Abstract
CuInGaSe2 crystals were synthesized using the traditional melt method. Various thicknesses of thin films were formed on glass substrates at room temperature using the thermal evaporation method. In the wavelength range of 250–2500 nm, the optical absorption spectra of thin films were examined. To calculate the optical band gap of the films, Taucs’ relation was applied. Calculations were made to determine how the film thickness affected the energy gap of CuInGaSe2 thin films. Estimation have been made to show fluctuations in the direct optical band gap values with variations in film thickness, but the indirect band gap energy varies systematically with thickness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Due to their application in ultraviolet (UV) optoelectronic applications, wide-bandgap (WBG) oxide semiconductors have recently attracted a lot of attention. These compounds have enabled previously unthinkable uses and prospects for a multitude of smart technologies and applications, including phototransistors, solar cells, touch-sensitive display technologies, optoelectronic devices, and UV emitters [1]. Cu(In,Ga)Se2 (CIGSe)-based solar cells have gained a lot of attention as a result of their commercialization [2]. The direct band gap of copper indium diselenide (CuInSe2 or CIS) at room temperature is about 1.02 eV, and it has a high absorption coefficient in the range of 104–105 cm−1, making it one of the most promising materials being researched for solar cell applications. Thin-film solar cells made of CuInSe2 have remarkable conversion efficiency [3,4,5,6]. Due to its high absorption coefficient, an absorber layer only has to be between 0.5 and 1.5 μm thick. Several vacuum and non-vacuum approaches, including single-stage and two-stage growth processes, can be used to create these chalcopyrite thin films. The thermal evaporation of chalcopyrite compounds is an important step towards forming CuInSe2 (CIS) and Cu(In,Ga)Se2 (CIGS) thin films on heated substrates [7]. In our work, we used the thermal evaporation technique to create CuInGaSe2 thin films in a variety of thicknesses. The produced films’ structure and nonlinear optical characteristics have been carefully taken into account.
2 Experimental
The modified Bridgman technique has been used to prepare CuInGaSe2 crystals (Cu 23%, In 19%, Ga 10%, 50% Se). In this procedure, the silica tube was charged with the stoichiometric total of the pure components Cu, In, Ga, and Se (6 N). CuInGaSe2 thin films with distinct thicknesses (50–300 nm) were developed on a glass substrate using the evaporation process in a vacuum of 10–4 Pa. At room temperature, all of the films were deposited. A Dektak 150 surface profiler was used to measure the thickness of the films. X-ray diffraction (XRD) was used to examine the structural characteristics of CuInGaSe films using a Shimadzu 6000 (XRD-6000) diffractometer and a CuK line (λ = 1.5405 Å) with 2Ɵ ranging from 50° to 90°. The voltage and current of the X-ray tube were 40 kV and 30 mA, respectively. The detector moved at a pace of 1° per minute. To further understand the impact thickness proportion, structural and optical analyses of the films were conducted. The optical absorption spectra of these thin films were examined in the 250–2500 nm wavelength range using a Jasco V-670 spectrophotometer to figure out their spectral dependence. Using these samples, an X-ray diffractogram was produced in order to identify the film’s structure and provide structural data. The range of the scanning angle (2θ) (CuK-Line) was 5–90. A computer controls the diffractometer, and the software programme gives us a qualitative analysis of the X-ray pattern.
3 Results and discussion
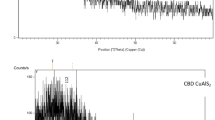
Figure 1 illustrates the XRD patterns of CuInGaSe2 films produced at various thicknesses. With 50-nm films, no apparent diffraction peaks can be seen, pointing to the film’s amorphous nature. At 100, 150, 200, 250, and 300 nm, the films typically exhibit a maximum diffraction peak at 29°. There are several peaks in the 2Ɵ range of 28–32°, at 37.5°, and around 60°. Due to the CuSe compound’s reaction with the nearby In and Ga atoms, the peak at 29° becomes stronger for the 200 nm thickness [8, 9].
According to the measured XRD patterns, the film’s grain expansion has improved at 200 nm. The CuInGaSe2 films had better crystal quality at 200 nm, where the diffraction peak intensity increased to its highest level. The waveguide method is taken into consideration for determining thin films’ absorption spectra in the spectral region of their transparency. At room temperature, the wavelength range of 200–900 nm is used to record the UV–vis absorption spectra of CuInGaSe2 films.
Figure 2 shows the relationship between CuInGaSe2 film absorbance and wavelength for various thicknesses (50, 100, 150, 200, 250, and 300 nm). At a thickness of 50 nm, a high absorption value was seen. In the UV range, it appears that the absorbance tends to decrease as the wavelength increases, a characteristic that is related to the absorbance of CuInGaSe2 films with high energy. The potential applications for these films in UV protection zones are highlighted by the greater optical absorption of CuInGaSe2 films in the UV region [10].
Experimentally, this easy equation can be used to obtain the absorption coefficient (α) [11]:
where thin film thickness (t) and absorbance (A) are specified.
The variation of the CuInGaSe2 thin films’ absorption coefficient for various thicknesses is shown in Fig. 3. Figure 3 depicts how the absorption coefficient varies with wavelength. According to published research, all films’ absorption coefficients dropped significantly around the fundamental absorption and then gradually at longer wavelengths [12].
According to the graph, for the thin films under investigation, the absorption coefficient (α) rises as photon energy rises.
The CuInGaSe2 thin films exhibit a low absorption coefficient at low energy. With a reduction in thickness, the absorption coefficient rises. This is related to a rise in layer density and grain size, and its high surface roughness can be explained by the light scattering effect [13]. By projecting the straight line of the graph to the zero-absorption coefficient that passed over the energy axis, the band gap was calculated using the optical absorbance data [14]. In order to calculate the band gaps, the formula has been used [15, 16]:
where the letters α, hυ, Eg, and A stand for the absorption coefficient, photon energy, band-gap energy, and proportionality constant, respectively. Known as the band-tailing parameter, constant A [17]. Figure 4 displays the plot of (hυ)2 vs hυ to determine the range of band gaps for the films. By extending the linear portion of the best fit of (αhυ)2 vs hυ = 0, the direct band gap was calculated. There is no systematic relationship in the Tauc plot between the direct band gap energy and thickness in the CuInGaSe2 films. In this study, the Eg values ranged as follows: 3.69, 3.56, 3.82, 3.63, 3.4, and 3.66 eV. Table 1 discusses the band gap change with film thickness. Figure 5 displays the optical indirect energy gap (Eg indirect) values that were derived by extrapolating the linear section of the plots to (αhυ)1/2 = 0 after evaluating the straight line of the (αhυ)1/2 plot against photon energy in the high absorption range. The Eg values in this investigation ranged from 2.5, 2.7, 2.85, 3.1, 3.1, and 3.5 eV, according to Table 1. With thickness, the indirect band gap energy systematically varies. Where the values of the energy gap rely generally on the crystal structure of the composites as well as the distribution and arrangement of atoms inside the crystal lattice.
The transmittance curve of the CuInGaSe2 films with respect to wavelength is shown in Fig. 6. Due to the logarithmic relationship between the absorbance and transmittance, as demonstrated by the following relationship, it should be noted that the transmittance is the opposite of the absorbance [18]:
where T is the transmittance.
4 Conclusions
CuInGaSe2 thin films with film thicknesses of 50, 100, 150, 200, 250, and 300 nm were deposited on glass substrates using thermal vacuum evaporation. Studies have been done on the optical characteristics. The measured band gap values for the direct and indirect transitions were calculated. The direct band gap energy does not change systematically with thickness, whereas the indirect band gap energy does. This finding shows that the 50 nm film is amorphous, but higher thicknesses yield crystalline films.
Data availability
The datasets generated during and/or analysed during the current study are available in the [Jazi Abdulwahed], [jaabdulwahed@uqu.edu.sa].
References
Zhang C, Liu G, Geng X, Wu K, Debliquy M (2020) Metal oxide semiconductors with highly concentrated oxygen vacancies for gas sensing materials: a review. Sens Actuators A Phys 309:112026. https://doi.org/10.1016/j.sna.2020.112026
Wang X-S, Fan Q-M, Tian Q-W, Zhou Z-J, Kou D-X, Zhou W-H, Meng Q-B, Zheng Z, Wu S-X (2019) CuInGaSe2 thin-film solar cells with 11.5% efficiency: an effective and low-cost way of Na-incorporation for grain-growth. Solar Energy 185:34–40. https://doi.org/10.1016/j.solener.2019.04.051. (ISSN 0038-092X)
Ramanathan K, Contreras MA, Perkins CL, Asher S, Hasoon FS, Keane J, Young D, Romero M, Metzger W, Noufi R, Ward J, Duda A (2003) Properties of 19.2% efficiency ZnO/CdS/CuInGaSe2 thin-film solar cells. Prog Photovoltaics Res Appl 11:225–230. https://doi.org/10.1002/pip.494
Contreras MA, Egaas B, Ramanathan K, Hiltner J, Swartzlander A, Hasoon F, Noufi R (1999) Progress toward 20% efficiency in Cu(In, Ga)Se2 polycrystalline thin-film solar cells. Prog Potovolt 7:311–316. https://doi.org/10.1002/(SICI)1099-159X(199907/08)7:4%3c311::AID-PIP274
Rau U, Schock H (1999) Electronic properties of Cu(In, Ga)Se2 heterojunction solar cells—recent achievements, current understanding, and future challenges. Appl Phys A 69:131–147. https://doi.org/10.1007/s003390050984
Tuttle JR, Contreras MA, Gillespie TJ, Ramanathan KR, Tennant AL (1995) Accelerated publication 171% efficient Cu(In, Ga)Se2-based thin-film solar cell. Prog Photovolt Res Appl 3:235–238. https://doi.org/10.1002/pip.4670030404
Chenene ML, Alberts V (2003) Structural and compositional properties of Cu(In, Ga)Se2 thin films prepared by the thermal evaporation of compound materials. J Phys D Appl Phys 36:56–61. https://doi.org/10.1088/0022-3727/36/1/308
Kim S, Kim WK, Kaczynski RM, Acher RD, Yoon S, Anderson TJ, Crisalle OD (2005) Reaction kinetics of CuInSe2 thin films grown from bilayer InSe/CuSe precursors. J Vac Sci Technol A 23:310. https://doi.org/10.1116/1.1861051
Hergert F, Hock R, Weber A, Purwins M, Palm J, Probst V (2005) In situ investigation of the formation of Cu(In, Ga)Se2 from selenised metallic precursors by X-ray diffraction—the impact of gallium, sodium and selenium excess. J Phys Chem Solids 66:1903. https://doi.org/10.1016/j.jpcs.2005.09.025
Abdullah OG, Tahir DA, Ahmad SS, Ahmad HT (2013) Optical properties of PVA: CdCl2 H2O polymer electrolytes. IOSR J Appl Phys 4:52–57. https://doi.org/10.9790/4861-0435257
Guneri E, Gode F, Ulutas C, Kirmizigul F, Altindemir G, Gumus C (2010) Properties of p-type SnS thin films prepared by chemical bath deposition. Chalcogenide Lett 7:685
Pankove JI (1971) Optical processes in semiconductors, vol 36. Prentice and Hall, New Jersey
Kushwaha N, Kushwaha VS, Shukla RK, Kumar A (2005) Effect of lead additive on photoconductive properties of se-te chalcogenide films. J Non-Cryst Solids 351(43–45):3414–3420. https://doi.org/10.1016/j.jnoncrysol.2005.09.011
Haque F, Rahman K, Akhtaruzzaman S, Abdullah H, Kiong TS, Amin N, Tiong SK (2018) Properties of sputtered ZnS thin films for photovoltaic application. Mater Res Express 5:096409
Islam MA, Hossain MS, Aliyu MM, Yusuf Sulaiman T, Razykov K, Sopian N (2014) Amin: structural, optical and electrical properties of in doped CdS thin films prepared from co-sputtering technique. J Ovonic Res 10(5):185–190
Gorji NE (2015) Oxygen incorporation into CdS/CdTe thin film solar cells. Opt Quantum Electron 47:2445–2453. https://doi.org/10.1007/s11082-015-0122-5
Khomchenko VA, Troyanchuk IO, Kurbakov AI. Laboratoire Leon Brillouin, CE Saclay, 91191 Gif-sur-Yvette (France)], Gamari-Seale H, Eremenko VV, Szymczak H, Szymczak R. Netherlands: p 2005. https://doi.org/10.1016/j.jmmm.2004.09.010
Fauzi NIM, Fen YW, Omar NAS, Saleviter S, Daniyal WMEMM, Hashim HS, Nasrullah M (2020) Nanostructured chitosan/maghemite composites thin film for potential optical detection of mercury ion by surface plasmon resonance investigation. Polymers (Basel) 12(7):1–13. https://doi.org/10.3390/polym12071497
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [KA]. The first draft of the manuscript was written by [JAMA] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdulwahed, J.A.M., Alshehri, K. The optical characteristics and co-evaporation processing of CuInGaSe2 thin film. J.Umm Al-Qura Univ. Appll. Sci. 10, 34–38 (2024). https://doi.org/10.1007/s43994-023-00073-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43994-023-00073-2