Abstract
The chemical looping single reactor (CLSR) system utilizing calcium ferrite as oxygen carriers represents an innovative approach to biogas reforming, which has the potential to enhance the efficiency and sustainability of biogas production. In this process, an oxy-combustion burner supplies the necessary endothermic heat while CO2 is simultaneously utilized, resulting in an autothermal process that generates high-purity syngas. Nearly 20% higher CO2 utilization can be achieved compared to the tri-reforming, owing to the higher driving force for CO2 oxidation in the counter-current moving bed and steam knock-out before inletting the flue gas into the reactor bottom. Additionally, the CLSR system is robust to variations in biogas to oxygen carrier ratios and CO2 concentration of the inlet feedstock, maintaining the outlet syngas purity within 1% variation. The effects of temperature, pressure, heat integration, and additional H2O/CO2 flow rate on the system performance are discussed, and the optimized scenarios are used for liquid fuel generation. The proposed process achieves about 13% reduction in syngas requirement compared to the conventional tri-reforming for the same amount of liquid fuel production. The feasibility of the CLSR system is further experimentally verified under various conditions. The results reveal the occurrence of CO2 counter-oxidation reactions on the surface of calcium ferrite oxygen carriers and demonstrate that higher temperatures are beneficial for the CH4 reforming reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The world’s energy consumption has dramatically risen, and currently, fossil fuel sources meet 80% of this energy demand [1]. However, the use of these non-renewable resources has contributed significantly to greenhouse gas emissions and ozone depletion that necessitates pollution control [2]. As a result, the energy industry has undergone a paradigm shift in recent years, moving towards renewable energy sources. Various alternatives to traditional fossil fuels, such as solar, wind, biomass, and geothermal sources, are being explored. Among these options, biogas is increasingly gaining attention as a highly attractive energy source due to its versatility in terms of feedstock sources and production methods, as well as its potential for financial and environmental benefits. According to the International Energy Agency's 2021 report, global biogas production will be 50% higher by 2040. It is stated in the report that biogas production is estimated to reach 260 million tons of oil equivalent (Mtoe) in 2040 [3].
Biogas typically consists of 65–75% methane, 35–25% carbon dioxide, and trace amounts of gases like hydrogen sulfide [1]. Due to the high concentration of methane, biogas has a calorific value of 21.5 MJ/m3, which makes it an excellent alternative for generating power and energy. Crude biogas can be utilized in two ways: direct and indirect utilization. Direct utilization involves biogas combustion such as cooking and lighting, whereas indirect utilization entails upgrading biogas to various products such as methanol, biofuels, and biopolymers. Due to the growing interest in sustainable carbon–neutral or negative chemical production, there has been a significant amount of research on biogas reforming technologies integrated with carbon capture. These technologies are being explored produce valuable chemical intermediates, such as syngas, which can be further converted into liquid fuels like naphtha, methanol, ammonia, and other important chemicals via the Fischer–Tropsch (F-T) process [4,5,6,7].
Among these technologies, dry reforming of biogas has received the most attention from researchers, as it can simultaneously utilize two carbon-based greenhouse gases: CH4 and CO2. The reaction scheme of dry biogas reforming is given by reaction (1).
Multiple catalysts have been explored for dry biogas reforming. Kalai et al. investigated La-promoted hydrotalcite-derived Ni catalysts at 600 °C [8]. The addition of La to the Ni-based hydrotalcite catalyst improved the CO2 and CH4 conversions and resisted deactivation. Moogi et al. recently tested perovskites (LaNiO3, LaCoO3), Ni/Al2O3, and Ni/La2O3 [9]. Specifically, the LaNiO3 catalyst synthesized using the citrate gel combustion method demonstrated superior reactants conversion CO2 (93.8%) and CH4 (97.2%) at 850 °C. Vo et al. studied the promoters addition to cobalt-based catalysts to inhibit carbon formation [10]. They reported that the addition of calcium as a promoter improved Co dispersibility and catalyst reducibility. The CH4 and CO2 conversions were reported as 84% and 89%. Despite the admirable efforts in catalyst research for dry reforming, the technology has not seen any commercial demonstration due to its high endothermic heat requirement (CH4 and CO2 have high bond-dissociation energies i.e., 435 and 526 kJ/mol, respectively) and side reactions leading to catalyst deactivation, complexity, and scale-up issues in catalyst synthesis [11, 12].
Another biogas reforming technology is steam or bi-reforming, which involves introducing steam as an additional oxidant in the presence of existing CO2 in biogas to produce syngas. The addition of steam has some notable advantages. First, it reduces the endothermicity of the reforming reaction and improves H2/CO ratio. Second, it helps to limit coking, thus leading to higher catalyst stability. The steam or bi-reforming reaction scheme can be described by reactions (1) and (2).
Tri-reforming is a biogas reforming technology that utilizes CO2, H2O and O2 as the oxidants to achieve autothermal reforming. It combines three processes: dry reforming, steam reforming, and partial oxidation reforming. The reaction scheme is provided by reactions (3–5).
Although tri-reforming has advantages such as high efficiency, autothermal operation, and economic benefit due to CO2 utilization, it is not widely used in industry. The main drawback of this process is the oxidation of catalysts leading to loss of reactivity. Additionally, there is a higher risk of secondary reactions due to the involvement of three oxidants. Furthermore, achieving autothermal operation while ensuring high syngas selectivity and purity remains a challenge due to the need for precise control over the three oxidants.
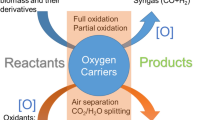
In this work, we report a novel Chemical Looping Single Reactor system (CLSR) for the conversion of biogas into liquid fuels. This technology is distinct from the catalytic reforming methods previously discussed. Herein, the biogas reacts with oxygen carriers which act as the source of oxygen to produce syngas [13,14,15,16,17,18,19,20,21]. By using the oxygen carrier such as metal oxide-based material to provide lattice oxygen for the partial oxidation of biogas, CLSR offers effective CO2 utilization, high thermal efficiency, production of value-added products with high selectivity and yield, flexibility in feedstock choice, and lower costs. Calcium ferrite Ca2Fe2O5 (C2F) is a stable oxygen carrier that exhibits exceptional properties in chemical looping reforming applications [22,23,24,25]. In addition, C2F is environmentally benign, making it an attractive option for sustainable processes [17, 26]. Hu et al. found that C2F has a brownmillerite-type structure and can produce a higher amount of hydrogen compared to other iron and calcium oxides, as well as pure iron oxide and calcium oxide [27]. The unique structure of C2F allows it to undergo complete regeneration in either CO2 or steam, eliminating the need for additional air oxidation steps that are typically required for other oxygen carriers [28,29,30,31]. Additionally, C2F exhibits fast oxygen ion transport in its bulk structure, resulting in improved solid–gas reaction kinetics [27]. The intermediate syngas produced from biogas reforming can be further converted into liquid fuels through Fischer–Tropsch (F-T) synthesis. This study provides a detailed analysis of the process and energy balance of the CLSR using C2F as the oxygen carrier for biogas conversion to liquid fuels as shown in Fig. 1. To ensure the CLSR system is carbon neutral, CO2/H2O is utilized to regenerate the C2F oxygen carrier. This novel system offers an unique advantage as it combines process intensification with carbon capture, while also efficiently converting renewable carbon-based biogas into syngas, which can then be used for F-T synthesis of liquid fuels.
2 Process description
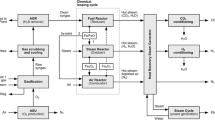
Figure 1 shows a schematic of the proposed CLSR system for syngas generation. Before the biogas is inlet to the CLSR system, it is treated for sulfur cleanup to remove all the sulfur species present in it [32]. The CLSR system uses a moving bed reactor, wherein the biogas and OCs travel enter from the top of the reactor and travel downwards co-currently to the middle of the reactor. Meanwhile, C2F OCs donate their lattice oxygen ([O]) to the biogas and reform it to produce syngas. The product syngas leaves from the middle of the reactor, whereas the solids flow downwards in the bottom half of the reactor. The product gases can be injected from the middle of the reactor by creation of dead space with the use of a dipleg or with the use of perforated tube or any state-of-the-art techniques for the gas extraction [33, 34]. Reactions occurring in the CLSR system are shown in the Eqs. (4,8, 9).
At the same time, some additional biogas is burned in an oxy-combustion burner to provide the heat required for the endothermic reactions occurring in the CLSR system [35, 36]. Biogas is burned with molecular oxygen supplied by an air separation unit (ASU). The outlet flue gas stream from this combustion process is then cooled to recover the usable heat and flashed to knock out water from the remaining flue gas stream. The flue gas stream, mainly CO2 at this point, is then reheated and injected from the bottom of the reactor to regenerate the reduced OCs from the top half zone of the CLSR system. Thus, the flue gas CO2 is sent counter-currently over reduced OCs to regenerate them back and utilize the generated CO2 in the process. It is to be noted that a conventional chemical looping system averts the use of an ASU, whereas an ASU is employed herein to generate a flue gas stream that is not diluted with the N2 present in the air. This allows for the direct utilization of the entire CO2 in the flue gas stream in the bottom half of the CLSR system.
Additional oxidant, either CO2 or H2O, is further sent from the bottom of the single reactor to regenerate the OCs completely, as well as adjust the product syngas yield and quality. The completely regenerated OCs from the bottom of the CLSR reactor are then sent back to the top using solid transport mechanisms such as a pneumatic conveying using a riser. The OCs travel to the reactor top along with the riser medium, whereafter the riser medium leaves from the top of the pot, whereas the OCs fall back to the top of the reactor. A detailed design of the riser and operation of the chemical looping system has been previously studied and reported in the literature [33, 34, 37].
Thus, some part of the biogas is directly used for the reforming reaction, whereas the remaining is used for oxy-combustion, and the CO2 generated from this process is used up in the bottom reactor zone. The system further utilizes additional CO2 or steam for complete regeneration of the OCs, thus leading to enhanced syngas production.
The syngas generated from the system is then used to produce liquid fuels using an F-T unit, as shown in Fig. 2. The F-T system considered in the current process uses an iron-based catalyst, wherein the syngas is first compressed to a pressure of 580 psia and a temperature of 192 °C [38]. The F-T process includes various steps for converting the syngas into liquid fuels, details of which can be found in the baseline report by NETL [38, 39].
3 Materials and methods
3.1 Aspen model setup and process calculations
The proposed CLSR process flowsheet is simulated using ASPEN Plus V11. A CLSR system consists of two zones: (a) the top half zone and (b) the bottom half zone. This system is simulated using a series of five RGIBBS reactor models, the details of which can be found in the supplementary information (SI) under section S1. A single RGIBBS reactor simulates the top part of the moving bed, wherein the OCs and the inlet biogas travel co-currently downward. A series of four RGIBBS reactors are used to simulate the counter-current flow of the regenerating gas with the reduced OCs occurring in the bottom half zone of the reactor. This way of simulating the co-current and counter-current moving bed has been reported by Fan et al, and has been used here accordingly [40, 41].
The RGIBBS reactor model works on the principle of minimization of Gibbs free energy and calculates the equilibrium composition of the components. The heater block is used to simulate both the heating and cooling of the flow streams. The inlet streams are heated to 400 °C before entering the system, and the outlet streams are cooled down to 120 °C to recover the useful heat. In addition to the heater block, a flash separator block is simulated, which is a single-stage separation process to separate vapor and liquid streams. The product syngas outlet from the reactor is cooled to 120 °C with a heater and then sent to the flash column operating at 35 °C for separating water from the syngas stream. The dry syngas stream is then compressed using a multistage compressor block consisting of four stages with intercoolers, and the discharge pressure is set at 580 Psia [39].
A design spec block is employed to manipulate the biogas flow rate to the oxy-combustion burner so that the heat from biogas combustion equals the endothermic heat required by the CLSR reactor zone. The corresponding oxygen flow rate was manipulated using a calculator block so that the oxygen flow rate is 1% excess of the stoichiometric requirement for complete combustion of the biogas.
The process simulation parameters are mentioned in SI under section S1 Table 1. Heat integration calculation and the heat exchanger network (HEN) design have been performed in ASPEN Energy Analyzer. Heat is extracted from the outlet streams, and the inlet streams are pre-heated using the extracted heat to increase the process efficiency. The outlet streams are cooled to 5 °C supersaturation temperature or 120 °C, whichever is lower, to recover the useful heat. For the HEN design, a minimum difference of 5 °C is considered for the design, and the cold utilities include cooling water, Low Pressure (LP), Medium Pressure (MP), and High Pressure (HP) steam generation.
The thermodynamics evaluation can be made with the help of the following parameters (Eqs. 8–11):
Solids Conversion (%) for this process specifically is calculated as follows [42]:
In the above equation, nx represents the molar flow rate of component x at the inlet and outlet of the reactor.
CO2 utilization % is defined as the ratio of the CO2 utilized in the system, i.e., the difference in the flow rate of the inlet and outlet CO2 of the system to the total CO2 inlet to the system, including the CO2 coming as a part of the inlet biogas.
The oxygen is supplied using an ASU, and its heat requirement of 0.41 MW/t/h is considered in the calculations [43]. Sections 4.1–4.3 further discuss the thermodynamic studies conducted in ASPEN Plus to optimize the system.
3.2 Fischer Tropsch black box setup
The black box model developed by NETL is used for simulating the F-T unit using an iron-based catalyst. The model also allows several input parameters to integrate the syngas generation unit. The inputs include syngas composition, gasifier island heat duty, electricity requirement, ASU oxygen requirement, and natural gas/hydrogen requirement [38].
All the output parameters, including the syngas composition, LP steam generation, ASU O2 requirement, and syngas compressor electricity requirement from the ASPEN flowsheet, are integrated into the model. The recycle tail gas (%) to the F-T reactor is set to a value such that the output electricity generated by the Natural Gas Combined Cycle (NGCC) gas turbine is almost zero. This ensures comparison only on the liquid fuels generation basis, and the variable of net export power is nullified. This is done manually by changing the recycle %, so the net export power is close to 0 for each case.
The F-T model is designed to achieve a liquid fuel production of ~ 50,000 bbl per day from the input syngas. The black box model outputs the syngas requirement, the utility requirement, and the fractions of different liquid fuels generated. The black box also has an integrated CO2 capture unit for capturing the CO2 and compressing it to 2214.7 psia for the CO2 pipeline. The black box model uses Rectisol Acid Gas Remover for capturing CO2. The outputs from the F-T process have been reported in Table 1. Further details on the black box setup for the current process can be found in the supplementary information section S2.
3.3 Sample preparation
The sample preparation was done as per the procedures Shah et al. used [22]. Supported C2F, with 60% by weight Ca2Fe2O5 and 40% by weight MgO, was prepared by mixing calcium oxide, iron oxide, and magnesium oxide (Noah Technologies, 99.9% pure, and 3–5 µm in size) in a 2:1 mol ratio for CaO-Fe2O3 and 3:2 wt ratio with MgO, using a solid-state synthesis technique [40, 44]. The MgO support was added to enhance and stabilize the performance of the C2F OC as previously optimized by Shah et al. Adding a support material, which is inert to both oxides, increases the resistance to sintering; thus, the OC maintains its mechanical strength over multiple redox cycles [45, 46]. The desired weight of the three oxide powders was measured and thoroughly mixed using a ball mill. This was done to activate them mechanically to achieve high surface energy and smaller grain size. Deionized water was then added to the mixture and converted into a paste. This paste was then dried at 150 °C in an oven for 60 min to obtain fine solids. These solids were further crushed and sintered at different temperatures for different times to obtain high purity Ca2Fe2O5 phase supported on MgO. The powdered mixture was sintered at 1150 °C for 10 h, following the procedure established by Shah et al. [22].
3.4 Thermogravimetric experiments
The reactivity and conversion of OC are tested for different biogas compositions using Setaram SETSYS Evolution Thermogravimetric Analyzer (TGA). The reduction–oxidation reactions were performed on these C2F OCs at 1000 °C, 900 °C, and 800 °C. The temperature of TGA was ramped at a rate of 55 °C/min from room temperature to the desired temperature. The total flow rate was maintained at 150 ml/min throughout the experiment. The helium flow rate was maintained at 50 ml/min for all experiments, which is used as a protective gas for the TGA balance. The protective gas ensures that reducing gases do not enter the sensitive weighing balance of TGA. A constant nitrogen flow was maintained at 100 ml/min when the temperature was ramped up. The reduction step was performed for 25 min with four different biogas compositions. The compositions of the biogas tested are summarized in supporting information under S1 Stream tables.
The oxidation step was performed in CO2 for 20 min to ensure the complete regeneration of OCs. A nitrogen flush of 10 min was performed between the two steps to ensure the complete removal of gases from the reactor. The formula for calculating the %reduction of OC particles is given by Eq. (13):
Wo, Wr, and Wi represent the weight of OC particles in the oxidized state, reduced state, and initial weight before the start of the experiment, respectively. 271.84 is the molecular weight of Calcium ferrite, and 48 is the weight of oxygen atoms.
4 Results
4.1 Optimal OC flowrate
First, finding a practically feasible OC flow rate while maintaining the optimal system performance for a given inlet feedstock flow rate is essential. Section For simulating the biogas, a 60:40 CH4:CO2 gas mixture is used, as previously done by Kong et al. [32]. Figure 3 shows the variation in the syngas purity, solids conversion in the reactor middle, and the carbon flow rate in the solids stream in the reactor middle for 1000° C and 1 bar for the variation in the [O]:C ratio. The [O]:C ratio is defined as the ratio amount of inlet lattice oxygen [O] that can be donated to by C2F material OC, and it is indicative of the OC flow rate for a given amount of inlet biogas flow rate. It should be noted that though a C2F molecule has 5 O atoms, only 3 can be donated, as the complete reduction of C2F leads to the formation of CaO and Fe. The CaO cannot be reduced to lower oxidation states in the current operating conditions.
Figure 3 shows that the syngas purity is initially ~ 100%, but the carbon deposition on the OCs is not zero for the [O]:C ratio lower than 0.21. As a result, operating in this regime would lead to carbon deposition on the OCs. This can be highly detrimental for the OCs, as it can lead to the loss of activity, poisoning of active sites, etc., as seen in several examples for other applications [47,48,49].
On further increasing the [O]:C ratio to 0.63, the syngas purity decreases and attains a constant value of around 92%. This is the region of interest as there is no carbon deposition, and a high syngas purity is achieved. In this region, the OCs form a mixture of Ca2Fe2O5 and Fe + CaO in the reactor middle, which is in equilibrium with the outlet syngas. Unlike Fe2O3, where multiple plateaus exist for syngas purity for the corresponding reduced species of Fe3O4, FeO, and Fe, Ca2Fe2O5 exhibits only a single plateau for syngas purity for the corresponding reduced species of Ca2Fe2O5/Fe/CaO. As a result, a substantial operational range of the [O]:C ratios are possible while maintaining a high syngas purity of 92% [50, 51].
By its inherent nature, biogas can have a significant composition variation depending on various upstream processing conditions [52,53,54,55]. Any biogas processing system needs to be designed so that these variations in the feedstock should have minimal effect on the quality of product syngas. Figure 4 shows the variation of the [O]:C ratio for three biogas compositions, containing 40%, 30%, and 20% CO2, while CH4 balances the remaining at 1000 °C and 1 bar. Three cases have been defined here as CH4%-CO2% hereafter. For example, an 80–20 case means the curve is obtained for biogas with an 80% CH4 and 20% CO2 composition.
Figure 4 shows that all three curves attain a plateau, with the syngas purity varying by 1%. Thus, for a 20% variation in the CO2 concentrations in the inlet biogas stream, the product quality changes only by 1% maximum. This is an example of the regulating nature of the OC, wherein the syngas at the outlet is in equilibrium with the solid phases present in the OCs in the middle of the reactor.
Figure 4 also shows the variation in the OC solid conversion as a function of the [O]:C ratio. As can be seen, the increase in the CH4 concentration in the biogas increases the solids conversion, i.e., the OC donates more lattice oxygen to maintain the syngas purity of ~ 92%. When there is an increase in the CO2 concentration of the inlet biogas, lower solids conversion is achieved as the OC donates a lower amount of lattice oxygen for the CH4 oxidation. In turn, the oxygen required for oxidizing CH4 is now extracted from CO2, which converts to CO. Thus, the C2F OC regulates the amount of lattice oxygen donated and the outlet syngas purity for the disturbances in the inlet biogas composition.
In terms of finding an optimal OC flow rate, it can be noted that the minimum [O]:C ratio required to operate at the plateau increases with an increase in the CH4 concentration [25]. This is because as the CH4 concentration increases, the amount of lattice oxygen required to be supplied by the OC also increases. Consequently, to ensure that the system operates in the plateau region for the highest variation in the biogas composition, a minimum [O]:C ratio of 0.63 is required as the overall minimum [O]:C ratio since this would ensure no carbon deposition even in the worst-case scenario, i.e., highest CH4 concentration of 80%.
Another advantage of operating on the plateau region is that the CLSR system is highly derisked as it is robust to minor disturbances in the [O]:C ratio. The [O]:C ratio indicates the ratio of the OC flow rate to the biogas flow rate. Therefore, the CLSR system can handle a considerable variation in the inlet biogas flow rate and maintain a high syngas purity at the outlet. For instance, the biogas supply decreases due to certain unknown reasons. The CLSR system can still be operated without disturbing the inlet OC flow rate as long as the new [O]:C operational ratio is on the plateau of the [O]:C curve in Fig. 4. The CLSR system can be operated robustly for CO2 in the range of 10–40%. In case of an increased syngas demand, a higher biogas flow rate can be sent to the reactor without perturbing the OC flow rate, and still, the same syngas purity can be achieved as long as the operating point is on the plateau region. Being able to operate at the OC flow rate is essential, as adjusting the OC flowrate according to the changes in the inlet feedstock may require manipulating a variety of factors typically associated with solids conveying, which can lead to unnecessary fluctuations in the system [56, 57]. Thus, by regulating the amount of lattice [O] donated by the OC, the CLSR system can achieve a vast turn-up and turn-down capability while maintaining the same OC flow rate.
An important aspect to note here is the effect of the support or inert material present in the OC. Typically for OC material, a vast range of support fractions in OC composition has been reported in the literature [45, 58, 59]. However, support materials are generally inert and do not participate in the reaction. As a result, they do not interfere with the heat of the reaction and hence are not considered herewith in the process simulations. Figure 5 shows the variation of the heat duty with the [O]:C ratio for a pure Ca2Fe2O5 and a supported sample used for experiments in the current study, containing 40% MgO as the inert support. The two curves completely overlap and thus prove the point mentioned above. Even though the changes in the support percent can cause changes in the reactor size and the reactivity of the OC, they do not affect the heat requirement calculated in this work. Secondly, the inert percent can change the temperature swings across the reactor in an adiabatic condition; however, it is assumed that the reactor is operating isothermally in the current system [41, 60]. Therefore, the effect of temperature swings is completely nullified as well.
As shown in Sect. 4.6, the OC can reduce to 11% to 22% for different biogas compositions. Also, as shown in Fig. 5, the changes in the OC reduction percentage do not affect the heat of the reaction as long as the system is operated in the plateau region. Therefore, an OC flow rate corresponding to the experimentally achievable solids-conversion is selected to make the system parameters obtained here practically feasible. Hence an OC flow rate of 1.44 kmol/h is selected, corresponding to a solids conversion of 15% for the 60–40 case, for further optimization. At this OC flow rate, the system is also stable in the plateau region for any biogas composition and minor perturbations to the OC flow rate.
Furthermore, this flow rate corresponds to a solids reduction of 15% for biogas containing 40% CO2, which is practically attainable. To be noted here is that the effect of heat loss has not been considered. It is assumed that the heat losses in a commercial system are negligible compared to the endothermic heat duties of the reaction and hence not considered.
4.2 Effect of temperature and pressure
Next, the variation in temperature and pressure is studied at the optimized OC flow rate in Sect. 4.2. Figure 6 shows the effect of variation in temperature and pressure on the outlet syngas purity. At a constant temperature, the syngas purity starts to decrease with increasing pressures because higher pressures favor the reverse reactions of biogas reforming, as Le Chatelier's principle predicted (Eqs. 8–9) [61]. The elevated pressure leads to unconverted CH4 in the outlet stream and decreased syngas purity, as also previously reported by Sandvik et al. [62].
Unlike natural gas, biogas typically occurs at atmospheric pressures, but the F-T process requires the feedstock syngas at elevated pressures [5]. Therefore, the biogas can be either compressed and then processed to generate syngas or the syngas can be generated at the atmospheric pressure in the CLSR system and then compressed to elevated pressure to generate liquid fuels. Since the syngas purity can decrease if the CLSR system is operated at elevated pressures, it is decided to operate the system at 1 bar pressure and then compress the syngas to elevated pressure [63].
The effect of temperature variation on syngas purity is not evident at 1 bar pressure, but it becomes more pronounced at higher pressures of 10 bar and 30 bar. The reforming reactions occurring in the CLSR system are endothermic, meaning higher temperatures would favor equilibrium conversion. Typically, the chemical looping applications operate at 800 °C to 1000 °C; therefore, a temperature of 1000 °C has been selected as the operating temperature [40, 44]. Section 4.6 further discusses the effect of temperature experimentally. The experiments also confirm the operation being preferred at 1000 °C compared to lower temperatures, as higher solids conversion can be achieved at a higher temperature.
Generally, operating at higher pressures can lead to higher gas density and a lower gas velocity perpetuating into a lower reactor volume. However, in a chemical looping system, in addition to the gas residence time, the reactor volume is typically decided by the solids inventory and residence time, which may not change significantly with an increase in pressure [37]. As a result, operating at elevated pressures may not lead to a decreased reactor volume and reduced capital costs, as would be typically the case in systems where gas residence time determines the reactor volume.
4.3 Bottom-half zone optimization
As mentioned in Sect. 2, the biogas reforming reaction that occurs in the top half zone of the CLSR system has an endothermic heat requirement of ~ 53 kWh/kmol of biogas (60–40 Case), and an oxy-combustion burner is used to supply the required heat by burning additional biogas and molecular oxygen.
The additional biogas requirement for accommodating the endothermic heat requirement is 0.55 kmol/(kmol inlet biogas) for the 60–40 case. It can be noted that additional biogas required to supply heat to the biogas reforming reaction is significantly high, i.e., an additional 55% of the biogas going in from the CLSR reactor top. However, the process utilizes the CO2 generated from the combustion within itself by converting it to CO in the bottom half zone of the CLSR system and thus increasing outlet syngas yield.
For the 60–40 case, the CO2 stream from the flue gas stream to the bottom of the CLSR reactor is not adequate to regenerate the OC completely. As a result, additional H2O or CO2 can be supplied to leverage the complete regeneration of the C2F OC and increase the syngas yield. This also offers an opportunity for additional CO2 utilization as the additional CO2 can be converted to CO and consequently into liquid fuels. Therefore, two different cases have been simulated: Case 1 with steam (H2O) and Case 2 with CO2 as the additional oxidant for the complete regeneration of OCs. Furthermore, Case 3 for tri reforming with the inputs of Case 2 is designed to compare the performance of the CLSR system with the conventional tri reforming, considering that Case 2 is the most desired operating case due to the highest achievable CO2 utilization.
Figure 7 shows the variation in product syngas flow rate and outlet OC flow rate as a function of additional inlet oxidant (H2O/CO2) for 1 kmol/h of inlet biogas from the CLSR reactor top and 0.55 kmol/h of biogas into the oxy-combustion burner (60–40 case). The syngas yield increases with an increase in the oxidant flow rate until it becomes constant with the complete regeneration of the OCs. As can be seen from Fig. 7, the OC is fully regenerated at the oxidation flow rate of 0.24 kmol/h in both cases.
Table 1 provides insight into the effect of H2O or CO2 as the additional oxidant on the H2:CO ratio. With no additional oxidant from the CLSR bottom, an H2:CO ratio of 0.72 is achieved. However, adding steam from the bottom increases the ratio to 0.86, whereas adding CO2 decreases the ratio to 0.64, while both increase the syngas yield by 7%. An H2:CO ratio can be manipulated between these two values by manipulating the proportion of the H2O and CO2 used as the oxidant.
Further increase in oxidant flow rate leads to a higher steam requirement when H2O is used as the additional oxidant or decreased syngas purity when CO2 is used as an additional oxidant. Therefore, the additional oxidant flow rate is set to the minimum required for complete regeneration, i.e., 0.24 kmol/h.
4.4 Heat integration
Though the biogas oxy-combustion provides heat for the endothermic heat of the CLSR reactor, further energy analysis is necessary to ensure the entire syngas generation island can be operated autothermally (with no external heat requirement) as well as to get an insight into the further improvement in process efficiency [64]. A heat integration analysis has been done in the Aspen Energy Analyzer, details of which can be found in Sect. 3.1. The HEN design is shown in Fig. 8 for Case 2. The HEN for Case 1 and Case 3 can be found in the supplementary information section S3. In all three cases, no external heat duty is required; additional LP steam utility is generated simultaneously. The non-recoverable heat is extracted from the system by cooling water from two flash columns used for water knock-out. The total non-recoverable heat in Case 2 is 47.8 MJ/h, whereas the LP steam generation is 97.91 MJ/h for Case 2 with 1 kmol/h of inlet biogas to the CLSR top. Thus, ~ 33% of the heat is non-recoverable, whereas the remaining 66% of the process heat can be recovered in the LP steam utility.
Table 1 shows the utilities generated for all 3 cases, normalized based on the lb-mol of syngas generated. Since heat is required in Case 1 for the additional steam oxidant, its corresponding effect can be seen in the utilities generated in Case 1 compared to Case 2, with higher LP steam output in Case 2. At the same time, the tri-reforming system in Case 3 generates a higher amount of utilities as compared to Case 1 and Case 2. This is because of the higher fraction of biogas-converted combustion products (H2O, CO2) in the tri-reforming, evident from the lower syngas purity in Case 3 owing to a higher fraction of CO2. The HEN shows no external input heat requirement in all three scenarios; thus, an autothermal condition within the syngas production system, including the oxy-combustion burner, is achieved.
4.5 Liquid fuels generation and comparison
A comparison with a conventional tri-reforming system is essential to realize the efficiency improvement for the current process. The three cases are designed as mentioned in Sect. 4.4: Case 1 and Case 2 are for the CLSR with H2O and CO2 as the additional feedstock from the bottom, respectively, whereas Case 3 is a simulation for tri reforming all the inlet gases for Case 2.
For simulating the reforming system, all inlet streams of Case 2, which include the feedstock biogas, the additional biogas coming for the oxy-combustion burner, the oxygen stream for the ASU, and the additional CO2 used for complete regeneration, are inlet to a single RGIBBS reactor to calculate the equilibrium product composition. Table 1 summarizes the performance of the three cases.
The syngas generated in cases 1, 2, and 3 are sent to an F-T process for liquid fuel production. For the same amount of liquid fuel production (~ 50,000 bbl/day), the syngas required in Case 2 is 5% higher than in Case 1. The syngas requirement in Case 3 is the highest, about 12% higher than in Case 1. This is because the CO2 present in the syngas from Case 3 is the highest, leading to a lower syngas purity and higher syngas requirement for the same amount of liquid fuel production.
This can be attributed to the higher driving force for CO2 utilization in a counter-current moving bed. As previously reported, the counter-current bed has a higher driving force than the co-current moving bed and hence can achieve higher H2O/CO2 conversions [65]. At the same time, the co-current moving bed is better suited for partial oxidation reactions as it can generate higher syngas yield than the counter-current moving bed. The proposed system capitalizes on both co-current and counter-current gas–solid movement to manipulate the conversion of the desired species. Furthermore, the C2F OC can completely regenerate in CO2/H2O, further enhancing the syngas yield compared to a single metal oxide like Fe2O3, which can only be regenerated until Fe3O4 in the presence of CO2/H2O [66].
The CLSR systems (Cases 1 and 2) have significantly higher CO2 utilization than the reforming process because the H2O is knocked out from the burning step, thus decreasing the competition for CO2 utilization in the CLSR bottom half zone. If more H2O is present in the CLSR bottom inlet gas, some H2O may react with the reduced OC, generating H2, thus competing with the CO2 oxidation reaction and decreasing CO2 utilization. Regarding the utilities generated from Cases 1, 2, and 3, the highest heat endothermic heat consumption can be seen in Case 1 due to the requirement for steam. However, Case 2 and Case 3 have a similar heat requirement; Case 3 has a higher exothermic heat than Case 2. Thus, Case 2 is the most desired as it offers the highest CO2 utilization while generating higher LP utility steam than Case 1.
In the current scenarios discussed here, it is considered that the biogas is a mixture of 60% CH4 and 40% CO2. However, this may be the worst-case scenario, as biogas typically have a higher CH4 content. In that case, a higher CO2 utilization can be achieved as the CH4 content of the inlet gas is higher.
Case 2 reports a lower H2:CO ratio than Case 3 because of no steam input. On the other hand, the H2:CO ratio in Case 2 is lower than that in Case 3 because of higher CO2 utilization, which generates CO, thus reducing the H2:CO ratio. For an F-T system with a Fe-based catalyst, the H2:CO ratio is more accommodating than the Co-based system, as the catalyst allows the water gas shift reaction to adjust the H2:CO ratio [39]. Therefore, though a lower H2:CO ratio is achieved in Case 2 compared to Case 3, the syngas requirement is lower in Case 2, while a higher CO2 utilization is also achieved.
4.6 Feasibility studies and future work
To evaluate the experimental feasibility of proposed CLSR process, TGA studies are conducted on a C2F OC synthesized using dry mixing techniques, as previously proposed by Shah et al. [22] (Sect. 3.3). This specific OC composition is used because it was previously established for natural gas reforming, with a > 99% methane conversion and ~ 98% syngas selectivity, with a near to thermodynamic limit performance. The focus of this work has been on developing the process and proving its feasibility rather than optimizing the material and proving its performance for the long term. Though the current work indicates the solid conversion achieved in the TGA, the moving bed chemical looping system has been previously shown to be operating close to the thermodynamic limit performance for CH4 reforming [50].
Figure 9 shows the solids conversion achieved in the TGA under different conditions. The C2F material can achieve a ~ 12% solids conversion in a 40% CO2 and 60% CH4 gas mixture and a solids conversion of 22% with an 80%-20% mixture of CH4-CO2 at 1000 °C in 25 min, which corresponds to the typical solids residence time in the chemical looping reactor systems [67]. This indicates that the OC flow rate for the corresponding solids conversion of 15% that was used in Sect. 4.1 can be practically achieved. Furthermore, it has been previously shown that the gases at the outlet of the looping systems are at equilibrium with the corresponding OC oxidation states [68]. Thus, at the practically achievable solids conversion of 15%, a high syngas purity of 92% can be achieved with the C2F material. It must be noted that though only solids conversion is reported here, though CO2 oxidation of the sample was conducted, and complete regeneration was seen as previously reported. Thus, the feasibility of the top half as well as the bottom half zone of the CLSR system is established [23].
It can also be noted that two competing reactions are occurring on the C2F material: CH4 reduction reaction and CO2 counter-oxidation. With an increase in the CH4 concentration, a deeper reduction becomes possible as the rate for the CO2 counter-oxidation is lower due to lower concentration. Therefore, further research on developing materials with higher selectivity towards CH4 reduction can be developed to achieve higher solids conversion. Material optimization studies in terms of dopants as well as supports can also be done to achieve a higher reduction with biogas and faster reaction kinetics [24].
Secondly, the effect of temperature on the reduction kinetics of the C2F material is evident in Fig. 9. The solids conversion significantly decreases with a decrease in the temperature, with a value of 1.33% at 900 °C and 0.08% at 800 °C for a 60–40 CH4-CO2 gas mixture. As a result, the CH4 reduction reaction is more activated at higher temperatures as compared to the CO2 counter-oxidation reaction. The rate of CH4 reduction increases faster with the temperature as compared to the CO2 oxidation, thereby leading to higher solids conversion at the higher temperature. This trend also justifies choosing 1000 °C and 1 bar have been used as optimal conditions for the CLSR unit. Operating at higher temperatures may lead to other challenges, such as loss of reactivity by sintering, agglomeration, and thermal stresses on the oxygen carriers, which may reduce their performance over a longer duration. However, further studies are necessary to understand the detailed effect of the temperature of the biogas reforming reaction on the C2F material performance.
The current work shows excellent promise for using biogas for liquid fuel production using this process and, with efforts for material development, can increase the chances of practical applications of this technology. However, the conditions are still very far from the maximum solids conversion that can be thermodynamically achieved. Further research on the material development can lead to better OC that can be reduced deeper and thus reduce the reactor size and particle inventory, leading to better process economics.
5 Conclusions
The proposed CLSR system for biogas reforming is integrated with F-T liquid fuel production for the current biogas-to-liquid fuels process. Especially with a fuel like biogas, where the feedstock variation can be a challenge, the CLSR system can handle a large variation in the feedstock composition while maintaining the syngas purity with less than 1% variation. Furthermore, the CLSR system can operate at a variable capacity while producing the same syngas quality for a large range of [O]:C ratio. Robustness to both of these variations can be attributed to the regulating property of the C2F OC, which manipulates the amount of donated [O] to offer robustness to the biogas CO2 content and extensive turn-down capability.
Though the system uses an oxy-combustion burner, with 55% additional biogas of the inlet biogas, to balance the endothermic heat of the top half of the CLSR system, the CO2 generated in the process is utilized in the bottom half of the reactor, along with a room for additional CO2 or H2O for syngas upgrading. Further heat integration shows the system can operate autothermally without external heat requirements and additional LP utility steam generation. Knocking out the steam from the burner flue gas helps to increase CO2 utilization by minimizing the competitive steam oxidation reaction on the reduced OCs.
Liquid fuel synthesis from the proposed CLSR system shows a reduction of ~ 13% in the syngas requirement for the same amount of liquid fuel production. Furthermore, the CO2 utilization for the CLSR system is about 20% higher than the conventional tri-reforming system. An advantage of the CLSR system over tri-reforming is the ability to remove the H2O so as to increase the CO2 uptake in the system. A variable H2:CO ratio of 0.64 to 0.86 can be achieved by varying the composition of the additional oxidant from the bottom of the CLSR system.
TGA experiments verify the feasibility of the process at various temperatures and CH4-CO2 composition conditions. The effect of the concentration of CO2 in the reacting gas mixture is evident and suggests to the presence of count-oxidation of OCs happening on the C2F material. Furthermore, the CH4 reduction increases significantly with temperatures, with higher temperatures favorable for better solids conversion. Further mechanistic studies are desired to understand the behavior of the biogas reaction on the OCs.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information.
Abbreviations
- ASU:
-
Air Separation Unit
- C2F:
-
Calcium Ferrite
- CLSR:
-
Chemical Looping Single Reactor
- F-T:
-
Fischer Tropsch
- HEN:
-
Heat Exchanger Network
- OC:
-
Oxygen Carrier
- TGA:
-
Thermogravimetric Analyzer
- NGCC:
-
Natural Gas Combined Cycle
- LP:
-
Low Pressure
- HP:
-
High Pressure
- MP:
-
Medium Pressure
References
Aghel B, Behaein S, Wongwises S, Shadloo MS. A review of recent progress in biogas upgrading: with emphasis on carbon capture. Biomass Bioenergy. 2022;160:106422. https://doi.org/10.1016/j.biombioe.2022.106422.
Kumar S, Mohapatra P, Joshi RK, Warburton M, Fan LS. Synergistic chemical looping process coupling natural gas conversion and NOx purification. Energy Fuels. 2023. https://doi.org/10.1021/acs.energyfuels.3c00254.
The outlook for biogas and biomethane to 2040 – Outlook for biogas and biomethane: Prospects for organic growth – Analysis. In: IEA. https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth/the-outlook-for-biogas-and-biomethane-to-2040. Accessed 27 Mar 2023
Evans G, Smith C. Biomass to liquids technology. Elsevier Enhanced Reader, p. 155–204
Zhao X, Joseph B, Kuhn J, Ozcan S. Biogas reforming to syngas: a review. iScience. 2020;23:101082. https://doi.org/10.1016/j.isci.2020.101082.
Gao Y, Jiang J, Meng Y, et al. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers Manage. 2018;171:133–55. https://doi.org/10.1016/j.enconman.2018.05.083.
Kale GR, Doke S, Anjikar A. Process thermoneutral point in dry autothermal reforming for CO2 utilization. J CO2 Util. 2017;18:318–25. https://doi.org/10.1016/j.jcou.2017.01.023.
Kalai DY, Stangeland K, Jin Y, et al. Biogas dry reforming for syngas production on La promoted hydrotalcite-derived Ni catalysts. Int J Hydrogen Energy. 2018;43:19438–50. https://doi.org/10.1016/j.ijhydene.2018.08.181.
Moogi S, Hyun Ko C, Hoon Rhee G, et al. Influence of catalyst synthesis methods on anti-coking strength of perovskites derived catalysts in biogas dry reforming for syngas production. Chem Eng J. 2022;437:135348. https://doi.org/10.1016/j.cej.2022.135348.
Vo C-M, Cao ANT, Saleh Qazaq A, et al. Toward syngas production from simulated biogas dry reforming: promotional effect of calcium on cobalt-based catalysts performance. Fuel. 2022;326:125106. https://doi.org/10.1016/j.fuel.2022.125106.
Hussien AGS, Polychronopoulou K. A review on the different aspects and challenges of the dry reforming of methane (DRM) reaction. Nanomaterials. 2022;12:3400. https://doi.org/10.3390/nano12193400.
Aramouni NAK, Touma JG, Tarboush BA, et al. Catalyst design for dry reforming of methane: analysis review. Renew Sustain Energy Rev. 2018;82:2570–85. https://doi.org/10.1016/j.rser.2017.09.076.
Joshi A, Mohapatra P, Joshi R, et al. Chapter 13—advances in chemical looping combustion technology. In: Brezinsky K, editor., et al., Combustion Chemistry and the Carbon Neutral Future. Amsterdam: Elsevier; 2023. p. 383–416.
Tang M, Xu L, Fan M. Progress in oxygen carrier development of methane-based chemical-looping reforming: a review. Appl Energy. 2015;151:143–56. https://doi.org/10.1016/j.apenergy.2015.04.017.
Mattisson T, Johansson M, Lyngfelt A. The use of NiO as an oxygen carrier in chemical-looping combustion. Fuel. 2006;85:736–47. https://doi.org/10.1016/j.fuel.2005.07.021.
Shen L, Zheng M, Xiao J, Xiao R. A mechanistic investigation of a calcium-based oxygen carrier for chemical looping combustion. Combust Flame. 2008;154:489–506. https://doi.org/10.1016/j.combustflame.2008.04.017.
Luo S, Zeng L, Fan L-S. Chemical looping technology: oxygen carrier characteristics. Annu Rev Chem Biomol Eng. 2015;6:53–75. https://doi.org/10.1146/annurev-chembioeng-060713-040334.
Gupta P, Li F, Velázquez-Vargas L, et al. Chemical looping particles. In: Chemical looping systems for fossil energy conversions. Hoboken: Wiley; 2010. p. 57–142.
Qin L, Cheng Z, Guo M, et al. Impact of 1% lanthanum dopant on carbonaceous fuel redox reactions with an iron-based oxygen carrier in chemical looping processes. ACS Energy Lett. 2017;2:70–4. https://doi.org/10.1021/acsenergylett.6b00511.
Liu Y, Qin L, Cheng Z, et al. Near 100% CO selectivity in nanoscaled iron-based oxygen carriers for chemical looping methane partial oxidation. Nat Commun. 2019;10:5503. https://doi.org/10.1038/s41467-019-13560-0.
Cheng Z, Baser DS, Nadgouda SG, et al. C2 selectivity enhancement in chemical looping oxidative coupling of methane over a Mg–Mn composite oxygen carrier by Li-doping-induced oxygen vacancies. ACS Energy Lett. 2018;3:1730–6. https://doi.org/10.1021/acsenergylett.8b00851.
Shah V, Cheng Z, Baser DS, et al. Highly selective production of syngas from chemical looping reforming of methane with CO2 utilization on MgO-supported calcium ferrite redox materials. Appl Energy. 2021;282:116111. https://doi.org/10.1016/j.apenergy.2020.116111.
Shah V, Mohapatra P, Fan L-S. Thermodynamic and process analyses of syngas production using chemical looping reforming assisted by flexible dicalcium ferrite-based oxygen carrier regeneration. Energy Fuels. 2020;34:6490–500. https://doi.org/10.1021/acs.energyfuels.0c00479.
Shah V, Cheng Z, Mohapatra P, Fan L-S. Enhanced methane conversion using Ni-doped calcium ferrite oxygen carriers in chemical looping partial oxidation systems with CO2 utilization. React Chem Eng. 2021;6:1928–39. https://doi.org/10.1039/D1RE00150G.
Shah V, Joshi R, Fan L-S. Thermodynamic investigation of process enhancement in chemical looping reforming of methane through modified Ca–Fe oxygen carrier utilization. Ind Eng Chem Res. 2020;59:15531–41. https://doi.org/10.1021/acs.iecr.0c03062.
Chan MSC, Liu W, Ismail M, et al. Improving hydrogen yields, and hydrogen: steam ratio in the chemical looping production of hydrogen using Ca2Fe2O5. Chem Eng J. 2016;296:406–11. https://doi.org/10.1016/j.cej.2016.03.132.
Hu Q, Shen Y, Chew JW, et al. Chemical looping gasification of biomass with Fe2O3/CaO as the oxygen carrier for hydrogen-enriched syngas production. Chem Eng J. 2020;379:122346. https://doi.org/10.1016/j.cej.2019.122346.
Fan L-S, Li F. Chemical looping technology and its fossil energy conversion applications. Ind Eng Chem Res. 2010;49:10200–11. https://doi.org/10.1021/ie1005542.
Bischi A, Langørgen Ø, Saanum I, et al. Design study of a 150kWth double loop circulating fluidized bed reactor system for chemical looping combustion with focus on industrial applicability and pressurization. Int J Greenhouse Gas Control. 2011;5:467–74. https://doi.org/10.1016/j.ijggc.2010.09.005.
Kong F, Li C, Zhang Y, et al. Hydrogen production from natural gas using an iron-based chemical looping technology: process modeling, heat integration, and exergy analysis. Energ Technol. 2020;8:1900377. https://doi.org/10.1002/ente.201900377.
Jerndal E, Mattisson T, Thijs I, et al. Investigation of NiO/NiAl2O4 oxygen carriers for chemical-looping combustion produced by spray-drying. Int J Greenhouse Gas Control. 2010;4:23–35. https://doi.org/10.1016/j.ijggc.2009.09.007.
Kong F, Swift J, Zhang Q, et al. Biogas to H2 conversion with CO2 capture using chemical looping technology: process simulation and comparison to conventional reforming processes. Fuel. 2020;279:118479. https://doi.org/10.1016/j.fuel.2020.118479.
Kim HR, Wang D, Zeng L, et al. Coal direct chemical looping combustion process: design and operation of a 25-kWth sub-pilot unit. Fuel. 2013;108:370–84. https://doi.org/10.1016/j.fuel.2012.12.038.
Zhang Y, Wang D, Pottimurthy Y, et al. Coal direct chemical looping process: 250 kW pilot-scale testing for power generation and carbon capture. Appl Energy. 2021;282:116065. https://doi.org/10.1016/j.apenergy.2020.116065.
Zhang Y, Vangaever S, Theis G, et al. Feasibility of biogas and oxy-fuel combustion in steam cracking furnaces: experimental and computational study. Fuel. 2021;304:121393. https://doi.org/10.1016/j.fuel.2021.121393.
Mohammadpour M, Ashjaee M, Houshfar E. Thermal performance and heat transfer characteristics analyses of oxy-biogas combustion in a swirl stabilized boiler under various oxidizing environments. Energy. 2022;261:125206. https://doi.org/10.1016/j.energy.2022.125206.
Hsieh T-L, Xu D, Zhang Y, et al. 250 kWth high pressure pilot demonstration of the syngas chemical looping system for high purity H2 production with CO2 capture. Appl Energy. 2018;230:1660–72. https://doi.org/10.1016/j.apenergy.2018.09.104.
Skone TJ, Grol E (2015) NETL Fischer-Tropsch Black Box Model
10.2. Fischer-Tropsch Synthesis. In: netl.doe.gov. https://netl.doe.gov/research/carbon-management/energy-systems/gasification/gasifipedia/ftsynthesis. Accessed 30 Mar 2023
Fan L-S. Chemical looping systems for fossil energy conversions. Hoboken: Wiley; 2010.
Joshi R, Pottimurthy Y, Shah V, et al. Coal-direct chemical looping process with in situ sulfur capture for energy generation using Ca–Cu oxygen carriers. Ind Eng Chem Res. 2021;60:11231–40. https://doi.org/10.1021/acs.iecr.1c01814.
Baser DS, Nadgouda SG, Joshi AS, Fan L-S. 110th anniversary: indirect partial oxidation of methane using a counter-current moving-bed chemical looping configuration for enhanced syngas production. Ind Eng Chem Res. 2019;58:16407–16. https://doi.org/10.1021/acs.iecr.9b02520.
Okeke IJ, Adams TA. Combining petroleum coke and natural gas for efficient liquid fuels production. Energy. 2018;163:426–42. https://doi.org/10.1016/j.energy.2018.08.058.
Fan L-S. Chemical looping partial oxidation: gasification, reforming, and chemical syntheses. 1st ed. Cambridge: Cambridge University Press; 2017.
Joshi A, Shah V, Mohapatra P, et al. Chemical looping—a perspective on the next-gen technology for efficient fossil fuel utilization. Adv Appl Energy. 2021;3:100044. https://doi.org/10.1016/j.adapen.2021.100044.
Zheng H, Jiang X, Gao Y, et al. Chemical looping reforming: process fundamentals and oxygen carriers. Discov Chem Eng. 2022;2:5. https://doi.org/10.1007/s43938-022-00012-3.
Ryu H-J, Lim N-Y, Bae D-H, Jin G-T. Carbon deposition characteristics and regenerative ability of oxygen carrier particles for chemical-looping combustion. Korean J Chem Eng. 2003;20:157–62. https://doi.org/10.1007/BF02697202.
Cho P, Mattisson T, Lyngfelt A. Carbon formation on nickel and iron oxide-containing oxygen carriers for chemical-looping combustion. Ind Eng Chem Res. 2005;44:668–76. https://doi.org/10.1021/ie049420d.
Zhu M, Chen S, Ma S, Xiang W. Carbon formation on iron-based oxygen carriers during CH4 reduction period in chemical looping hydrogen generation process. Chem Eng J. 2017;325:322–31. https://doi.org/10.1016/j.cej.2017.05.027.
Luo S, Zeng L, Xu D, et al. Shale gas-to-syngas chemical looping process for stable shale gas conversion to high purity syngas with a H2: CO ratio of 2: 1. Energy Environ Sci. 2014;7:4104–17. https://doi.org/10.1039/C4EE02892A.
Joshi RK, Shah V, Fan L-S. Acetic acid production using calcium ferrite-assisted chemical looping gasification of petroleum coke with in situ sulfur capture. Energy Fuels. 2020;34:16560–71. https://doi.org/10.1021/acs.energyfuels.0c03408.
Eklund B, Anderson EP, Walker BL, Burrows DB. Characterization of landfill gas composition at the fresh kills municipal solid-waste landfill. Environ Sci Technol. 1998;32:2233–7. https://doi.org/10.1021/es980004s.
Rasi S, Veijanen A, Rintala J. Trace compounds of biogas from different biogas production plants. Energy. 2007;32:1375–80. https://doi.org/10.1016/j.energy.2006.10.018.
Jaffrin A, Bentounes N, Joan AM, Makhlouf S. Landfill biogas for heating greenhouses and providing carbon dioxide supplement for plant growth. Biosys Eng. 2003;86:113–23. https://doi.org/10.1016/S1537-5110(03)00110-7.
Jung S, Lee J, Moon DH, et al. Upgrading biogas into syngas through dry reforming. Renew Sustain Energy Rev. 2021;143:110949. https://doi.org/10.1016/j.rser.2021.110949.
He P, Fan L-S. A general methodology and the correlation for the prediction of the solids flow rates through the L-valves. Powder Technol. 2020;360:278–88. https://doi.org/10.1016/j.powtec.2019.09.090.
Wang D, Joshi A, Fan L-S. Chemical looping technology—a manifestation of a novel fluidization and fluid-particle system for CO2 capture and clean energy conversions. Powder Technol. 2022;409:117814. https://doi.org/10.1016/j.powtec.2022.117814.
Chen X, Wang L, Lin Y, et al. Migration of lattice oxygen during chemical looping dry reforming of methane with Ca2Fe2O5/Zr0.5Ce0.5O2 oxygen carrier. Fuel Process Technol. 2023;244:107706. https://doi.org/10.1016/j.fuproc.2023.107706.
Ismail M, Liu W, Chan MSC, et al. Synthesis, application, and carbonation behavior of Ca2Fe2O5 for chemical looping H2 production. Energy Fuels. 2016;30:6220–32. https://doi.org/10.1021/acs.energyfuels.6b00631.
Brody L, Neal L, Liu J, Li F. Autothermal chemical looping oxidative dehydrogenation of ethane: redox catalyst performance, longevity, and process analysis. Energy Fuels. 2022;36:9736–44. https://doi.org/10.1021/acs.energyfuels.2c01293.
Knox K. Le Châtelier’s Principle. J Chem Educ. 1985;62:863. https://doi.org/10.1021/ed062p863.
Sandvik P, Kathe M, Wang W, et al. High-pressure chemical looping reforming processes: system analysis for syngas generation from natural gas and reducing tail gases. Energy Fuels. 2018;32:10408–20. https://doi.org/10.1021/acs.energyfuels.8b01834.
Sandvik P, Wang W, Kathe M, et al. Operating strategy of chemical looping systems with varied reducer and combustor pressures. Ind Eng Chem Res. 2019;58:5228–35. https://doi.org/10.1021/acs.iecr.8b06248.
Kong F, Tong A, Kathe MV, et al. Process intensification by applying chemical looping in natural gas to dimethyl ether conversion process—implications for process design education. Chem Eng Process Process Intensif. 2019;143:107566. https://doi.org/10.1016/j.cep.2019.107566.
Kathe M, Fryer C, Sandvik P, et al. Modularization strategy for syngas generation in chemical looping methane reforming systems with CO2 as feedstock. AIChE J. 2017;63:3343–60. https://doi.org/10.1002/aic.15692.
Li F, Zeng L, Fan L-S. Biomass direct chemical looping process: process simulation. Fuel. 2010;89:3773–84. https://doi.org/10.1016/j.fuel.2010.07.018.
Park C, Hsieh T-L, Pottimurthy Y, et al. Design and operations of a 15 kW th subpilot unit for the methane-to-syngas chemical looping process with CO2 utilization. Ind Eng Chem Res. 2020;59:6886–99. https://doi.org/10.1021/acs.iecr.9b05577.
Hsieh T-L, Zhang Y, Xu D, et al. Chemical looping gasification for producing high purity, H2-rich syngas in a cocurrent moving bed reducer with coal and methane cofeeds. Ind Eng Chem Res. 2018;57:2461–75. https://doi.org/10.1021/acs.iecr.7b04204.
Author information
Authors and Affiliations
Contributions
RKJ, PM, IKK and SGS and ZC wrote the main manuscript text and JP prepared figures. LI and LK performed simulations. All authors contributed to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joshi, R.K., Mohapatra, P., Kudva, I.K. et al. Biogas conversion to liquid fuels via chemical looping single reactor system with CO2 utilization. Discov Chem Eng 3, 13 (2023). https://doi.org/10.1007/s43938-023-00029-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43938-023-00029-2