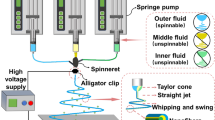
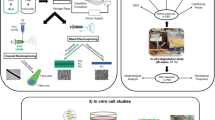
Abstract
Treatment of critical size defects is quite challenging, often requiring autologous bone grafts for bone regeneration. A massive volume of autologous bone is essential during this process to fill the defect leading to donor site morbidity. Although 3D printed PCL scaffolds are frequently utilised for bone correction procedures, there have been reports of delayed PCL biodegradation and inadequate bone tissue formation. To enhance the regenerative potential, in this study, silk in the form of silk fibroin microfibers are reinforced into the PCL matrix to form the composite. Two silk variations were used: Antheraea mylitta and Bombyx mori, and has been proven to promote cell proliferation, adhesion, and osteogenic potential. This work creates 8 mm critical size defects in a rabbit calvaria model to test for the first time ever the ability of 3D printed PCL-silk scaffolds to regenerate bone tissue. Micro-CT imaging and histological examination performed 6 and 12 weeks after implantation revealed that the PCL-silk scaffold-augmented defects considerably outgrew their PCL-scaffold-only counterparts and the control group in terms of neo-bone formation. By 6 weeks, PCL-silk scaffolds had 47.4–50.3% of bone growth that was twice as high as PCL scaffolds alone (16.7–19.9%). Similarly, by 12 weeks, the PCL-silk group had four times more (80–87.3%) new bone tissue production than the PCL group (18.6–22.4%). The promise of silk fibroin-reinforced PCL biomaterial for pre-clinical and clinical studies for craniofacial reconstructive applications is thus supported by these results.
Graphical Abstract








Similar content being viewed by others
Data Availability
The raw data required to reproduce these findings are available at authors and can be shared upon request.
References
T. Guda et al., Methods to analyze bone regenerative response to different rhBMP-2 doses in rabbit craniofacial defects. Tissue Eng. Part C Methods 20(9), 749–760 (2014). https://doi.org/10.1089/ten.tec.2013.0581
M.Y. Yen-Hong Lin, Y.C. Chiu, Y.F. Shen, Y.H. Andrew Wu, Shie, Bioactive calcium silicate/poly-ε-caprolactone composite scaffolds 3D printed under mild conditions for bone tissue engineering. J. Mater. Sci: Mater. Med. 29(11), 1–13 (2018). https://doi.org/10.1007/s10856-017-6020-6
Y.C. Chiu, H.Y. Fang, T.T. Hsu, C.Y. Lin, M.Y. Shie, The characteristics of mineral trioxide aggregate/polycaprolactone 3-dimensional Scaffold with osteogenesis properties for tissue regeneration. J. Endod. 43(6), 923–929 (2017). https://doi.org/10.1016/j.joen.2017.01.009
D. Steffens et al., 3D-printed PCL scaffolds for the cultivation of mesenchymal stem cells. J. Appl. Biomater. Funct. Mater. 14(1), e19–e25 (2016). https://doi.org/10.5301/jabfm.5000252
J.S. Park et al., Fabrication and characterization of 3D-printed bone-like β-tricalcium phosphate/polycaprolactone scaffolds for dental tissue engineering. J. Ind. Eng. Chem. 46, 175–181 (2017). https://doi.org/10.1016/j.jiec.2016.10.028
C. Murphy, K. Kolan, W. Li, J. Semon, D. Day, M. Leu, 3D bioprinting of stem cells and polymer/bioactive glass composite scaffolds for bone tissue engineering. Int. J. Bioprint. 3(1), 54–64 (2017). https://doi.org/10.18063/IJB.2017.01.005
N.A. For, M. Devices, M. L., A.-E. K. N., P.-G. A. J. Flores-Cedillo, Multiwall carbon nanotubes / polycaprolactone scaffolds seeded with human dental pulp stem cells for bone tissue regeneration. J. Mater. Sci.: Mater. Med. 27(35), 1–12 (2016). https://doi.org/10.1007/s10856-015-5640-y
S.D. Stanislav Evlashin, P. Dyakonov, S.K. Mikhail Tarkhov, S. Rodionov, A. Shpichka, M. Kostenko, P. Timashev, I. Akhatov, I. Sergeichev, Flexible polycaprolactone and polycaprolactone / graphene scaffolds for tissue engineering. Materials 12(2991), 1–11 (2019). https://doi.org/10.3390/ma12182991
A. Seyedsalehi, L. Daneshmandi, M. Barajaa, J. Riordan, C.T. Laurencin, Fabrication and characterization of mechanically competent 3D printed polycaprolactone—reduced graphene oxide scaffolds. Sci. Rep. (2020). https://doi.org/10.1038/s41598-020-78977-w
B. Yuan et al., In vitro and in vivo study of a novel nanoscale demineralized bone matrix coated PCL/β-TCP scaffold for bone regeneration. Macromol. Biosci. (2021). https://doi.org/10.1002/mabi.202000336
S.F. Hashemi, M. Mehrabi, A. Ehterami, A.M. Gharravi, F.S. Bitaraf, M. Salehi, In-vitro and in-vivo studies of PLA / PCL / gelatin composite scaffold containing ascorbic acid for bone regeneration. J. Drug Deliv. Sci. Technol. (2021). https://doi.org/10.1016/j.jddst.2020.102077
J. Henkel et al., Bone regeneration based on tissue engineering conceptions-a 21st century perspective. Bone Res. 1, 216–248 (2013). https://doi.org/10.4248/BR201303002
L. Moroni, A. Nandakumar, F.B. de Groot, C.A. van Blitterswijk, P. Habibovic, Plug and play: combining materials and technologies to improve bone regenerative strategies. J. Tissue Eng. Regen. Med. 9(7), 745–759 (2015). https://doi.org/10.1002/term.1762
L. Moroni, D. Hamann, L. Paoluzzi, J. Pieper, J.R. de Wijn, C.A. van Blitterswijk, Regenerating articular tissue by converging technologies. PLoS One (2008). https://doi.org/10.1371/journal.pone.0003032
E.P.E. Silva et al., In vivo study of conductive 3D printed PCL/MWCNTs scaffolds with electrical stimulation for bone tissue engineering. Biodes. Manuf. 4(2), 190–202 (2021). https://doi.org/10.1007/s42242-020-00116-1
S.A. Park et al., In vivo evaluation of 3D-printed polycaprolactone scaffold implantation combined with β-TCP powder for alveolar bone augmentation in a beagle defect model. Materials (2018). https://doi.org/10.3390/ma11020238
M. Shahrezaee, M. Salehi, S. Keshtkari, A. Oryan, A. Kamali, B. Shekarchi, In vitro and in vivo investigation of PLA/PCL scaffold coated with metformin-loaded gelatin nanocarriers in regeneration of critical-sized bone defects. Nanomedicine 14(7), 2061–2073 (2018). https://doi.org/10.1016/j.nano.2018.06.007
S.S.R. Bojedla et al., Three-dimensional printing of customized scaffolds with polycaprolactone-silk fibroin composites and integration of gingival tissue-derived stem cells for personalized bone therapy. ACS Appl. Bio. Mater. 5(9), 4465–4479 (2022). https://doi.org/10.1021/acsabm.2c00560
P.S. Gomes, M.H. Fernandes, Rodent models in bone-related research: the relevance of calvarial defects in the assessment of bone regeneration strategies. Lab. Anim. 45(1), 14–24 (2011). https://doi.org/10.1258/la.2010.010085
H. Lee, C.H. Jang, G.H. Kim, A polycaprolactone/silk-fibroin nanofibrous composite combined with human umbilical cord serum for subacute tympanic membrane perforation; an in vitro and in vivo study. J. Mater. Chem. B 2(18), 2703–2713 (2014). https://doi.org/10.1039/c4tb00213j
S.S.R. Bojedla, S. Chameettachal, S. Yeleswarapu, M. Nikzad, S.H. Masood, F. Pati, Silk fibroin microfiber-reinforced polycaprolactone composites with enhanced biodegradation and biological characteristics. J. Biomed. Mater. Res. A 110(7), 1386–1400 (2022). https://doi.org/10.1002/jbm.a.37380
S.S.R. Bojedla et al., Three-dimensional printing of customized scaffolds with polycaprolactone-silk fibroin composites and integration of gingival tissue-derived stem cells for personalized bone therapy. ACS Appl. Bio. Mater. (2022). https://doi.org/10.1021/acsabm.2c00560
F. Mottaghitalab, H. Hosseinkhani, M.A. Shokrgozar, C. Mao, M. Yang, M. Farokhi, Silk as a potential candidate for bone tissue engineering. J. Control. Release 215, 112–128 (2015). https://doi.org/10.1016/j.jconrel.2015.07.031
M. Farokhi et al., Silk fibroin/hydroxyapatite composites for bone tissue engineering. Biotechnol. Adv. 36(1), 68–91 (2018). https://doi.org/10.1016/j.biotechadv.2017.10.001
S. Miyamoto et al., Bombyx mori silk fibroin scaffolds for bone regeneration studied by bone differentiation experiment. J. Biosci. Bioeng. 115(5), 575–578 (2013). https://doi.org/10.1016/j.jbiosc.2012.11.021
M. Xu, X. Zhang, S. Meng, X. Dai, B. Han, X. Deng, Enhanced critical size defect repair in rabbit mandible by electrospun gelatin/β-TCP composite nanofibrous membranes. J. Nanomater. (2015). https://doi.org/10.1155/2015/396916
S.H. Jegal et al., Functional composite nanofibers of poly(lactide-co-caprolactone) containing gelatin-apatite bone mimetic precipitate for bone regeneration. Acta Biomater. 7(4), 1609–1617 (2011). https://doi.org/10.1016/j.actbio.2010.12.003
S. Wang et al., Tuning pore features of mineralized collagen/PCL scaffolds for cranial bone regeneration in a rat model. Mater. Sci. Eng. C (2020). https://doi.org/10.1016/j.msec.2019.110186
F. Pati, T.H. Song, G. Rijal, J. Jang, S.W. Kim, D.W. Cho, Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials 37, 230–241 (2015). https://doi.org/10.1016/j.biomaterials.2014.10.012
N. Saulacic, M. Fujioka-Kobayashi, Y. Kimura, A.I. Bracher, C. Zihlmann, N.P. Lang, The effect of synthetic bone graft substitutes on bone formation in rabbit calvarial defects. J. Mater. Sci. Mater. Med. (2021). https://doi.org/10.1007/s10856-020-06483-6
O.P. Lappalainen et al., Micro-CT analysis of bone healing in rabbit calvarial critical-sized defects with solid bioactive glass, tricalcium phosphate granules or autogenous bone. J. Oral Maxillofac. Res. (2016). https://doi.org/10.5037/jomr.2016.7204
C.A.Y. Takauti, F. Futema, R.B. de Brito Junior, A.C. Abrahao, C. Costa, C.S. Queiroz, Assessment of bone healing in rabbit calvaria grafted with three different biomaterials. Braz. Dent. J. 25(5), 379–384 (2014). https://doi.org/10.1590/0103-6440201302383
S. Viguet-Carrin, P. Garnero, P.D. Delmas, The role of collagen in bone strength. Osteoporos. Int. 17(3), 319–336 (2006). https://doi.org/10.1007/s00198-005-2035-9
P. Bhattacharjee et al., Silk scaffolds in bone tissue engineering: an overview. Acta Biomaterialia. 63, 1–17 (2017). https://doi.org/10.1016/j.actbio.2017.09.027
Acknowledgements
We acknowledgement Dr. Jerald Mehesh Kumar, Principal scientist, CSIR-CCMB, India for the pathological interpretation.
Funding
The authors would like to acknowledge the financial support from Department of Science and Technology, Ministry of Science and Technology, Govt. of India under the FIST program (SR/FST/LSI-683/2016(C)).
Author information
Authors and Affiliations
Contributions
Conceptualization: SSRB, FP, VK; Methodology: SSRB; Validation: SSRB, VK, AMA; Investigation: SSRB; Writing—original draft preparation: SSRB; Writing—review and editing: MN, SHM, SR, FP; Visualization: SSRB, VK, AMA; Supervision: FP; Project administration & funding acquisition: FP. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
Animal selection, supervision, preparation, and surgical protocol, were set based on the routines approved by the Jeeva Life Sciences, Hyderabad, India (Reference no. IAEC/JLS/16/07/21/38).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bojedla, S.S.R., Kattimani, V., Alwala, A.M. et al. Augmented Repair and Regeneration of Critical Size Rabbit Calvaria Defects with 3D Printed Silk Fibroin Microfibers Reinforced PCL Composite Scaffolds. Biomedical Materials & Devices 1, 942–955 (2023). https://doi.org/10.1007/s44174-023-00072-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44174-023-00072-1