracellular enzymatic responses to warming. Soil Biol Biochem 120:212–221. https://doi.org/10.1016/j.soilbio.2018.02.014
Liang C, Schimel JP, Jastrow JD (2017) The importance of anabolism in microbial control over soil carbon storage. Nat Microbiol 2:17105–17110. https://doi.org/10.1038/nmicrobiol.2017.105
Liu XZ, Liu Y, Zhang L, Yin R, Wu GL (2021) Bacterial contributions of bio-crusts and litter crusts to nutrient cycling in the Mu Us Sandy Land. CATENA 199:105090. https://doi.org/10.1016/j.catena.2020.105090
Liu JB, Zhu KC, Zhang C, Zhang XC, Chen N, Jia HZ (2022) Microscale spatiotemporal variation and generation mechanisms of reactive oxygen species in the rhizosphere of ryegrass: Coupled biotic-abiotic processes. Environ Sci Technol 56:16483–16493. https://doi.org/10.1021/acs.est.2c06167
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49:219–286. https://doi.org/10.1016/S0065-2911(04)49005-5
Melton ED, Swanner ED, Behrens S, Schmidt C, Kappler A (2014) The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat Rev Microbiol 12:797–808. https://doi.org/10.1038/nrmicro3347
Michlits H, Lier B, Pfanzagl V, Djinovic-Carugo K, Furtmuller PG, Oostenbrink C, Obinger C, Hofbauer S (2020) Actinobacterial Coproheme Decarboxylases Use Histidine as a Distal Base to Promote Compound I Formation. ACS Catal 10:5405–5418. https://doi.org/10.1021/acscatal.0c00411
Mopper K, Zhou X (1990) Hydroxyl radical photoproduction in the sea and its potential impact on marine processes. Science 250:661–664. https://doi.org/10.1126/science.250.4981.661
Murphy SA, Meng S, Solomon BM, Dias D, Shaw TJ, Ferry JL (2016) Hydrous ferric oxides in sediment catalyze formation of reactive oxygen species during sulfide oxidation. Front Mar Sci 3:1–12. https://doi.org/10.3389/fmars.2016.00227
Page SE, Sander M, Arnold WA, McNeill K (2012) Hydroxyl radical formation upon oxidation of reduced humic acids by oxygen in the dark. Environ Sci Technol 46:1590–1597. https://doi.org/10.1021/es203836f
Page SE, Kling GW, Sander M, Harrold KH, Logan JR, McNeill K, Cory RM (2013) Dark formation of hydroxyl radical in arctic soil and surface waters. Environ Sci Technol 47:12860–12867. https://doi.org/10.1021/es4033265
Petigara BR, Blough NV, Mignerey AC (2002) Mechanisms of hydrogen peroxide decomposition in soils. Environ Sci Technol 36:639–645. https://doi.org/10.1021/es001726y
Qiu HS, Ge TD, Liu JY, Chen XB, Hu YJ, Wu JS, Su YR, Kuzyakov Y (2018) Effects of biotic and abiotic factors on soil organic matter mineralization: Experiments and structural modeling analysis. Eur J Soil Biol 84:27–34. https://doi.org/10.1016/j.ejsobi.2017.12.003
Redanz S, Cheng XQ, Giacaman RA, Pfeifer CS, Merritt J, Kreth J (2018) Live and let die: Hydrogen peroxide production by the commensal flora and its role in maintaining a symbiotic microbiome. Mol Oral Microbiol 33:337–352. https://doi.org/10.1111/omi.12231
Rodrigues C, Borges R, Lima VN, Madeira LM (2018) p-Nitrophenol degradation by Fenton’s oxidation in a bubble column reactor. J Environ Manage 206:774–785. https://doi.org/10.1016/j.jenvman.2017.11.032
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kogel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56. https://doi.org/10.1038/nature10386
Singh M, Sarkar B, Biswas B, Bolan NS, Churchman GJ (2017) Relationship between soil clay mineralogy and carbon protection capacity as influenced by temperature and moisture. Soil Biol Biochem 109:95–106. https://doi.org/10.1016/j.soilbio.2017.02.003
Tong M, Yuan SH, Ma SC, Jin MG, Liu D, Cheng D, Liu XX, Gan YQ, Wang YX (2016) Production of abundant hydroxyl radicals from oxygenation of subsurface sediments. Environ Sci Technol 50:214–221. https://doi.org/10.1021/acs.est.5b04323
Trusiak A, Treibergs LA, Kling GW, Cory RM (2018) The role of iron and reactive oxygen species in the production of CO2 in arctic soil waters. Geochim Cosmochim Acta 224:80–95. https://doi.org/10.1016/j.gca.2017.12.022
Vaughan PP, Blough NV (1998) Photochemical formation of hydroxyl radical by constituents of natural waters. Environ Sci Technol 32:2947–2953. https://doi.org/10.1021/es9710417
Waggoner DC, Chen HM, Willoughby AS, Hatcher PG (2015) Formation of black carbon-like and alicyclic aliphatic compounds by hydroxyl radical initiated degradation of lignin. Org Geochem 82:69–76. https://doi.org/10.1016/j.orggeochem.2015.02.007
Wang HH, Yue C, Mao QQ, Zhao J, Ciais P, Li W, Yu Q, Mu XM (2020) Vegetation and species impacts on soil organic carbon sequestration following ecological restoration over the Loess Plateau. China Geoderma 371:114389. https://doi.org/10.1016/j.geoderma.2020.114389
Witzgall K, Vidal A, Schubert DI, Hoschen C, Schweizer SA, Buegger F, Pouteau V, Chenu C, Mueller CW (2021) Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat Commun 12:4115. https://doi.org/10.1038/s41467-021-24192-8
Yu GH, Kuzyakov Y (2021) Fenton chemistry and reactive oxygen species in soil: Abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth-Sci Rev 214:103525. https://doi.org/10.1016/j.earscirev.2021.103525
Yu CL, Zhang YT, Lu YX, Qian A, Zhang P, Cui YP, Yuan SH (2021) Mechanistic insight into humic acid-enhanced hydroxyl radical production from Fe(II)-bearing clay mineral oxygenation. Environ Sci Technol 55:13366–13375. https://doi.org/10.1021/acs.est.1c02461
Yu WJ, Huang WJ, Weintraub-Leff SR, Hall SJ (2022) Where and why do particulate organic matter (POM) and mineral-associated organic matter (MAOM) differ among diverse soils? Soil Biol Biochem 172:108756. https://doi.org/10.1016/j.soilbio.2022.108756
Zeng DH, Hu YL, Chang SX, Fan ZP (2009) Land cover change effects on soil chemical and biological properties after planting Mongolian pine (Pinus sylvestris var. mongolica) in sandy lands in Keerqin, northeastern China. Plant Soil 317:121–133. https://doi.org/10.1007/s11104-008-9793-z
Zhang T, Hansel CM, Voelker BM, Lamborg CH (2016) Extensive dark biological production of reactive oxygen species in brackish and freshwater ponds. Environ Sci Technol 50:2983–2993. https://doi.org/10.1021/acs.est.5b03906
Zhang W, Qiao W, Gao D, Dai Y, Deng J, Yang G, Han X, Ren G (2017) Relationship between soil nutrient properties and biological activities along a restoration chronosequence of Pinus tabulaeformis plantation forests in the Ziwuling Mountains, China. CATENA 161:85–95. https://doi.org/10.1016/j.catena.2017.10.021
Zhang BG, Li YN, Fei YM, Cheng YT (2021a) Novel Pathway for Vanadium(V) Bio-Detoxification by Gram-Positive Lactococcus raffinolactis. Environ Sci Technol 55:2121–2131. https://doi.org/10.1021/acs.est.0c07442
Zhang S, Lv J, Han R, Wang Z, Christie P, Zhang S (2021b) Sustained production of superoxide radicals by manganese oxides under ambient dark conditions. Water Res 196:117034. https://doi.org/10.1016/j.watres.2021.117034
Zhang Y, Zhang N, Qian A, Yu C, Zhang P, Yuan S (2022) Effect of C/Fe molar ratio on H2O2 and •OH production during oxygenation of Fe(II)-humic acid coexisting systems. Environ Sci Technol 56:13408–13418. https://doi.org/10.1021/acs.est.2c01312
Zhao YP, Xiang W, Huang CL, Liu Y, Tan Y (2021) Production of hydroxyl radicals following water-level drawdown in peatlands: A new induction mechanism for enhancing laccase activity in carbon cycling. Soil Biol Biochem 156:108241. https://doi.org/10.1016/j.soilbio.2021.108241
Zhao G, Wu B, Zheng X, Chen B, Kappler A, Chu C (2022) Tide-triggered production of reactive oxygen species in coastal soils. Environ Sci Technol 56:11888–11896. https://doi.org/10.1021/acs.est.2c03142
Zhu BZ, Zhao HT, Kalyanaraman B, Frei B (2002) Metal-independent production of hydroxyl radicals by halogenated quinones and hydrogen peroxide: An ESR spin trapping study. Free Radical Biol Med 32:465–473. https://doi.org/10.1016/S0891-5849(01)00824-3
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 42277030, 42207451), and the Introduction Plan for High end Foreign Experts (Grant No. 110000207920220115).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Fuhao Liu, Kecheng Zhu, Zhiqiang Wang, Jinbo Liu, Zheng Ni, Yuanyuan Ding, Chi Zhang and Hanzhong Jia. The first draft of the manuscript was written by Fuhao Liu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Bo Pan
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Further details about reagents and methods (Text S1-S5), and two tables (Table S1-S2) and eleven figures (Fig. S1-S11).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, F., Zhu, K., Wang, Z. et al. Production of reactive oxygen species and its role in mediating the abiotic transformation of organic carbon in sandy soil under vegetation restoration. Carbon Res. 2, 35 (2023). https://doi.org/10.1007/s44246-023-00074-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-023-00074-0