Abstract
Salt stress causes osmotic stress, ion toxicity and oxidative stress, inducing the accumulation of abscisic acid (ABA) and excessive reactive oxygen species (ROS) production, which further damage cell structure and inhibit the development of roots in plants. Previous study showed that vitamin B6 (VB6) plays a role in plant responses to salt stress, however, the regulatory relationship between ROS, VB6 and ABA under salt stress remains unclear yet in plants. In our study, we found that salt stress-induced ABA accumulation requires ROS production, in addition, salt stress also promoted VB6 (including pyridoxamine (PM), pyridoxal (PL), pyridoxine (PN), and pyridoxal 5′-phosphate (PLP)) accumulation, which involved in ROS scavenging and ABA biosynthesis. Furthermore, VB6-deficient maize mutant small kernel2 (smk2) heterozygous is more susceptible to salt stress, and which failed to scavenge excessive ROS effectively or induce ABA accumulation in maize root under salt stress, interestingly, which can be restored by exogenous PN and PLP, respectively. According to these results, we proposed that PN and PLP play an essential role in balancing ROS and ABA levels under salt stress, respectively, it laid a foundation for VB6 to be better applied in crop salt resistance than ABA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil salinity is one of the most inclusive abiotic stresses, limits crop growth and production (Asgher et al., 2017) and causes osmotic stress, which is followed by ion poisoning and oxidative stress (Bagri et al., 2018; Hao et al., 2021; Hernandez et al., 2001; Mittler, 2017). Osmotic stress affects the accumulation of ABA, which leads to the closure of stomata and greatly reduces photosynthesis, ultimately leading to plant growth inhibition. ABA is the key plant phytohormone in resisting the harsh conditions caused by abiotic stress (Banerjee and Roychoudhury, 2017; Mehrotra et al., 2014; Vishwakarma et al., 2017). Abiotic stress, such as drought stress, salt stress and heat stress, strongly induces ABA biosynthesis in rice and maize (Dinler et al., 2014; Huang et al., 2018; Lu et al., 2019). Moreover, ABA produces a defense response by inducing the accumulation of ROS in plant cells (Sakamoto et al., 2008), which further causes deleterious effects on plant cells. In brief, although ABA and ROS regulate each other under abiotic stress, the mechanism is still unclear. Abiotic stress triggers the expression of a series of ABA biosynthesis genes, including zeaxanthin oxidase (ZEP), molybdenum cofactor sulfurase (MCSU) and ABA-aldehyde oxidase (AAO) (Vishwakarma et al., 2017). The first step of ABA biosynthesis is initiated by ZEP in Nicotiana plumbaginifolia (Marin et al., 1996), which is involved in stress regulation by inducing ABA accumulation in seed germination (Xiong et al., 2001; Xiong and Zhu, 2003). Viviparous14 (Vp14), which has the same function as 9-cis-epoxycarotenoid dioxygenase (NCED) in maize, was first cloned in 1996 by Tan et al. (Schwartz et al., 2003; Tan et al., 1997). AAO3 is responsible for the final step of the ABA biosynthesis process with the help of a molybdenum (Mo) cofactor, oxidizing the abscisic aldehyde to abscisic acid (Vishwakarma et al., 2017). Previous results indicated that pyridoxal phosphate (PLP) coenzyme was essential for the activity of molybdenum cofactor sulfurase (Heidenreich et al., 2005). The concept of oxidative stress, which is a biochemical process caused by the imbalance between the production of antioxidants and oxidants, which further induces cell damage and changing cellular physiology (Rosado-Perez et al., 2018), was formulated in 1985 (Sies, 2015; Sies and Cadenas, 1985).
ROS is a general term for a group of substances with strong oxidation in plants, mainly including singlet oxygen (1O2), hydroxyl radical (OH−), superoxide anion (O2−) and hydrogen peroxide (H2O2) (Ahanger et al., 2017; Waszczak et al., 2018). Over the past decade, ROS were thought to regulate various biological processes, and was induced by virous environmental stresses (Jimenez-Quesada et al., 2016; Leng et al., 2017; Saini et al., 2018; Tognetti et al., 2017; Xie et al., 2014; You et al., 2014). Salt stress-induced ROS are secondary damage signal that ultimately affect the membrane structure, root development and hormone metabolism inside and outside the plants cell (Ahmad et al., 2008; Bienert et al., 2006; Schmidt et al., 2013; Yang et al., 2007; You and Chan, 2015). Previous studies have shown that ROS disrupt auxin transport, thereby affecting the gravitropism of primary roots (PRs) (Joo et al., 2001; Joo et al., 2005). ROS also regulate cell proliferation and differentiation in the root (Tsukagoshi et al., 2010). Furthermore, in vivo ROS homeostasis plays a central role in cell division, differentiation and regulating lateral root (LR) emergence (Biswas et al., 2019; Orman-Ligeza et al., 2016; Xu et al., 2018). However, excessive ROS accumulation damages plant cellular structure and negatively modulates the development of lateral root emergence under high salt stress (Ahanger et al., 2017). In addition, exogenous hydrogen peroxide treatment inhibited primary root (PR) elongation and the emergence of lateral roots (LRs) (Orman-Ligeza et al., 2016), which also indicated that excessive ROS negatively regulated root development. ROS metabolism also contributes to root growth under drought stress (Dalal et al., 2018). In summary, these studies show that ROS are a double-edged sword for plant development, and maintaining the dynamic balance of ROS in plants is crucial for root development.
Vitamin B6 (VB6) contains pyridoxamine (PM), pyridoxal (PL), pyridoxine (PN), and their 5′-phosphorylated forms (Drewke and Leistner, 2001; Yang et al., 2017), which were identified as singlet oxygen antioxidants (Bilski et al., 2000). In addition, pyridoxal 5′-phosphate (PLP) plays an essential role in a variety of enzyme systems as a cofactor (Rosenberg, 2012). Previous results showed that oxidative stress strongly induced the expression of pyridoxine biosynthesis 1.2 (PDX1.2), which participates in VB6 biosynthesis in Arabidopsis (Moccand et al., 2014). More interestingly, overexpression of the PDX-II gene also enhanced potato tolerance to salt stress (Bagri et al., 2018). In addition, VB6 biosynthesis-deficient mutant plants are more sensitive to salt stress (Gonzalez et al., 2007; Titiz et al., 2006). In maize, the VB6 biosynthesis-deficient plants small kernel2 (smk2) homozygous mutants exhibit an embryonic lethal phenotype, and SMK2 has the similar function with Arabidopsis VB6 biosynthesis gene PDX2.1 (Yang et al., 2017). However, the mechanism of SMK2 in regulating salt-resistant still remains unclear.
Root system is an extremely important nutrient absorption organ for plants and sensitive to environmental change (Kolb et al., 2017; Shahzad and Amtmann, 2017; Su et al., 2017; Sun et al., 2017). The root system contains PRs, LRs and adventitious roots (Olatunji et al., 2017). LRs are more sensitive than PR in responding to salt stress, and the growth and development of LRs are regulated by many plant hormones, including ethylene, auxin, abscisic acid and cytokinin (Ilina et al., 2018; Jing and Strader, 2019; Lu et al., 2019; Qin and Huang, 2018). Under salt stress, ABA severely inhibited the development of maize LRs (Lu et al., 2019). Based on the abovementioned studies, ROS, ABA and VB6 play essential roles in plant root development under salt stress, however, the relationship among them remained unclear yet. Here, we further explored the role of VB6 in balancing salt stress-induced ROS and ABA content in maize roots.
In our study, we found that salt stress simultaneously induced ROS production, ABA and VB6 (including PM, PL, PN, PLP) accumulation in maize roots. Furthermore, PN exogenous can eliminate salt stress-induced ROS accumulation and enhance root resistance to salt stress. In addition, VB6-deficient smk2 heterozygous plants were more susceptible to salt stress and failed to scavenge excessive ROS and induce ABA accumulation under salt stress. Further study showed that exogenous PN rescued the salt stress-susceptible phenotype of heterozygous smk2, on the other hand, exogenous PLP restored salt stress-induced ABA accumulation in heterozygous smk2 by acting as a coenzyme to promote AAO activity, which is involved in ABA biosynthesis in maize roots. In summary, PN and PLP act as antioxidants and coenzymes, respectively, which can eliminate excessive ROS and regulate the biosynthesis of ABA under salt stress, so as to balance the accumulation of ROS and ABA and make maize roots better adapt to salt stress.
Results
ROS production is required for ABA accumulation induced under salt stress
Many studies have shown that ROS are involved in high salinity-inhibited root growth (Golldack et al., 2014; Miller et al., 2010). Our previous work revealed that ABA inhibited LR emergence by affecting the polar location of PIN1 in maize (Lu et al., 2019). To further reveal the regulatory relationship between ROS and ABA under salt stress, the ROS inducer methyl viologen (MV), which inhibits electron transfer in the electron transfer chain and leads to ROS production (Li et al., 2017), was used to mimic oxidative stress. We observed that the elongation of PRs and the number of LRs were strongly inhibited with the increase of MV, and we choose 10 μM MV for the following experiments (Fig. S1). Our results showed that ROS marker fluorescence signal was stronger than that of control under NaCl or MV treatment (Fig. 1A). And the green immunofluorescence of ABA showed significant enhancement after NaCl and MV treatment (Fig. 1B). Similarly, quantitative analysis of the fluorescence intensity of ROS clearly showed that the fluorescence intensity under 100, 200 mM NaCl and 100 μM MV treatments was 27%, 68% and 115% higher than that of the control, respectively (Fig. 1C), and the ABA immunofluorescence intensity under 100, 200 mM NaCl and 100 μM MV treatment increased by 50%, 110% and 108% (Fig. 1C), respectively. In addition, the ABA biosynthesis genes (AAO3, ZEP and VP14) were significantly upregulated after NaCl and MV treatment (Fig. 1D). Furthermore, NaCl and MV treatment significantly inhibited PR elongation and strongly decreased the number of LRs (Fig. 1E and F). Therefore, exogenous catalase (CAT), a ROS scavenger, partially recovered PR elongation and the number of LRs under salt stress (Fig. 1E and F). And CAT significantly decreased the ROS marker fluorescence intensity (Fig. 1A and C), the ABA immunofluorescence intensity and the expression of ABA biosynthesis genes in maize roots (Fig. 1B-D). These results suggested that ROS play an essential role for ABA accumulation and the inhibition of root growth under salt/oxidative stress.
ROS regulate the accumulation of ABA in lateral roots induced by salt stress. A, B ROS and ABA fluorescence in LRs of 6-d-old seedlings subjected to H2O (Control), 100 mM NaCl (N), 100 mM NaCl + 100 μM CAT (Catalase), 200 mM NaCl, 200 mM NaCl + 100 μM CAT, 10 μM MV (Methyl viologen) and 10 μM MV + 100 μM CAT for 24 h. ROS and ABA fluorescence were detected by using the fluorescent dye H2DCFDA and immunofluorescence in LRs, respectively. Bar = 50 μm. The white thread represents the profile of the LR. C ROS and ABA fluorescence intensity of A and B. D qRT–PCR analysis of AAO3, ZEP and VP14 expression in maize roots of 6-d-old seedlings subjected to H2O (Control), 100 mM NaCl (N), 100 mM NaCl + catalase (CAT, C), 200 mM NaCl, 200 mM NaCl + CAT, 10 μM MV (Methyl viologen) and 10 μM MV + CAT for 24 h. E Length of PRs after different treatments for 4 d. PRs is from independent maize seedling (n ≥ 6). F Number of LRs (n ≥ 6) after different treatments for 4 d. LRs is from independent maize seedling (n ≥ 6). Image J software was used for quantifying fluorescence intensity. The different letters represent significant differences (P < 0.05, based on one-way ANOVA)
ROS is involved in NaCl induced-VB6 accumulation
Previous study also showed that VB6 biosynthesis genes were important for oxidative stress and salt stress responses in plants (Bagri et al., 2018; Titiz et al., 2006). Thus, we hypothesize that VB6 is also induced by salt stress. To prove our hypothesis, we detected the expression of VB6 biosynthesis genes (SMK2 and PDX1.1) and VB6 content under salt stress in maize root. The expression of SMK2 and PDX1.1 was dramatically upregulated, especially the expression of SMK2, which increased 8-, 5- and 22-fold compared with that of the control under 10 μM MV, 100 and 200 mM NaCl treatment (Fig. 2A), respectively. As expected, exogenous CAT also restored the expression of the SMK2 and PDX1.1 induced by NaCl (Fig. 2A), and 10 μM exogenous MV also induced the expression of SMK2 and PDX1.1. We also measured the content of VB6, including PL, PN, PM and PLP, by using high-performance liquid chromatography (HPLC) in maize roots. NaCl treatment significantly increased the four VB6 isoforms accumulation in maize root (Fig. 2B), which was marked decreased by exogenous CAT (Fig. 2B). Similarly, exogenous CAT partially restored the expression of ABA biosynthesis genes (AAO3, ZEP, VP14) and VB6 biosynthesis genes (SMK2 and PDX1.1) and the content of different components of VB6 induced by MV (Fig. 1D, 2A and B). These results suggest ROS production is essential for salt/oxidative stress-induced VB6 accumulation.
Salt and oxidative stress induced VB6 accumulation in maize roots. A qRT–PCR analysis of SMK2 and PDX1.1 expression in maize roots of 6-d-old seedlings subjected to H2O (Control), 100 mM NaCl (N), 100 mM NaCl + CAT, 200 mM NaCl, 200 mM NaCl + CAT, 10 μM MV (Methyl viologen) and 10 μM MV + CAT for 24 h. B HPLC detection of the contents of PL, PN, PM, and PLP after different treatments for 24 h. The different letters represent significant differences (P < 0.05, based on one-way ANOVA)
Exogenous PN decreased ROS and ABA accumulation
VB6 and its derivatives were identified as antioxidants by quenching singlet oxygen, especially PN, which has a strong singlet oxygen scavenging capacity (Bilski et al., 2000). To elucidate the function of different components of VB6, we performed the following experiments. Firstly, we compared the ability of PN, PM PL and PLP in scavenging ROS. To test the ability of PN to scavenge ROS, PN was applied under NaCl and MV treatment. Supplementation with exogenous PN dramatically decreased the ROS and ABA fluorescence intensity and brightness under NaCl or MV treatment (Fig. 3A-D, Fig. S2), respectively. Furthermore, PN also stronger rescued the inhibition of LRs and PRs development by NaCl (200 mM) than PL and PM, while PLP failed to restore the growth of LR and PR under the 200 mM NaCl treatment (Fig. S3), similarly, PLP also failed scavenging ROS accumulation (Fig. S2). Similarly, exogenous PN supplementation also promoted PR elongation and increased the number of LRs under the NaCl and MV treatment (Fig. 3E and F), indicating that PN effectively scavenge ROS accumulation induced by NaCl. In addition, PN also significantly decreased the expression of ABA biosynthesis genes (AAO3, ZEP and VP14) induced by NaCl and MV (Fig. S4). These results indicate that PN may be involved in scavenging excessive ROS and ABA biosynthesis under salt treatment.
Exogenous PN reduced ROS and ABA accumulation under salt stress. A, B ROS and ABA fluorescence in LRs of 6-d-old seedlings subjected to 200 mM NaCl (N), 200 mM NaCl + 100 μM PN, 10 μM MV (methyl viologen) and 10 μM MV + 100 μM PN for 24 h. ROS and ABA fluorescence were detected by using the fluorescent dye H2DCFDA and immunofluorescence in LRs, respectively. Bar = 50 μm. The white thread represents the profile of the LR. C ABA fluorescence intensity of (A). D ROS fluorescence intensity of (B). Image J software was used for quantifying fluorescence intensity. The different letters represent significant differences (P < 0.05, based on one-way ANOVA). E Length of PRs after different treatments for 4 d. PRs is from independent maize seedling (n ≥ 6). F Number of LRs after different treatments for 4 d. LRs is from independent maize seedling (n ≥ 6). Different letters represent significant differences (P < 0.05, based on one-way ANOVA)
smk2 heterozygous plants were more sensitive to salt stress
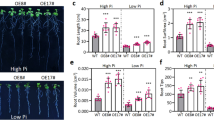
Next, smk2 heterozygous plants was identified and used to further reveal the effect of endogenous VB6 on salt resistance and antioxidation in maize (Fig. S5). Our results showed that the heterozygous smk2 phenotype exhibited a similar phenotype to that of the wild type (W22) under normal conditions (Fig. 4A). However, the developmental state of heterozygous smk2 was weaker than that of the control under salt stress (Fig. 4A). More seriously, PRs length and the density of lateral roots in heterozygous smk2 exhibited an extensive reduction compared with that of the W22 under salt stress (Fig. 4A-C). Furthermore, we observed showed that the expression of SMK2 in smk2 heterozygous was dramatically decreased compared with that of W22 under salt stress or MV treatment (Fig. S6). We further detected the content of VB6 by using HPLC, and we observed that both NaCl and MV significantly increase VB6 (PL, PN, PM and PLP) content in W22 plants, but failed to do so in smk2 heterozygous (Fig. 4D), which indicated that SMK2 positively regulates VB6 accumulation and maize root growth under salt stress.
smk2 heterozygous plants were more sensitive than the W22 plants to salt stress. A Phenotypes of W22 and smk2 heterozygous suffered from H2O (Control), 100 mM NaCl (N), 200 mM NaCl and 10 μM MV (methyl viologen), − and + means with or without 100 μM PN. Bar = 1 cm. The white dotted lines show the enlarged part. B LRs number of (A) after different treatments for 4 d. LRs are from independent maize seedling (n ≥ 6). C The PRs length of (A) after different treatments for 4 d. PRs are from independent maize seedling (n ≥ 6). D HPLC detection of the contents of PL, PN, PM, and PLP after different treatments for 24 h in W22 and smk2 heterozygous plants. The different letters represent significant differences (P < 0.05, based on one-way ANOVA)
Furthermore, we detected the accumulation of ROS in W22 and smk2 heterozygotes in the presence of NaCl and MV. We observed that NaCl and MV induced more ROS accumulation in smk2 than that of W22 (Fig. 5A and B). In addition, the application of exogenous PN reduced ROS accumulation to a level similar to that of W22 (Fig. 5A and B), suggesting that PN is essential for plants to scavenge excessive ROS induced by salt stress. However, PLP failed to scavenging excessive ROS or restoring ABA biosynthesis genes (AAO3, ZEP and VP14) expression under salt/oxidative stress (Figs. S2 and S7), which suggest that PLP plays a different role than PN.
PN is extremely essential for smk2 heterozygous plants to scavenge excessive ROS induced by NaCl and MV. A ROS fluorescence in LRs of 6-d-old smk2 heterozygous and W22 seedlings subjected to 100 mM NaCl (N), 200 mM NaCl, 10 μM MV (methyl viologen) or added with 100 μM PN treatments for 24 h by using the fluorescent dye H2DCFDA. Bar = 50 μm. The white thread represents the profile of the LR. B ROS fluorescence intensity of (A). Image J software was used for quantifying fluorescence intensity. The different letters represent significant differences (P < 0.05, based on one-way ANOVA)
PLP is essential for abscisic aldehyde oxidase activity
Previous results showed that PLP serves as an ABA3 cofactor, which encodes molybdenum cofactor sulfurase (Mendel and Bittner, 2006). In A. thaliana, ABA3 directly promotes the activity of aldehyde oxidase (AAO) (Bittner et al., 2001), which is involved in the conversion of ABA-aldehyde to ABA (Vishwakarma et al., 2017). As expected, we found that AAO activity in W22 was much higher than that of smk2 heterozygous after NaCl or MV treatment (Fig. 6A). Furthermore, exogenous PLP partially recovered AAO activity under MV and NaCl treatment in smk2 (Fig. 6B). Similarly, MV and NaCl failed to induce ABA accumulation in smk2 roots, and exogenous PLP also restored the accumulation of ABA in smk2 heterozygous LRs (Fig. 6C). Moreover, previous results showed that the content of PLP was increased 1.75-, 2.6- and 3.5-fold by NaCl (100 mM, 200 mM) and MV in W22 but was disabled in smk2 heterozygous plants (Fig. 4D). These results indicate that PLP is essential for AAO activity, which further promotes ABA accumulation under oxidative stress.
PLP was essential for AAO activity. A AAO activity of smk2 heterozygous and W22. 6-d-old maize seedlings were treated with 100 mM NaCl (N), 200 mM NaCl and 10 μM MV (methyl viologen) for 24 h. The maize seedlings roots were harvested for detection of AAO activity. B AAO activity of smk2 heterozygous. 6-d-old maize seedlings were treated with H2O (Control), 200 mM NaCl (N), 10 μM MV (methyl viologen), 200 mM NaCl + 100 μM PLP and 10 μM MV + 100 μM PLP for 24 h. The maize seedlings roots were harvested for detection of AAO activity. C ABA detection in smk2 heterozygous and W22 maize LRs. 6-d-old smk2 heterozygotes and W22 subjected to H2O (Control), 200 mM NaCl (N), 10 μM MV (Methyl viologen), 200 mM NaCl + 100 μM PLP and 10 μM MV + 100 μM PLP for 24 h. ABA fluorescence was detected by immunofluorescence in LRs. Bar = 50 μm. The white thread represents the profile of the LR
Discussion
Previous studies showed that VB6 biosynthesis gene was involved in plant defense against abiotic stress. VB6 biosynthesis-deficient mutant pdx1.3 is sensitive to salt stress in Arabidopsis (Titiz et al., 2006), and overexpression of PDX genes enhanced plant resistance to salt stress (Bagri et al., 2018; Raschke et al., 2011), which is consistent with our research results. In addition, PDX1.1 confers plant resistance to ammonium-induced oxidative stress by mediating VB6 biosynthesis (Liu et al., 2022b). However, whether the involvement of VB6 in the salt stress response requires the participation of ROS and ABA has not been reported. In summary, our study reveals that the important role of PN and PLP in balancing the dynamic equilibrium of ROS and ABA under salt/oxidative stress in maize.
ROS acts as a bridge in salt stress-induced ABA and VB6 accumulation
Previous research indicated that both ROS and ABA were also induced by salt stress (Hao et al., 2021), and our study also indicated that VB6 accumulated under salt stress (Fig. 2B). ABA biosynthesis genes, such as ZEP and VP14, and ABA contents, were significantly induced by salt/oxidative stress (Fig. 1A and F). ROS scavenger CAT obviously decreased the expression of ABA biosynthesis and ABA contents (Fig. 1A and F). These results indicate that salt stress induces ABA accumulation by enhancing the expression of ABA biosynthesis genes, and this process requires excessive ROS accumulation. In addition, we also found that salt/oxidative stress upregulated the expression of VB6 biosynthesis genes SMK2 and increased VB6 content, which was inhibited by CAT (Fig. 2A and B). Accordingly, ROS act as a positive inducing signaling molecule involved in salt-induced VB6 and ABA accumulation under salt stress.
PN scavenges excess ROS accumulation to alleviates the inhibition of root development by salt stress
ROS act as a secondary messenger to regulating plant growth and development under various stresses (Bailey-Serres and Mittler, 2006). There are two ROS scavenging mechanisms in plants: an enzymatic scavenging system, including superoxide dismutase (SOD) and CAT (Gill and Tuteja, 2010), and a nonenzymatic scavenging system, mainly containing ascorbic acid, carotenoids, tocopherols, and flavonoids glutathione (You and Chan, 2015). Interestingly, our data showed that exogenous PN effectively decreased the production of excessive ROS induced by salt/oxidative stress (Figs. S2, Fig. 3A and D). Similarly, overexpression of the VB6 biosynthesis gene (PDX-II) enhanced potato tolerance to abiotic stresses (Bagri et al., 2018). In Arabidopsis, pdx1.1 and pdx1.3 are essential for root growth and development, and pdx1.3 is more sensitive than Col-0 to salt stress (Titiz et al., 2006). Similarly, we found that salt stress induces high-level accumulation of PN, which was compromised in smk2 heterozygous (Fig. 4D). In addition, smk2 heterozygous roots were more sensitive to salt stress and oxidation stress, and supplementation with exogenous PN recovered the salt tolerance of smk2 (Fig. 4A-C). Moreover, we also observed that smk2 heterozygous accumulates more ROS than that of W22 under salt/oxidative stress, while exogenous application of PN can greatly reduce ROS accumulation (Fig. 5). Similarly, exogenous PN decreased salt/oxidative stress-induced the upregulation of ABA biosynthesis genes expression and ABA accumulation, which further relieved the inhibition phenotype of maize roots by salt/oxidative stress (Fig. 3, Figs. S3 and S4). Accordingly, we proposed that SMK2 plays an essential role for plant defense against salt/oxidative stress, PN decrease the accumulation of ABA by scavenging excessive ROS, which further regulate root development under salt stress.
PLP plays a novel regulatory role in ABA biosynthesis
Our results showed that ROS accumulated to higher levels and ABA biosynthesis genes were also induced in smk2 heterozygous under NaCl or MV treatment (Fig. 5 and Fig. S7), which indicates that VB6 act as an antioxidant to scavenging excessive ROS and further regulating the accumulation of ABA. However, PLP failed to scavenging excessive ROS, relieving the inhibitory effect of salt stress on roots or decreased the upregulation expression of ZEP, AAO3 and VP14 induced by salt stress (Figs. S2, S3 and S7), which indicate PLP play a novel role in plant-salt/oxidative stress interaction. PLP is an active form of PL involved in over 170 enzymatic reactions as a cofactor (Rosenberg, 2012). PLP was indeed identified to be the molybdenum cofactor sulfurase ABA3-bound chromophore by binding with a NifS-like domain (Heidenreich et al., 2005). ABA3 directly activates aldehyde oxidase (AO), which was proposed to be involved in the conversion of ABA-aldehyde to ABA in plants (Bittner et al., 2001). Here, we found that salt/oxidation stress increased the activity of AAO in the W22 but failed to induce AAO activity in smk2 heterozygous (Fig. 6A). Moreover, exogenous PLP recovered the AAO activity induced by salt/oxidative stress in smk2 heterozygous (Fig. 6A). Furthermore, salt/oxidative stress failed to inducing the accumulation of ABA in smk2 heterozygous (Fig. 6C), which could be restored by exogenous PLP in maize roots (Fig. 6C). Our latest report suggests that VB6 regulates ABA biosynthesis to close stomata, thereby enhancing rice resistance to bacterial leaf streak (Liu et al., 2022a), which suggests that PLP may play a role in plant-pathogen interaction. Overall, PLP plays an essential role in plant-pathogen and plant-adversity interaction by directly promoting AAO activity to further regulating ABA biosynthesis.
Model of VB6 modulation of plant antioxidant stress
In conclusion, we generated the following possible model to better understand ROS-VB6-ABA interactions under salt/oxidative stress (Fig. 7). Salt/oxidative stress induces ROS accumulation, which next upregulates ABA- and VB6 biosynthesis-associated genes (ZEP, VP14 and SMK2), and further increase VB6 (PL, PN, PM and PLP) content and ABA accumulation in maize roots. As feedback regulation, PN scavenges excessive ROS accumulation and alleviates its oxidative damage to maize roots. On the other hand, PLP acts as a coenzyme to activate AAO activity, which further promotes ABA biosynthesis. In a word, VB6 acts as a bridge to mediate the regulation of ROS on ABA accumulation under salt stress, so as to make maize roots better adapt to salt stress. Finally, our study revealed the role of VB6 in balancing the dynamic equilibrium of ROS and ABA under salt/oxidative stress.
Possible model of VB6 involved in the plant response to salt stress. On the one hand, salt stress-induced ROS accumulation leads to oxidative damage and root inhibition, which further induces the expression of ZEP, AAO3 and VP14 and upregulates the VB6 (PL, PN, PM, PLP) content in maize roots. As a feedback mechanism, PN could scavenge excessive ROS to reduce the damage of oxidative stress to maize roots. On the other hand, PLP promoted AAO activity, further improving ABA production to inhibit root development and enhance plant salt stress tolerance
Materials and methods
Plant growth condition
All maize seeds that were used in this study were surface-sterilized before use. The background of smk2 heterozygous mutant used in this study is W22, and that of other maize seeding are B73 background, smk2 homozygous mutants were embryo lethal, so we used smk2 heterozygous mutants for the experiments (Yang et al., 2017). Specific operations were as follows: all maize seeds were surface-sterilized with 75% ethyl alcohol for 60 s, washed three times with deionized water, transferred to a 3% (v/v) NaClO solution for 10–15 min and washed 6–7 times in deionized water. All steps involved shaking and thorough mixing. Next, seeds were placed on a petri plate, which was covered by sterile absorbent cotton gauze, for germination and a proper amount of sterile double distilled water was added to keep the plate wet under a 16-h-light/8-h-dark photoperiod at 28 °C. Three days later, seedlings were transferred into Hoagland’s nutrient solution supplemented with different treatments.
ROS detection
The ROS-specific fluorescence probe H2DCFDA (Invitrogen) was used to detect endogenous ROS. The maize seedling roots were washed with deionized water three times, and then the seedling roots were cut and sliced on clean glass slides. The transects of maize roots were placed into a 5 μM staining solution and was vacuumed for 15 min. Then, washed these samples for three times and imaged by a Zeiss LSM880 (Zeiss) confocal microscope (excitation, 488 nm; emission, 530 nm). For 3,3-diaminobenzidine (DAB) staining, the seedlings roots were put into 0.5 mg/ml DAB staining solution, vacuum for 0.5 hours (h). Then the samples were washed for three times with deionized water and reacted with H2O2 for 6–8 h under light. When the deep brown deposition appears in maize roots, transfer the samples into boiled ethanol and decolorizing three times. Finally, maize roots were imaged by a SMZ25 stereoscopic microscope (Nikon, Japan).
Phenotypic analysis
Seedlings were transferred into 50%-strength Hoagland’s nutrient solution. The PR length was measured by a straightedge, and the number of LRs, which is emerged in PRs rather than adventitious roots, was counted with fine-tipped tweezers every day. The number of statistics was not less than 6 per experiment, and three parallel experiments were performed.
Immunofluorescence assay of ABA
The immunofluorescence experiments used previous methods with some modifications (Ondzighi-Assoume et al., 2016). Maize seedling roots were washed three times to clean the roots. Then, roots were cut off from maize seedlings and placed on slides, and slicing was performed by hand to cut out the transverse section of the root. Next, the LR transverse sections were put into ABA stationary liquid and vacuum infiltrated for 2 h in an ice box. Then, the samples were transformed into a 4 °C refrigerator and left overnight, washed the samples for three times with 10 mM PBS (pH = 7.2) and then hyalinized in 10 mM PBS, including 0.2% pectinase, 0.2% cellulose, 3% (w/v) nonfat milk powder and 1% Triton X-100, for one hour at room temperature. The transverse sections were washed for three times and incubated with 1/3000 anti-ABA monoclonal antibody on a shaker at 4 °C overnight. Next, the samples were washed for many times to be cleaned and incubated with secondary antibody conjugated with Alexa Fluor 488 for 2 h at room temperature, and the formula of the incubation solution was the same except for the antibody. The transverse sections were washed 6–7 times to thoroughly remove antibody residues and blocked using Citifluor AF1 (Ted Pella, Inc.). Finally, pictures were imaged by using Zeiss LSM880 (excitation at 488 nm, emission at 510–560 nm). Fluorescence intensity was quantified using an analysis particle tool (ImageJ).
Plant RNA extraction and quantitative PCR analysis
Total RNA of maize roots (~ 2000 ng) was extracted by using the TRIzon Reagent Kit (CWBIO). 1 μg total RNA was reverse transcribed for quantitative PCR by using a HiScript III 1st Strand cDNA Synthesis Kit (+gDNA wiper) Kit (Vazyme). Quantitative PCR analyses were conducted by using a SYBR Green Fast qPCR Mix (ABclonal). Target genes expression was calculated following the previous report (Chen et al., 2021).
Vitamin B6 detection by HPLC
VB6 was extracted from maize roots. A 1200 HPLC series was used to quantitatively detect VB6 content. Three independent maize roots were obtained from three different experiments. VB6 was extracted by 1‰ (v/v) hydrochloric acid in darkness. The chromatographic column used by HPLC was a ZORBAXSB-C18 4.6*50 mm (Agilent Technologies, USA), the operating temperature was 25 °C, both mobile phases A and B were 1‰ (v/v) hydrochloric acid, the flow rate was 0.8 mL/min, the detection wavelength was 280 nm. Standard pyridoxal hydrochloride (CAS No: 65–22-5), pyridoxine hydrochloride (CAS No: 58–56-0), pyridoxamine dihydrochloride (CAS No: 524–36-7) and pyridoxal 5′-phosphate (CAS No:853645–22-4) were purchased from Sigma.
Detection of AAO activity
AAO activity was detected refer to previously recent articles with minor modifications (Liu et al., 2022a). Maize roots were pretreated with NaCl, MV and other treatments and harvested, next, transfer the samples into liquid nitrogen and ground to a powder in a mortar, the powder was suspended in extraction buffer to extract total proteins, which were then subjected to native PAGE. The bands indicating AAO activity were developed with abscisic aldehyde (Sigma-Aldrich) in the reaction buffer (1 mM EDTA, 0.01 M flavin adenine dinucleotide, 2 mM dithiothreitol, 1 mM sodium molybdate, 0.05 M Tris HCl [pH = 7.5]) at 25–30 °C in the dark for 0.5 h.
Availability of data and materials
All data and materials are available in the paper and online supplemental files.
Abbreviations
- ABA:
-
Abscisic acid
- ROS:
-
Reactive oxygen species
- VB6 :
-
Vitamin B6
- PM:
-
Pyridoxamine
- PL:
-
Pyridoxal
- PN:
-
Pyridoxine
- PLP:
-
Pyridoxal 5′-phosphate
- SMK2:
-
Small kernel2
- LR:
-
Lateral root
- PR:
-
Primary root
- MV:
-
Methyl viologen
- HPLC:
-
High-performance liquid chromatography
- AAO:
-
ABA-aldehyde oxidase
- ZEP:
-
Zeaxanthin oxidase
- VP14:
-
Viviparous14
- PDX:
-
Pyridoxine biosynthesis gene
- DAB:
-
3,3-diaminobenzidine
- MCSU:
-
Molybdenum cofactor sulfurase
- NCED:
-
9-cis-epoxycarotenoid dioxygenase
- SOD:
-
superoxide dismutase
- CAT:
-
Catalase
References
Ahanger MA, Tomar NS, Tittal M, Argal S, Agarwal RM (2017) Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol Plants 23:731–744. https://doi.org/10.1007/s12298-017-0462-7
Ahmad R, Kim MD, Back KH, Kim HS, Lee HS, Kwon SY, Murata N, Chung WI, Kwak SS (2008) Stress-induced expression of choline oxidase in potato plant chloroplasts confers enhanced tolerance to oxidative, salt, and drought stresses. Plant Cell Rep 27:687–698. https://doi.org/10.1007/s00299-007-0479-4
Asgher M, Per TS, Masood A, Fatma M, Freschi L, Corpas FJ, Khan NA (2017) Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ Sci Pollut Res Int 24:2273–2285. https://doi.org/10.1007/s11356-016-7947-8
Bagri DS, Upadhyaya DC, Kumar A, Upadhyaya CP (2018) Overexpression of PDX-II gene in potato (Solanum tuberosum L.) leads to the enhanced accumulation of vitamin B6 in tuber tissues and tolerance to abiotic stresses. Plant Sci 272:267–275. https://doi.org/10.1016/j.plantsci.2018.04.024
Bailey-Serres J, Mittler R (2006) The roles of reactive oxygen species in plant cells. Plant Physiol 141:311. https://doi.org/10.1104/pp.104.900191
Banerjee A, Roychoudhury A (2017) Abscisic-acid-dependent basic leucine zipper (bZIP) transcription factors in plant abiotic stress. Protoplasma 254:3–16. https://doi.org/10.1007/s00709-015-0920-4
Bienert GP, Schjoerring JK, Jahn TP (2006) Membrane transport of hydrogen peroxide. Biochim Biophys Acta 1758:994–1003. https://doi.org/10.1016/j.bbamem.2006.02.015
Bilski P, Li MY, Ehrenshaft M, Daub ME, Chignell CF (2000) Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem Photobiol 71:129–134. https://doi.org/10.1562/0031-8655(2000)0712.0.co;2
Biswas MS, Fukaki H, Mori IC, Nakahara K, Mano J (2019) Reactive oxygen species and reactive carbonyl species constitute a feed-forward loop in auxin signaling for lateral root formation. Plant J 100:536–548. https://doi.org/10.1111/tpj.14456
Bittner F, Oreb M, Mendel RR (2001) ABA3 is a molybdenum cofactor sulfurase required for activation of aldehyde oxidase and xanthine dehydrogenase in Arabidopsis thaliana. J Biol Chem 276:40381–40384. https://doi.org/10.1074/jbc.c100472200
Chen MX, Lu CC, Sun PC, Nie YX, Tian Y, Hu QJ, Das D, Hou XX, Gao B, Chen X et al (2021) Comprehensive transcriptome and proteome analyses reveal a novel sodium chloride responsive gene network in maize seed tissues during germination. Plant Cell Environ 44:88–101. https://doi.org/10.1111/pce.13849
Dalal M, Sahu S, Tiwari S, Rao AR, Gaikwad K (2018) Transcriptome analysis reveals interplay between hormones, ROS metabolism and cell wall biosynthesis for drought-induced root growth in wheat. Plant Physiol Biochem 130:482–492. https://doi.org/10.1016/j.plaphy.2018.07.035
Dinler BS, Demir E, Kompe YO (2014) Regulation of auxin, abscisic acid and salicylic acid levels by ascorbate application under heat stress in sensitive and tolerant maize leaves. Acta Biol Hung 65:469–480. https://doi.org/10.1556/abiol.65.2014.4.10
Drewke C, Leistner E (2001) Biosynthesis of vitamin B6 and structurally related derivatives. Vitam Horm 61:121–155. https://doi.org/10.1016/s0083-6729(01)61004-5
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Golldack D, Li C, Mohan H, Probst N (2014) Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci 5:151. https://doi.org/10.1007/978-3-030-40277-8_2
Gonzalez E, Danehower D, Daub ME (2007) Vitamer levels, stress response, enzyme activity, and gene regulation of Arabidopsis lines mutant in the pyridoxine/pyridoxamine 5′-phosphate oxidase (PDX3) and the pyridoxal kinase (SOS4) genes involved in the vitamin B6 salvage pathway. Plant Physiol 145:985–996. https://doi.org/10.1104/pp.107.105189
Hao S, Wang Y, Yan Y, Liu Y, Wang J, Chen S (2021) A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 7:132. https://doi.org/10.3390/horticulturae7060132
Heidenreich T, Wollers S, Mendel RR, Bittner F (2005) Characterization of the NifS-like domain of ABA3 from Arabidopsis thaliana provides insight into the mechanism of molybdenum cofactor sulfuration. J Biol Chem 280:4213–4218. https://doi.org/10.1074/jbc.m411195200
Hernandez JA, Ferrer MA, Jimenez A, Barcelo AR, Sevilla F (2001) Antioxidant systems and O(2)(.-)/H(2)O(2) production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol 127:817–831. https://doi.org/10.1104/pp.010188
Huang X, Hou L, Meng J, You H, Li Z, Gong Z, Yang S, Shi Y (2018) The antagonistic action of Abscisic acid and Cytokinin signaling mediates drought stress response in Arabidopsis. Mol Plant 11:970–982. https://doi.org/10.1016/j.molp.2018.05.001
Ilina EL, Kiryushkin AS, Semenova VA, Demchenko NP, Pawlowski K, Demchenko KN (2018) Lateral root initiation and formation within the parental root meristem of Cucurbita pepo: is auxin a key player? Ann Bot 122:873–888. https://doi.org/10.1093/aob/mcy052
Jimenez-Quesada MJ, Traverso JA, Alche Jde D (2016) NADPH oxidase-dependent superoxide production in plant reproductive tissues. Front Plant Sci 7:359. https://doi.org/10.3389/fpls.2016.00359
Jing H, Strader LC (2019) Interplay of auxin and Cytokinin in lateral root development. Int J Mol Sci 20:486. https://doi.org/10.3390/ijms20030486
Joo JH, Bae YS, Lee JS (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126:1055–1060. https://doi.org/10.1104/pp.126.3.1055
Joo JH, Yoo HJ, Hwang I, Lee JS, Nam KH, Bae YS (2005) Auxin-induced reactive oxygen species production requires the activation of phosphatidylinositol 3-kinase. FEBS Lett 579:1243–1248. https://doi.org/10.1016/j.febslet.2005.01.018
Kolb E, Legue V, Bogeat-Triboulot MB (2017) Physical root-soil interactions. Phys Biol 14:065004. https://doi.org/10.1088/1478-3975/aa90dd
Leng Y, Yang Y, Ren D, Huang L, Dai L, Wang Y, Chen L, Tu Z, Gao Y, Li X et al (2017) A Rice PECTATE LYASE-LIKE gene is required for plant growth and leaf senescence. Plant Physiol 174:1151–1166. https://doi.org/10.1104/pp.16.01625
Li Y, Wang Y, Xue H, Pritchard HW, Wang X (2017) Changes in the mitochondrial protein profile due to ROS eruption during ageing of elm (Ulmus pumila L.) seeds. Plant Physiol Biochem 114:72–87. https://doi.org/10.1016/j.plaphy.2017.02.023
Liu H, Lu C, Li Y, Wu T, Zhang B, Liu B, Feng W, Xu Q, Dong H, He S et al (2022a) The bacterial effector AvrRxo1 inhibits vitamin B6 biosynthesis to promote infection in rice. Plant Commun 3:100324. https://doi.org/10.1016/j.xplc.2022.100324
Liu Y, Maniero RA, Giehl RFH, Melzer M, Steensma P, Krouk G, Fitzpatrick TB, von Wiren N (2022b) PDX1.1-dependent biosynthesis of vitamin B6 protects roots from ammonium-induced oxidative stress. Mol Plant 15:820–839. https://doi.org/10.1016/j.molp.2022.01.012
Lu C, Chen MX, Liu R, Zhang L, Hou X, Liu S, Ding X, Jiang Y, Xu J, Zhang J et al (2019) Abscisic acid regulates auxin distribution to mediate maize lateral root development under salt stress. Front Plant Sci 10:716. https://doi.org/10.3389/fpls.2019.00716
Marin E, Nussaume L, Quesada A, Gonneau M, Sotta B, Hugueney P, Frey A, Marion-Poll A (1996) Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J 15:2331–2342. https://doi.org/10.1002/j.1460-2075.1996.tb00589.x
Mehrotra R, Bhalothia P, Bansal P, Basantani MK, Bharti V, Mehrotra S (2014) Abscisic acid and abiotic stress tolerance - different tiers of regulation. J Plant Physiol 171:486–496. https://doi.org/10.1016/j.jplph.2013.12.007
Mendel RR, Bittner F (2006) Cell biology of molybdenum. Biochim Biophys Acta 1763:621–635. https://doi.org/10.1016/j.bbamcr.2006.03.013
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mittler R (2017) ROS Are Good. Trends Plant Sci 22:11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Moccand C, Boycheva S, Surriabre P, Tambasco-Studart M, Raschke M, Kaufmann M, Fitzpatrick TB (2014) The pseudoenzyme PDX1.2 boosts vitamin B6 biosynthesis under heat and oxidative stress in Arabidopsis. J Biol Chem 289:8203–8216. https://doi.org/10.1074/jbc.m113.540526
Olatunji D, Geelen D, Verstraeten I (2017) Control of endogenous auxin levels in plant root development. Int J Mol Sci 18. https://doi.org/10.3390/ijms18122587
Ondzighi-Assoume CA, Chakraborty S, Harris JM (2016) Environmental nitrate stimulates Abscisic acid accumulation in Arabidopsis root tips by releasing it from inactive stores. Plant Cell 28:729–745. https://doi.org/10.1105/tpc.15.00946
Orman-Ligeza B, Parizot B, de Rycke R, Fernandez A, Himschoot E, Van Breusegem F, Bennett MJ, Perilleux C, Beeckman T, Draye X (2016) RBOH-mediated ROS production facilitates lateral root emergence in Arabidopsis. Development 143:3328–3339. https://doi.org/10.1242/dev.136465
Qin H, Huang R (2018) Auxin controlled by ethylene steers root development. Int J Mol Sci 19:3656. https://doi.org/10.3390/ijms19113656
Raschke M, Boycheva S, Crevecoeur M, Nunes-Nesi A, Witt S, Fernie AR, Amrhein N, Fitzpatrick TB (2011) Enhanced levels of vitamin B(6) increase aerial organ size and positively affect stress tolerance in Arabidopsis. Plant J 66:414–432. https://doi.org/10.1111/j.1365-313x.2011.04499.x
Rosado-Perez J, Aguiniga-Sanchez I, Arista-Ugalde TL, Santiago-Osorio E, Mendoza-Nunez VM (2018) The biological significance of oxidative stress, effects of fruits as natural edible antioxidants. Curr Pharm Des 24:4807–4824. https://doi.org/10.2174/1381612824666190114164758
Rosenberg IH (2012) A history of the isolation and identification of vitamin B(6). Ann Nutr Metab 61:236–238. https://doi.org/10.1159/000343113
Saini S, Kaur N, Pati PK (2018) Reactive oxygen species dynamics in roots of salt sensitive and salt tolerant cultivars of rice. Anal Biochem 550:99–108. https://doi.org/10.1016/j.ab.2018.04.019
Sakamoto H, Matsuda O, Iba K (2008) ITN1, a novel gene encoding an ankyrin-repeat protein that affects the ABA-mediated production of reactive oxygen species and is involved in salt-stress tolerance in Arabidopsis thaliana. Plant J 56:411–422. https://doi.org/10.1111/j.1365-313x.2008.03614.x
Schmidt R, Mieulet D, Hubberten HM, Obata T, Hoefgen R, Fernie AR, Fisahn J, San Segundo B, Guiderdoni E, Schippers JH et al (2013) Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell 25:2115–2131. https://doi.org/10.1105/tpc.113.113068
Schwartz SH, Qin X, Zeevaart JA (2003) Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol 131:1591–1601. https://doi.org/10.1104/pp.102.017921
Shahzad Z, Amtmann A (2017) Food for thought: how nutrients regulate root system architecture. Curr Opin Plant Biol 39:80–87. https://doi.org/10.1016/j.pbi.2017.06.008
Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol 4:180–183. https://doi.org/10.1016/j.redox.2015.01.002
Sies H, Cadenas E (1985) Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond Ser B Biol Sci 311:617–631. https://doi.org/10.1098/rstb.1985.0168
Su SH, Gibbs NM, Jancewicz AL, Masson PH (2017) Molecular mechanisms of root Gravitropism. Curr Biol 27:R964–R972. https://doi.org/10.1016/j.cub.2017.07.015
Sun CH, Yu JQ, Hu DG (2017) Nitrate: a crucial signal during lateral roots development. Front Plant Sci 8:485. https://doi.org/10.3389/fpls.2017.00485
Tan BC, Schwartz SH, Zeevaart JA, McCarty DR (1997) Genetic control of abscisic acid biosynthesis in maize. Proc Natl Acad Sci U S A 94:12235–12240. https://doi.org/10.1073/pnas.94.22.12235
Titiz O, Tambasco-Studart M, Warzych E, Apel K, Amrhein N, Laloi C, Fitzpatrick TB (2006) PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J 48:933–946. https://doi.org/10.1111/j.1365-313x.2006.02928.x
Tognetti VB, Bielach A, Hrtyan M (2017) Redox regulation at the site of primary growth: auxin, cytokinin and ROS crosstalk. Plant Cell Environ 40:2586–2605. https://doi.org/10.1111/pce.13021
Tsukagoshi H, Busch W, Benfey PN (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143:606–616. https://doi.org/10.1016/j.cell.2010.10.020
Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M et al (2017) Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci 8:161. https://doi.org/10.3389/fpls.2017.00161
Waszczak C, Carmody M, Kangasjarvi J (2018) Reactive oxygen species in plant signaling. Annu Rev Plant Biol 69:209–236. https://doi.org/10.1146/annurev-arplant-042817-040322
Xie HT, Wan ZY, Li S, Zhang Y (2014) Spatiotemporal production of reactive oxygen species by NADPH oxidase is critical for Tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell 26:2007–2023. https://doi.org/10.1105/tpc.114.125427
Xiong L, Gong Z, Rock CD, Subramanian S, Guo Y, Xu W, Galbraith D, Zhu JK (2001) Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Dev Cell 1:771–781. https://doi.org/10.1016/s1534-5807(01)00087-9
Xiong L, Zhu JK (2003) Regulation of abscisic acid biosynthesis. Plant Physiol 133:29–36. https://doi.org/10.1104/pp.103.025395
Xu N, Chu Y, Chen H, Li X, Wu Q, Jin L, Wang G, Huang J (2018) Rice transcription factor OsMADS25 modulates root growth and confers salinity tolerance via the ABA-mediated regulatory pathway and ROS scavenging. PLoS Genet 14:e1007662. https://doi.org/10.1371/journal.pgen.1007662
Yang Y, Xu S, An L, Chen N (2007) NADPH oxidase-dependent hydrogen peroxide production, induced by salinity stress, may be involved in the regulation of total calcium in roots of wheat. J Plant Physiol 164:1429–1435. https://doi.org/10.1016/j.jplph.2006.08.009
Yang YZ, Ding S, Wang Y, Li CL, Shen Y, Meeley R, McCarty DR, Tan BC (2017) Small kernel2 encodes a Glutaminase in vitamin B6 biosynthesis essential for maize seed development. Plant Physiol 174:1127–1138. https://doi.org/10.1104/pp.16.01295
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092. https://doi.org/10.3389/fpls.2015.01092
You J, Zong W, Hu H, Li X, Xiao J, Xiong L (2014) A STRESS-RESPONSIVE NAC1-regulated protein phosphatase gene rice protein phosphatase18 modulates drought and oxidative stress tolerance through abscisic acid-independent reactive oxygen species scavenging in rice. Plant Physiol 166:2100–2114. https://doi.org/10.1104/pp.114.251116
Acknowledgments
We thank Prof. Tan Baocai from Shandong University for providing smk2 heterozygous seeds. We thank all colleagues involved in this work for their contributions to this article.
Funding
This work was supported by National Natural Science Foundation of China (U2106230), National Natural Science Foundation of China (32072500, 31801867, 31872925), the Program for Scientific Research Innovation Team of Young Scholar in Colleges and Universities of Shandong Province (2019KJE011), Shandong Provincial Key Research and Development Plan (2021TZXD007, 2019GNC106152, 2020CXGC010803), the Funds of Shandong ‘Double Top’ Program.
Author information
Authors and Affiliations
Contributions
Chongchong Lu: Formal analysis, Validation, Data curation, Data Curation, Writing - Original Draft, Visualization. Yuan Tian: Formal analysis, Investigation. Xuanxuan Hou: Formal analysis, Resources. Xin Hou: Investigation, Resources. Zichang Jia: Investigation. Min Li: Investigation, Resources. Mingxia Hao: Formal analysis, Investigation, Resources. Yanke Jiang: Investigation. Qingbin Wang: Writing - review & editing. Qiong Pu: Writing - review & editing. Ziyi Yin: Investigation, Resources, Writing - review & editing. Yang Li: Investigation, Resources, Writing - review & editing. Baoyou Liu: Resources. Writing - review & editing. Xiaojing Kang: Investigation, Writing - review & editing. Guangyi Zhang: Writing - review & editing. Xinhua Ding: Conceptualization, Funding acquisition, Resources, Writing - original draft, Writing - review & editing, Project administration. Yinggao Liu: Conceptualization, Funding acquisition, Resources, Writing - original draft, Writing - review & editing, Supervision. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human or animal subjects.
Consent for publication
All authors agree to publish.
Competing interests
Author QBW is employed by Shandong Pengbo Biotechnology Co., LTD, Tai’an, Shandong, China. The remaining authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Primers for this article. Fig. S1. MV inhibit maize root development. (A) The phenotype of maize root subjected to 0, 0.1, 1 and 10 μM MV. (B) Number of the lateral roots (LRs) of (A). LRs is from independent maize seedling (n ≥ 6). The different letters represent significant differences (P < 0.05, based on one-way ANOVA). (C) The length of the primary roots (PRs) of (A). PRs is from independent maize seedling (n ≥ 6). Fig. S2. ROS is detected using DAB staining in maize roots. Seven-day-old maize roots subjected to 0, 200 mM NaCl (N), 200 mM NaCl supplemented with 100 μM PN, PM, PL and PLP or 10 μM MV, 10 μM MV supplemented with 100 μM PN, PM, PL and PLP for 24 h. DAB staining is used for ROS staining. Fig. S3. Exogenous PN enhance maize roots resistance to salt stress. (A) The phenotype of maize roots subjected to 200 mM NaCl, 200 mM NaCl + 100 μM PN, 200 mM NaCl + 100 μM PM, 200 mM NaCl + 100 μM PL and 200 mM NaCl + 100 μM PLP for 4 d. (B) PRs length of (A). PRs is from independent maize seedling (n ≥ 6). (C) Number of lateral roots of (A). LRs is from independent maize seedling (n ≥ 6). The different letters represent significant differences (P < 0.05, based on one-way ANOVA). Fig. S4. The relative expression of ZEP, AAO3 and VP14. Maize roots are subjected to H2O (Control), 100 mM NaCl (N), 200 mM NaCl + 100 μM PN, 10 μM Methyl viologen (MV) and 10 μM MV + 100 μM PN for 24 h, qRT-PCR is used to detecting the target genes expression. Fig. S5. PCR genotyping of smk2 heterozygosity. Number 1–12 represent the different strains of maize. Number 1, 2, 3, 4, 6, 9, 10, 11 and 12 are identified as smk2 heterozygosity. Fig. S6. The relative expression of SMK2. Maize roots are subjected to H2O (Control), 100 mM NaCl (N), 200 mM NaCl and 10 μM MV (Methyl viologen). qRT-PCR is used to detecting the target genes expression. Fig. S7. The relative expression of ZEP, AAO3 and VP14. Maize roots are subjected to H2O (Control), 100 mM NaCl (N), 200 mM NaCl + 100 μM PLP, 10 μM Methyl viologen (MV) and 10 μM MV + 100 μM PLP for 24 h, qRT-PCR is used to detecting the target genes expression.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, C., Tian, Y., Hou, X. et al. Multiple forms of vitamin B6 regulate salt tolerance by balancing ROS and abscisic acid levels in maize root. Stress Biology 2, 39 (2022). https://doi.org/10.1007/s44154-022-00061-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44154-022-00061-2