Abstract
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) has revolutionized scientific research over the past few decades and has provided a unique platform in ongoing technological developments. Undoubtedly, there has been a bloom chiefly in the field of biological sciences with this emerging technology, and has enabled researchers to generate critical data in the field of disease diagnoses, drug development, dereplication. It has received well acceptance in the field of microbial identification even at strain level, as well as diversified field like biomolecule profiling (proteomics and lipidomics) has evolved tremendously. Additionally, this approach has received a lot more attention over conventional technologies due to its high throughput, speed, and cost effectiveness. This review aims to provide a detailed insight regarding the application of MALDI-TOF MS in the context of medicine, biomolecule profiling, dereplication, and microbial ecology. In general, the expansion in the application of this technology and new advancements it has made in the field of science and technology has been highlighted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The progression of modern mass spectrometry has amplified the perception of entire biological systems by examining varied sets of biomolecules such as carbohydrates, lipids, proteins, and amino acids thereby facilitating the characterization and quantification of thousands of proteins, biologically active metabolites and unique microbial signatures for identifying microbes at genus and species level (Pomastowski et al. 2019; Wang et al. 2022). The advancement of high resolution mass analyzers like ion cyclotron resonance analyzer (Campuzano et al. 2020), orbitrap analyzer (Wörner et al. 2020) and tandem mass spectrometry techniques (Smaoui et al. 2020) has provided structural information from mass values, in transforming the era of modern mass spectrometry. The advancement of new instruments, and new data analysis, processing and visualization tools has enhanced research based on mass spectrometric analysis (Jang and Kim 2018). This high-tech capability of MALDI-TOF favors its potential applications in multiple areas including medical diagnostics, dereplication, biomolecule profiling and microbial ecology
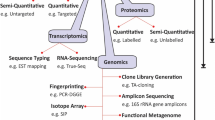
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) has significantly improved the potential to identify and differentiate microorganisms (Weis et al. 2020). Mass spectrometry is promptly replacing phenotypic and biochemical analytical approaches for microbial identification. With the evolution of the soft ionization techniques, the unique spectral fingerprints can promptly identify bacteria, fungi and yeast at the genus and species level with much ease and accuracy (Alizadeh et al. 2021; Tsuchida et al. 2020). This ability has been extensively explored by the microbiologists and has become a promising tool in clinical microbiology. Compared to traditional PCR and other immunoassay-based approaches, Yan et al. recent work verified the resilience of this technology to identify new Corona virus efficiently (Yan et al. 2021). Apart, from this it has also proven a reliable procedure for identifying microorganisms from many ecosystems (Ashfaq et al. 2022; Santos et al. 2016; Freitas et al. 2022). The high throughput profiling using this approach of the diverse microbial biomolecules can unravel their key function in ecosystems (Weigt et al. 2018). The ribosomal protein spectra are microbe specific, as in case of rep-PCR, and hence these could be a potential tool for dereplication (Barthélemy et al. 2020). Additionally, this technique has also its application in the accurate identification of organisms isolated from varied environments such as biofilm habitats (Gaudreau et al. 2018), cellular phones (Noumi et al. 2020), spacecraft surfaces (Seuylemezian et al. 2018) and in nosocomial settings (De Florio et al. 2018). Furthermore, it has revolutionized the field of disease diagnostics by identifying the varied mammalian cell types, circulating immune cells without using cell specific antibodies thus making diagnostic procedures more effective and affordable (Munteanu and Hopf 2013). MALDI-TOF MS has also shown the potential results for profiling biomolecules and has led to the identification of numerous biomarkers for cancer (Sivoňová et al. 2021) and other diseases (Zawada et al. 2021). It also analyzes the fingerprints of biological fluids, cells and, tissues under normal or altered conditions. Biomolecule profiling reveals the state of health and has a role in identifying different microbial species such as mycobacterial, bacterial, yeasts and viruses (Croxatto et al. 2012; Gonzalo et al. 2020). Here in this review, we tried to summarize the recent advances that this technique has brought in numerous scientific fields. New heights have been achieved in the health sector, ecology, disease detection and, diagnostic fields (Fig. 2). The expansion in the application of this emerging technique has simplified the hectic procedures of identification of new microbial strains and issues related to the disease diagnosis, have been resolved with much ease and cost effectiveness. The robustness and versatility of this technique makes it worth to discuss.
Principle of MALDI-TOF MS
MALDI and ESI are common ionization methods employed in modern mass spectrometry. In ESI, ions are produced using electron spray, where a high voltage is exerted to a liquid to generate an aerosol. While as in MALDI, samples are first co-crystallized with a matrix. The matrix is preferentially composed of small acidic molecules with strong optical absorption. The ions are created by subjecting the matrix embedded sample to the laser beam, then desorption and then ionization. The matrix has a role in absorbing the laser light energy and induces the vaporization of the analyte to produce charged ions (singly charged). The singly charged ions are accelerated at a fixed potential and are set apart from one another based on mass to charge ratio (m/z) in the mass analyzer. The charged analytes are detected and measured by a detector (Karas and Krüger 2003; Duncan et al. 2016)
The Matrix is usually a crystallized compound, the most common being cyano-4-hydroxycinnamic acid (CHCA), sinapinic acid, and 2,5-dihydroxybenzoic acid (DHB) (Hou et al. 2019; Choi et al. 2019). DHB is mostly used for the analysis of glycans and glycoproteins as well as often used for the analysis of peptides. CHCA is used mostly for peptides having a mass range (< 2500 Da), and SA is good for the analysis of high mass proteins and peptides (> 2500 Da). One of these crystallized molecules is used to make a solution in a mixture of an organic solvent such as ethanol or acetonitrile. Highly purified water allow both hydrophilic and hydrophobic molecules to dissolve in a solution. Trifluoroacetic acid (TFA) is usually added as a counter ion source to generate [M+H] ions. Mostly matrix compounds are of low molecular weight and vaporize easily but are large enough so that they may not evaporate during experimentation. They are mostly acids and thus act as a proton source for ionization of an analyte. They absorb laser light either in the UV or the IR range. The matrix solution is mixed with the analyte and spotted onto the MALDI plate. The solvents in the solution are vaporized, leaving only matrix and analyte molecules.
The laser is targeted at matrix crystals and, absorption of laser energy takes place first, desorption and then ionization occurs during this event. The hot plume generated during this process contains many ionized and neutral matrix molecules, deprotonated and protonated matrix molecules, nanodroplets etc. The matrix charges the analyte by transferring the proton to the analyte. An ion obtained after this process comprises of starting neutral molecule [M] with ions removed in case of removed proton [M−H]− or added in case of added proton [M+H]+ and is termed asa quasimolecular ion. MALDI is capable of generating singly or multiply charged ions ([M+nH]n+) based on the laser intensity, nature of the ion, and the voltage used (Knochenmuss 2006). Ion yield is measured to range from 10−4 to 10−7 (Lu et al. 2015). The charged analytes are then detected and measured by the time of flight (TOF) analyzer (Fig. 1).
Diagnosis
Pathogens identification
Diagnosis of pathogens is important for initiating the administration of antibiotics and improving treatment outcomes. Conventional techniques for the diagnosis of microorganisms use gene sequencing or phenotypic identification methods, which are time-consuming. Contrastingly, MALDI-TOF MS is a rapid, low-cost, and reproducible technique that has been employed to identify pathogens. Based on specific protein and peptide profiles from cells, MALDI-TOF MS offers differential identification of bacteria, Mycobacteria, filamentous fungi, yeast, and protozoan parasites.
In clinical diagnosis, identification of microorganisms is mainly based on microscopy, biochemical tests, interpretation of phenotypic characteristics, or molecular biology. These procedures are time-consuming and require expertise. MALDI-TOF technology permits the identification of large variety of microbial species in reduced time with less microbial biomass. Bacterial isolate colonies, picked from agar medium are smeared in a thin film on MALDI target plate, overlaid with DHB (for Vitek MS instrument) or CHCA (for Bruker instrument) and put into a mass spectrometer for data analysis and identification is carried out by comparing it with the reference database. Bacterial species with fewer differences in their ribosomal protein sequences, such as strains of Propionibacterium acnes, Stenotrophomonas maltophilia or streptococcus pneumonia, Shigella spp., and E. coli can be misidentified by this technique. The accuracy of identification depends on a number of database entries therefore, improvement in database entries of characterized species will permit approximately 100% identification rate for Mycobacteria, Neisseria, Salmonella, Clostridia, Campylobacter, and Helicobacter pylori (Wieser et al. 2012). A study by Veen et al. (2010) showed the identification of 327 clinical isolates by using this technique [Van Veen et al (2010)]. This study revealed the identification at genus level to about 95.1% and at the species level to about 85.6%. In another study by the same group of researchers, where 980 clinical isolates of yeast and bacteria displayed the correct identification to about 92.2% using MALDI-TOF MS. By using this technique, correct species identification was seen in Enterobacteriaceae, gram-negative bacteria, Staphylococci, Streptococci and HACCEK group of bacteria (Haemophilus, Actinobacillus, Cardiobacterium, Capnocytophaga, Eikenella and Kingella) to about 97.7%, 92%, 94.3%, 84.8%, and 84%, respectively (Singhal et al. 2015).
Yeast colonies are picked and treated with 70% ethanol, the suspension is pelleted and then dried. After drying, 70% of formic acid and acetonitrile are used to make a suspension. Centrifugation isdone, and one microlitre of supernatant is laid on the MALDI target plate, covered with a matrix, and then introduced into the mass spectrometer for analysis. Vitek and Bruker MS contain reference spectra of yeasts like Cryptococcus neoformans strains and many Candida spp. Identification of yeast isolates using MALDI-TOF MS showed 92.5% accuracy in clinical isolates of Trichosporon, Cryptococcus, Candida, Geotrichum, Saccharomyces, Blastoschizomyces spp., and Pichia (Hagen et al. 2015). Another study, 12 different species were identified from 61 yeast isolates with great accuracy (Singhal et al. 2015). Similarly, in another study, identification of 24 yeast isolates comprising of 12 different species was reported using this technique (Bizzini et al. 2010). Bruker and Shimadzu systems of MALDI-TOF MS and their respective related databases and software, Saramis and, were tested to identify yeast species in clinical diagnosis (Bader et al. 2011). Species identification was similar for Bruker/Biotyper (97.6%) and for Shimadzu/Saramis (96.1%) were comparable with biochemical tests (96.9%). No misidentifications were reported by Saramis, and fewer misclassifications were seen with Biotyper as compared to classical approaches. Stevenson et al. (2010) by using Bruker MALDI-TOF system has established a library of the spectral databases for 109 yeast reference strains representing eight genera and 44 species to assess the ability of MALDI-TOF MS to identify yeast species rapidly.
The Identification of Mycobacterium tuberculosis is generally carried out through antigen detection and PCR. Identification of M. tuberculosis by MALDI-TOF MS has been observed to decrease turn-around time, cost and labor compared to nucleic-acid-based methods. Before identification, Mycobacterium species require sample processing so to deactivate the pathogen. Inactivation is followed by sonication, which causes cell disruption. A study reported the correct identification approx 88.8% at the Genus level and 82.2% at the species level (Machen et al. 2013). However, for closely related strains, misidentifications can occur, such as for M. tuberculosis, M. africanum, M. bovis and M. microtiand for M. abscessus, M. bolletiiand M. massiliense, for which reference mass spectra databases are not fully available (Lotz et al. 2010; Kodana et al. 2016).
For fungal colonies, 75% ethanol solution is used, centrifuged, and suspended in acetonitrile and formic acid. Supernatant is centrifuged and laid on MALDI target plate (Schulthess et al. 2014). Various clinical fungi are distinguished by using MALDI-TOF MS, which mainly include Fusarium, Penicillium, Aspergillus, and dermatophytes (Singhal et al. 2015). First study where fungal identification was made by using MALDI-TOF MS was carried out by Welham et al. (2000); Giebel et al. (2010). Three fungal species, Scytalidiumdimidiatum, Trychophyton rubrum, and Penicillium spp. displayed distinct spectral fingerprints that permit the accurate distinction between species (L’Ollivier and Ranque 2017). Using this approach, fungal spores were used to identify various Penicillium species (Reeve et al. 2019). In another study, 12 species of Penicillium were correctly identified by MALDI-TOF in which fungal samples were resuspended in trifluoroacetic acid/acetonitrile solvent prior to this analysis (Hettick et al. 2008b). By using the same extraction method Hettick et al. (2008a, b) got mass spectral fingerprints of 12 Aspergillus species, and five strains of Apergillus flavus furthermore, the results communicated that 12 species of Aspergillus were 95% accurately identified at the strain level. However, Hettick et al. (2008a, b) found that this approach could not discriminate between Aspergillus niger and A. chevalieri (Hettick et al. 2008a, b). Another study showed that different species of Aspergillus sp., could be identified by their spores (Rodrigues et al. 2011). The characterization of various Fusarium spp. has also been observed by MALDI-TOF MS in many studies. A study where nine Fusarium spp. having 62 isolates were subjected to both MALDI-TOF MS and molecular identification. Using the Biotyper database, 13 isolates of five Fusarium spp. were correctly identified to about 92%, four Fusarium strains were not identified and one Fusarium sp. namely F. pseudonygamai was misidentified and this was due to the absence of reference spectra in database (Marinach-Patrice et al. 2009). By analyzing spores of five Fusarium spp. (mycotoxin-producing), MALDI-TOF MS was successful in correct identification with high reproducibility (Passarini et al. 2013). Dermatophytes such as Arthrodermabenhamiae, Trychophyton tonsurans, T. interdigitale and T. rubrum were identified by using Saramis database (Singhal et al. 2015).
A study revealed that MALDI-TOF MS was used to detect human herpesviruses from various biological specimens (Singhal et al. 2015). It has been investigated that MALDI-TOF MS combined with antibody magnetic nanoparticles was suitable for the diagnosis of influenza viruses (Dinali et al. 2017). This technique is also applied to screen virus subtypes, suggesting a better technique f or the early diagnosis of influenza viruses. This assay detects the intrinsic physical properties of molecules. MALDI-TOF MS, in association with multiplex PCR has been used for analyzing eight human enteric viruses such as poliovirus, norovirus, astrovirus, echovirus, coxsackievirus, reovirus, and hepatitis E virus (Piao et al. 2012). This technique has a sensitivity that ranges from 100 to 1000 copies/reaction and determines that this method could be used for the detection of co-infections with several viruses. MALDI-TOF MS has been used to diagnose foot, hand and mouth disease that are caused by enteroviruses such as echovirus, poliovirus and coxsackievirus A and B (Peng et al. 2013). The diagnosis of these pathogens assists in thorough surveillance of enteroviruses to simultaneously comprehend the molecular epidemiology of enteroviruses analyzing various genotypes. The main advantage of this approach is the detection of several genotypes simultaneously, pioneering new potentialities for the diagnosis and discovering epidemiological and pathological characteristics. Reports suggested that MALDI-TOF MS has been used for the detection of Hepatitis B virus (HBV) and Hepatitis C virus (HCV), and identified all eight HBV genotypes (Camarasa and Cobo 2018). Due to prolonged treatment with antiviral therapy, some mutations in HBV may cause lamivudine resistance. This method has been suitable for detecting YMDD mutants (Abbaszadeh et al. 2018). MALDI-TOF has also been used to study genotypes of these viruses and detect the sequence variations in the target genome. In another study, this approach determines the mutations of influenza A (H5) viruses for monitoring purposes (Camarasa and Cobo 2018) (Fig. 2).
Biomarker detection for disease diagnosis
Currently, biochemical tests use chemical reagents to diagnose disease biomarkers. However, these diagnostic procedures often suffer from negatives or false positives in the presence of other biological compounds in the body fluids. These shortcomings may be overcome by mass spectrometric approaches to detect the disease biomarkers in biological fluids. For the characterization of large biomolecules, the fluid is generally diluted in different ratios while mixing with the matrix (Yamazaki et al. 2020; Anderson et al. 2012). Biomolecule profiling gives an insight into physiological, developmental, and pathological changes in the body (Wei et al. 2021). Principal component analysis (PCA) is usually involved in identifying differences in the patterns of biomolecules between samples. In association with MALDI-TOF/MS, each mass spectrum is defined by a point in 2D or 3D PCA plots, where similar characteristics in mass spectra are clustered (Jiang et al. 2019; Lasch and Noda 2017). Using proteomic tools like MALDI-TOF/TOF combined with 2D electrophoresis, the protein expression profiles of different stages of Ovarian cancer (OC) and the normal ovarian epithelium tissues have been studied. For e.g. the expression of gila maturation factor beta (GMGB) was analyzed using MALDI-TOF combined with 2D electrophoresis. The results revealed that the expression of GMFB was significantly increased in advanced stages of OC compared to normal or early stages of malignancies (Zuo et al. 2014). This technique has been fruitful in identifying wide variety of proteins and peptides predictive biomarkers thus paving way to develop effective personalized cancer therapies (Sargent et al. 2005). Another study carried by Andresen et al. (2021) concluded MALDI-TOF MSI as a promising and innovative diagnostic tool in prostrate cancer. The study confirmed specific differences in the lipid metabolism of cancerous and non-cancerous epithelium, and stroma tissue types thus providing diagnostic and prognostic biomarkers for prostate cancer (Andersen et al. 2021)
Dereplication
The MALDI-TOF MS facilitates the identification of new diversity of microorganisms and also dereplication of a multitude of isolates. Dereplication refers categorizing of unknown bacterial isolates by identifying similar isolates at a specific taxonomic level (Yamazaki et al. 2020) and analyzing the multitude of microorganisms present in a specific habitat. It classifies bacterial isolates at the species, subspecies and strain level (Abbaszadeh et al. 2018). The advantage of using MALDI-TOF MS over other techniques is that it produces a protein fingerprint that permits the algorithm-driven pattern of spectra based on the absence or presence of peaks (Dice coefficient, Pearson product-moment correlation coefficient) (Demirev and Sandrin 2016). On the other hand, spectra analysis can be done using a curve-based algorithm without including peak, using Pearson product-moment correlation coefficient. Various studies have revealed the role of MALDI-TOF MS in identifying the microorganisms at the strain level (Santos et al. 2016). A study where 456 bacterial isolates were isolated from sponges, (marine) and were recognized at the strain level using MALDI-TOF MS. For instance, from marine sponges, a bacteria, namely Pseudoalteromonas, was isolated and was differentiated by MALDI-TOF MS spectra (Stets et al. 2013). Using phenotypic markers, MALDI-TOF MS differentiate every closely related species with high accuracy. A study using MALDI-TOF MS in association with the Dice similarity coefficient was effectively used to categorize isolates of E. coli from avian, bovine and, canine sources (Santos et al. 2016). In another study different strains of Arthrobacter were identified using the Pearson coefficient. Various species and strains of Bacillus spores were differentiated by using MALDI-TOF MS (Santos et al. 2016; Wenning et al. 2014). Burkholderia pseudomallei isolated from six clinical and five environmental isolates were grouped by using this technique (Niyompanich et al. 2015). Besides that, source-specific markers of clinical and environmental B. pseudomallei were assessed. ClinPro Tools (CPT) software allows access to mass spectrometry data for rapid and comprehensive evaluation and to mine potential biomarkers in complex protein profiles. Out of 10 mass ions, 6 were specified as environmental-identifying biomarker ions, whereas the rest were assigned as clinical-identifying biomarker ions. MALDI-TOF MS was used to describe the grouping of bacteria capable of degrading alkylphenol polyethoxylate. These isolates were analyzed using MALDI-TOF MS, PCR-RFLP and gyrB sequence. MALDI-TOF MS, and gyrB yielded similar results and differentiated isolates based on their potency to degrade the compound (Ichiki et al. 2008). Halophytic prokaryotes isolated from solar saltern sediments were also categorized using MALDI-TOF MS. In this study, clustering of mass spectra was done and was separated into groups delineated using the Jaccard algorithm (peak based) (Spitaels et al. 2016; Munoz et al. 2011). MALDI-TOF MS was also used as a dereplication tool in analyzing the Burkholderiacaledonica isolated from soil (Verstraete et al. 2014). Bacterial isolates were assessed for novel carotenoids by using MALDI-TOF MS (Stafsnes et al. 2013). A distance cutoff valve of 500 was set for delineation of mass spectral clusters, and it was observed that not all species were delineated appropriately. Ghyselinck et al. (2011) studied the comparison of taxonomic resolution of (GTG)5-PCR fingerprinting and MALDI-TOF MS in an analysis of potato rhizosphere isolates and aimed to differentiate between strains based on the reproducibility of both techniques by assessing some of the isolates in triplicate (Spitaels et al. 2016; Ghyselinck et al. 2011). Doan et al. (2012) studied the comparison of MALDI-TOF MS and (GTG)5-PCR for the dereplication of LAB isolates from the meat, sequence analyses using the pheS gene were done for precise identification of strains (Doan et al. 2012). Application of MALDI-TOF MS for dereplication of beer spoilage isolates; 348 isolates were recovered from different types of beer cultured onto different growth media and under different conditions (Wieme et al. 2014). In another study where bacterial and yeast isolates were taken from fermented lambic beer and dereplicated using the database. Delineation of MALDI-TOF MS clusters were done, and identification of the representative isolates of each cluster was carried out by using 16SrRNA sequence analysis for bacteria, 26SrRNA sequence analysis for yeasts, and housekeeping genes for both groups. The MALDI-TOF MS-based dereplication combined with sequence analysis-based, identification was predominant for the differentiation of microbial community (Spitaels et al. 2014). In a similar study, where 1300 yeast and bacterial isolates were dereplicated and identified by MALDI-TOF MS (Spitaels et al. 2015). The mass spectra obtained were then used for the construction of the database. The application of this technique showed that the isolates with comparable mass spectra were grouped together to represent one species, but this classification did not always prove to be reliable; sometimes, completely different clusters may also stand in for the same species. A study demonstrated that MALDI-TOF MS effectively categorized environmental isolates of E. Coli than rep-PCR and can discriminate between strains (Santos et al. 2016). This technique determines a large spectrum of proteins and differentiates between closely related species and group organisms at subspecies level (Grenga et al. 2019). A study suggested that some isolates belonging to various genera such as Bacillus, Stenotrophomonas, Pseudomonas, and Rhodococcus were grouped and were differentiated upto strain level by using MALDI-TOF MS however, rep-PCR failed to produce such results (Ghyselinck et al. 2011). MALDI-TOF MS can classify serotypes of Listeria monocytogenes in three lineages (Ojima-Kato et al. 2016), differentiated subspecies of Francisellatularensis (Regoui et al. 2020) as well as discriminated the strains of Legionella (Blanco et al. 2021). Tanigawa et al. (2010) used mass spectral analysis of ribosomal proteins to distinguish between different Lactococcus species and subspecies. The results discriminated the L. lactis subsp. cremoris from L. lactis subsp. Lactis in a more reliable way than any technique before (Tamang et al. 2016).
Biomolecule profiling
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry is an approach that involves rapid and high-efficiency screening of ions that are generated from molecules when subjected to ionization detected in biological samples, including crude extracts and intact cells (Clark et al. 2013; Cho et al. 2015). MALDI-TOF MS profiling identifies the most intense low molecular mass molecular ions (< m\z 20,000) and doesn’t directly identify the proteins. MALDI-TOF MS profiling leads to identifying several biomarkers and records fingerprints of biological fluids, cells, and tissues under normal or altered conditions. It has emerged as a powerful technique in the field of microbiological diagnostics, identifying a wide spectrum of pathogenic microbes for example, clinical, mycobacterial (Oswald-Richter et al. 2012), bacterial (Patel 2015), viruses (Calderaro et al. 2014), environmental yeasts (Agustini et al. 2014) and entomopathogenic soil fungi (Lopes et al. 2014). New frontiers have been explored besides microbial species level identification, such as direct identification of pathogens from positive blood cultures, subtyping, and drug susceptibility detection (Alizadeh et al. 2021)
Protein profiling
Mass spectrometry provides a sensitive technique, higher throughput, and is very reproducible. MALDI-TOF MS is an important technique for the identification of microorganisms by identifying proteins. A study identified Bacillus spore species by MALDI-TOF/TOF MS using a top-down approach. Fragmentation of protein spore biomarkers and their identification by comparing their spectra with a reference database. Recognition of protein spore biomarkers enabled the identification of bacillus spore species such as Bacillus cereus and Bacillus globigii (Abbaszadeh et al. 2018; Jeong et al. 2014). Identification of various spore proteins were done and used to characterize various Bacillus species. MALDI-TOF MS can also be used to detect metabolic pathways and new functional genes. For instance, MALDI-TOF, in combination with two-dimensional electrophoresis (2-DE), provides an understanding of the metabolism of Synechocystis sp. PCC 6803 (Kurian et al. 2006; Tabandeh et al. 2012).
MALDI-TOF MS can also be applied to map the proteins of an ecosystem. Microorganisms alter their protein expression profiles when they are subjected to changes in environmental variables. A study was conducted where proteomic approaches were employed to study the protein expression profiles of microbial communities (Santos et al. 2016). 2-DE in combination with MALDI-TOF, was used to determine the alteration in protein expression of Shewanellaoneidensis MR-1 (Santos et al. 2016).
Alteration in disease-associated protein expression can decrease or increase in the amount of expression of protein to change in the posttranslational modifications on specific protein (Rodríguez-Piñeiro et al. 2006; Souček et al. 2006) Protein phosphorylation and glycosylation have received more attention as their role as a source of biomarkers in early stages cancer (Thomas et al. 2021). Glycoproteins like PSA in prostate cancer, CA125 in ovarian cancer, and Her2/neu in breast cancer are clinical biomarkers and are therapeutic targets in cancer (Costa 2017; Badr et al. 2014). MALDI–TOF MS has been used in the serum and plasma proteomic patterns of ovarian cancers. Four potential ovarian cancer serum biomarkers have been identified namely kininogen-1, complement 3, transthyretin inter-alpha-trypsin inhibitor heavy chain H4 (Swiatly et al. 2017). Analysis revealed the role of MS-based glycomic profiling of serum in diagnosing oesophageal adenocarcinoma (Dong et al. 2018). Profiling of serum phosphorylated peptides by MALDI-TOF MS is used to detect hepatocellular carcinoma. The expression of peptides was different between healthy and patient individuals (Hu et al. 2009). MALDI-TOF MS technique revealed that degAla-FPA acts as a biomarker for gastric cancer; furthermore, the changes in protein glycosylation identified the serum of patients help in the early diagnosis of this disease. Another study by Gomes et al. (2013) using MALDI-TOF MS showed the identification of circulating proteins, displaying abnormal glycans, and furnished the presence of serum proteins carrying altered O-glycosylation in patients with lesions of gastric carcinoma (Gomes et al. 2013). MALDI-TOF MS is also important for protein profiling and early diagnosis of the bladder (Meng et al. 2013), gastric (Dai et al. 2016), and colorectal cancers (Zhu et al. 2013; Pietrowska et al. 2012). Another study revealed that MALDI-TOF was able to differentiate between malignant and normal states of organs by serum protein profiling and to detect colorectal cancer (de Noo et al. 2006). This technique identified the serum protein patterns that show high specificity and sensitivity in identifying bladder cancer patients (Frantzi et al. 2015). MALDI-TOF MS spectra of tissue biopsies can be used to analyze cancer-associated biochemical changes. Normal gastric and oesophageal tissues produce spectra characterized by increased calgranulins levels. MALDI-TOF spectra of lipids and polypeptides differentiate between the normal esophagus and oesophageal adenocarcinoma and between the normal stomach and gastric cancer (Singhal et al. 2013). MALDI-TOF MS identified 10 membrane-associated proteins are highly expressed in the invasion group of lung cancer (Chen et al. 2012).
MALDI-TOF MS has been used for the plant metabolite profiling (Amorim-Carrilho et al. 2014). No database regarding protein profiles of plant organs or tissues under physiological states is available. The application of this technique in plant proteomics is in discovering protein markers. Identifying protein markers can help breeders select cultivars better acclimatized to a variety of abiotic and biotic stresses or in diverse developmental stages.
Lipid profiling
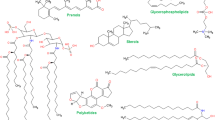
Lipid profiling involves the detection of lipid distributions in living beings. The quantitative (May et al. 2012) and qualitative (Nollet and Toldrá 2012) variations in the lipid profiles of various organisms have been employed to differentiate microorganisms. MALDI-TOF MS has resulted in the analysis of biological families like oligonucleotides, peptides, and lipids and plotted in 2D graphs based on drift time and m/z ratio. With this approach, 2D graphs have been produced in which lipids were separated from peptides (Bush et al. 2012). A study revealed that phospholipid’s mixtures were analyzed and allowed for 2D separation based on their ion mobility and m/z ratio which mainly was because of to radyl chain length, cationization of discrete species, and degree of unsaturation (Cajka and Fiehn 2016). Using MALDI-TOF/TOF MS, studies have been conducted on Sphingolipids and glycerophospholipids in rat brain tissue for the structural characterization of different lipid-like sphingomyelin and Phosphatidylcholine species in tissues (Lee et al. 2012). Structural information facilitating the identification of acyl groups of phosphatidylcholine species was furnished by mass spectral peaks. Studies by (Müller et al. 2015) showed that, by using negative ion mode, lipid species such as phosphatidylserine (PS), phosphatidylethanolamine (PE), phosphatidylinositol PI and sphingomyelin (SM) were identified in rat brain. MALDI-ion trap MS has been used for lipid studies based on MS2 and MS3 data, and PS, PC, SM, and cerebroside species from sciatic and spinal cord nerve tissue were identified (Garrett et al. 2007; Aichler and Walch 2015)
Phosphoinositides are phospholipids with inositol as one of the groups a head group. They have a role in cell signaling, cytoskeletal dynamics, membrane trafficking and calcium homeostasis (Martini et al. 2014). MALDI-TOF-MS has been used to analyze phosphatidylinositols in murine brain extracts (Schneider 2015). Through ESI-MS, composition of fatty acids in lysophosphoinositides in plasma from ovarian cancer were analyzed (Zhang et al. 2015). The tandem mass spectrometer (MS/MS) in combination with collision-induced dissociation can analyze and quantify phosphoinositides that are less abundant with a degree of unsaturation and fatty acid composition (Balla 2013). Another experiment revealed the profiling of phospholipids from rat brain tissue and an overlay of two spectra of the cerebral caudate-putamen of the brain with DHB matrix with cesium (red area) and without cesium (blue area) in positive ion mode. Depending on the drift time of lipid species due to head group, radyl chain, and cationization, 22 phospholipid species were identified, including PEs, PIs, PCs, PSs, and SMs (Cajka and Fiehn 2016).
Lipid profiling of six basidiospores species; Agaricusbisporus, Auriacularia auricula, Hypsizygusmarmoreus, Pleurotusostreatus, Lentinus edodes, and Volvriellavolvacea were carried out by MALDI-TOF MS. Results revealed that phosphatidic acid (PA) and phosphatidylcholine (PC) were dominant lipid components detected almost in all basidiospores. Other phospholipids like PS, PE, and PG were observed in most spores. Diacylglycerol was detected in A. bisporus, A. auricular, and P. ostreatus and triacylglycerol was only observed in H. marmoreus. These results showed the species-specific lipid profiles of fungal spores and can be used to discriminate between fungal spores (Li et al. 2013).
Cholesterol is an important part of biological membranes besides membrane proteins and glycerophospholipids. As far as determining the cholesterol by MALDI-TOF MS is concerned, less data is available because these metabolites are determined easily either by enzymatic kits or by liquid chromatography (LC) coupled to MS. For cholesteryl esters, very few studies have been done in which DHB was used as MALDI matrix (Leopold et al. 2018). In another study apolar lipid constituents like diacylglycerol (DAG), Triacylglycerol (TAG), sterols, and cholesteryl esters were determined by MALDI-TOF MS (Holcapek et al. 2015). A study by Zaima et al. showed that cholesterol linoleate and cholesterol oleate in human and mouse lipid-rich regions were determined by imaging mass spectrometry using DHB as a matrix (Zaima et al. 2011).
Microbial ecology
Microorganisms are ubiquitously present and almost occupy all environments where macroscopic life exists. These represent the sole inhabitant of the extreme environs as well. Although minute in size, their immense diversity regulates the major ecological functions and thus are intimately associated with environmental sustainability. As per the data generated from the metagenomic and cultivation–independent studies, it has been revealed that the majority portion of this enormous diversity is yet unknown. Thus to exploit their ecological capacities to the fullest and to understand their function new strategies are being employed. MALDI-TOF-MS has gained a lot of popularity pertaining to its affordability and applicability especially in microbial ecology (Patil et al. 2015; Tong et al. 2015). The applications of this technique in the environmental remediation research, microbial diversity studies, and proteomic and metabolomic studies have been discussed as under:
Bioremediation research
Bioremediation is regarded as a promising approach to eliminating or reducing hazardous waste from the environment (Ullah et al. 2015). The application of MALDI-TOF-MS in this research area mainly focuses on the screening and identifying the site-specific microbes for the degradation if various chemical compounds. Evaluating the various microbes for the degradation process is based on their global protein expression (Sharma et al. 2019), which eventually enables the researchers to employ highly potential microorganisms for the remediation process. Numerous researches have been done to identify numerous microbes involved in the degradation of various contaminants. For example, different bacterial species were isolated from the soils contaminated with biphenyls, and pesticides. The identification of bacterial sp. up to species level was achieved using MALDI-TOF-MS, technique, and the results concurred with the 16SrRNA genotyping results (Lovecka et al. 2015; Uhlik et al. 2011). Different enzymes and the proteins involved in the catalysis of contaminants have been identified using the MALDI-TOF-MS technique. A study carried out by (Tomás‐Gallardo et al. 2006) identified specific proteins in Rhodococcus sp. which is involved in the degradation of phthalate and protocatechuic acid (PCA). Furthermore, in another study membrane proteome of Acinetobacter radioresistens S13 was profiled by the 2-DE/MALDI-TOF-MS technique, and the results confirmed the expression of novel proteins expressed only during aromatic pollution (Pessione et al. 2003). MALDI-TOF-MS has allowed researchers to study and identify the protein involved in the biotransformation. For example (Wilmes et al. 2008), was able to identify the proteins involved in the chemical transformation in the mixed culture activated sludge system; the proteins screened have been identified essential in phosphorus removal. In another study, three bacterial species, Bacillus cereus, Bacillus sonorensis and Pseudomonas stuztzeri have been identified using the MALDI-TOF-MS technique in the soils heavily contaminated with the oils in the extreme climatic condition of the Qatar Gulf area (Al-Kaabi et al. 2018). Likewise, the MALDI-TOF-MS technique was employed to identify bacteria isolated from copper contaminated environments of Sassego Mine, Brazil. The 16Sr DNA method was also used for identification purposes. The results confirmed the MALDI-TOF-MS technique as an effective tool for the identifying bacteria isolated from copper-contaminated sites (Avanzi et al. 2017). In a recent study from the saffron soils of the Kashmir Himalaya region, four lead tolerant bacterial species were identified using MALDI-TOF-MS and 16S rRNA genotype sequencing, namely Staphylococcus equorum, Staphylococcus warneri, Bacillus safensis, and Bacillus thuringiensiswith B. thuringiensis as the highest metal sequester (65.3%) and S. warneri was observed as the least metal sequester (52.8%) (Nazir et al. 2020).
Proteomics and metabolomics
MALDI-TOF-MS has been proved as a high-throughput technique as it enables the screening of novel proteins thus acting as an important tool in environmental proteomics aiding in the characterization and identification process (Toby et al. 2016). For instance, a study carried out by Demirev et al. (2005) resulted in identifying intact Bacillus spore species, namely Bacillus globigii and Bacillus cereus (Toby et al. 2016). Fragmented ion spectra of whole protein biomarkers were studied using this technique and by comparing it to the proteome database, the results were obtained. Several other studies employ the MALDI-TOF-MS technique to identify a wide variety of proteins expressed during stress conditions. For example, work carried out by Heim et al. (2003), used the MALDI-TOF-MS tool and identified twenty-five proteins expressed in Pseudomonas putida during iron limitation conditions. In another study, 2-DE MALDI-TOF MS/MS technique was utilized, and differentially expressed proteins were identified in the bacterial community when exposed to cadmium stress (Franzosa et al. 2015). There has been extensive literature supporting the MALDI-TOF-MS and proteomic studies; however, this technique serves as an important tool in metabolomic studies as. This approach is highly fruitful in studying the bioremediation processes. It is very necessary to analyze the metabolic pathway and identify the catabolic byproducts thus determining the potential of different microorganisms in degrading a contaminant (Lellis et al. 2019). A study carried out by Edward and Kennedy (2005) screened more than 100 metabolites from E. coli using this technique (Zenobi 2013). Similarly, in another study, MALDI-TOF-MS was used to study the pigment composition for the identification and characterizations of bacterial sp. The results furnished by this study identified bacteriochlorophyll a and homologs of bacteriochlorophyll c, a characteristic feature of Chlorobiumtepidum thus indicating its presence (Morgan-Kiss et al. 2009).
Detection of pathogenic microbes
The pathogenic bacteria and fungi represent a constant threat to the terrestrial and aquatic biodiversity, including crops. It is crucial to detect and identify the pathogenic microbes for proper diagnostic and disease management procedures, for which MALDI-TOF-MS serves as an effective tool. For instance, Puccinia triticina causing wheat leaf rust (a notorious pathogen of wheat worldwide) has been studied using a proteomic approach (Kosová et al. 2014). Similarly, MALDI-TOF-MS technique has been proven a reliable tool in detecting numerous plant pathogens like Serpulalacrymans, S. himantioides, Coniophoraputeana, C. marmorata, Antrodiavaillantii, and A. sinuosa (Drissner and Freimoser 2017). Also, it has been regarded as a rapid and easy technique compared to genotype sequencing method to identify species-specific profiling of Candida sp. (Reddel et al. 2019). Moreover, this technique was also found effective in the identification and discrimination of eight polypore species of genre Fomitopsis, Rhodofomes, Fomes, and Ganoderma in, Czech Republic (Pristaš et al. 2017). It has also successfully aided in the identification of two closely related Vibrio sp., V. furnissii and V. fluvialis, both representing human pathogen associated with the consumption of contaminated seafood and contaminated water (Schirmeister et al. 2014). The identification of water-borne pathogens, chiefly Helicobacter pylori, Salmonella typhimurium, Yersinia enterocolitica, Legionella pneumophila, and Campylobacter jejuni was achieved by the MALDI-TOF-MS technique, and the results were confirmed by 16SrRNA sequencing (Lee et al. 2014).
Biotyping
MALDI-TOF-MS finds its application in microbial biotyping chiefly to elucidate the bacterial taxonomy, differentiation, and characterization (Sandrin et al. 2013) with more accuracy and ease when compared to 16SrRNA genotyping. A study carried out by (Dieckmann et al. 2005) isolated and differentiated Pseudoalteromonas sp. from marine sponges and characterized them even if they differed only by 1 bp out of 400 bp or by 3–4 bp out of 1500 bp. The results obtained by MALDI-TOF-MS spectra were more resolved when compared to conventional genotype sequencing. Similarly, in another study MALDI-TOF-MS tool differentiated at least thirty Vibrio strains isolated from two waste water treatment plants (Eddabra et al. 2012). Furthermore, a study carried out by (Crossay et al. 2017), performed MALDI-TOF-MS proteomic biotyping technique to identify Arbuscular mycorrhizal fungi (AMF, Glomeromycota) important for the plant growth. The results furnished that the nineteen isolates belonged to fourteen genera and five families also, the intraspecific differentiation was revealed for majority of species.
Future perspectives
MALDI-TOF MS has become a popular tool for microbial identification in clinical, microbiological laboratories. This approach has also been used as a dereplication tool in other microbiological fields. A commercial database system permits the identification of isolates or strains during dereplication, but due to a lack of reference databases for a few isolates or strains, many or most species/strains fail to identify. For such species and isolates, commercial databases will need to be broadened with an appropriate number of reference strains to enable the identification of isolates during dereplication studies. The accuracy and quality of spectra can be affected by sample preparation methods, the type of material analyzed and the matrix components. Improving sample-processing procedures and enhancing, the spectral database leads to highly reliable outcomes for the identification of microbes. The development of new sample processing methods and newer algorithms will enhance the MALDI-TOF MS applications. Dereplication of microorganisms at the strain level demands further studies. Flexible algorithms are needed to filter out common peaks and focus mostly on differential peaks in the spectra. For biomarker characterization, consistent analysis, accurate quantification, and analytical reproducibility requires further improvement for better analysis.
References
Abbaszadeh, S.M., Miri, S.M., Naderi, R.: An effective nutrient media for asymbiotic seed germination and in vitro seedling development of phalaenopsis ‘Bahia Blanca.’ J. Ornament. Plants 8(3), 183–192 (2018)
Agustini, B.C., Silva, L.P., Bloch, C., Bonfim, T.M., da Silva, G.A.: Evaluation of MALDI-TOF mass spectrometry for identification of environmental yeasts and development of supplementary database. Appl. Microbiol. Biotechnol. 98(12), 5645–5654 (2014)
Aichler, M., Walch, A.: MALDI Imaging mass spectrometry: current frontiers and perspectives in pathology research and practice. Lab. Investig. 95(4), 422–431 (2015)
Al-Kaabi, N., Al-Ghouti, M.A., Oualha, M., Mohammad, M.Y., Al-Naemi, A., Sølling, T.I., Zouari, N.: A MALDI-TOF study of bio-remediation in highly weathered oil contaminated soils. J. Petroleum Sci. Eng. 168, 569–576 (2018)
Alizadeh, M., Yousefi, L., Pakdel, F., Ghotaslou, R., Rezaee, M.A., Khodadadi, E., Kafil, H.S.: MALDI-TOF mass spectroscopy applications in clinical microbiology. Adv. Pharmacol. Pharm. Sci. (2021)
Amorim-Carrilho, K., Cepeda, A., Fente, C., Regal, P.: Review of methods for analysis of carotenoids. TrAC Trends Anal. Chem. 56, 49–73 (2014)
Andersen, M.K., Høiem, T.S., Claes, B.S., Balluff, B., Martin-Lorenzo, M., Richardsen, E., Rye, M.B.: Spatial differentiation of metabolism in prostate cancer tissue by MALDI-TOF MSI. Cancer Metab. 9(1), 1–13 (2021)
Anderson, N.W., Buchan, B.W., Riebe, K.M., Parsons, L.N., Gnacinski, S., Ledeboer, N.A.: Effects of solid-medium type on routine identification of bacterial isolates by use of matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 50(3), 1008–1013 (2012)
Ashfaq, M.Y., Da’na, D.A., Al-Ghouti, M.A.: Application of MALDI-TOF MS for identification of environmental bacteria: a review. J. Environ. Manag. 305, 114359 (2022)
Avanzi, I.R., Gracioso, L.H., Baltazar, M.D.P.G., Karolski, B., Perpetuo, E.A., do Nascimento, C.A.O.: Rapid bacteria identification from environmental mining samples using MALDI-TOF MS analysis. Environ. Sci. Pollut. Res. 24(4), 3717–3726 (2017)
Bader, O., Weig, M., Taverne-Ghadwal, L., Lugert, R., Gross, U., Kuhns, M.: Improved clinical laboratory identification of human pathogenic yeasts by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Microbiol. Infect. 17(9), 1359–1365 (2011)
Badr, H.A., AlSadek, D.M., Darwish, A.A., ElSayed, A.I., Bekmanov, B.O., Khussainova, E.M., Li, C.-Z.: Lectin approaches for glycoproteomics in FDA-approved cancer biomarkers. Expert Rev. Proteomics 11(2), 227–236 (2014)
Balla, T.: Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93(3), 1019–1137 (2013)
Barthélemy, M., Guérineau, V., Genta-Jouve, G., Roy, M., Chave, J., Guillot, R., Eparvier, V.: Identification and dereplication of endophytic Colletotrichum strains by MALDI TOF mass spectrometry and molecular networking. Sci. Rep. 10(1), 1–16 (2020)
Bizzini, A., Durussel, C., Bille, J., Greub, G., Prod’Hom, G.: Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J. Clin. Microbiol. 48(5), 1549–1554 (2010)
Blanco, S., Sanz, C., Gutiérrez, M.P., Simarro, M., López, I., Escribano, I., López, J.C.: A new MALDI-TOF approach for the quick sequence type identification of Legionella pneumophila. J. Microbiol. Methods 188, 106292 (2021)
Bush, M.F., Campuzano, I.D., Robinson, C.V.: Ion mobility mass spectrometry of peptide ions: effects of drift gas and calibration strategies. Anal. Chem. 84(16), 7124–7130 (2012)
Cajka, T., Fiehn, O.: Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal. Chem. 88(1), 524–545 (2016)
Calderaro, A., Arcangeletti, M.-C., Rodighiero, I., Buttrini, M., Gorrini, C., Motta, F., De Conto, F.: Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry applied to virus identification. Sci. Rep. 4(1), 1–10 (2014)
Camarasa, C.G., Cobo, F.: Application of MALDI-TOF mass spectrometry in clinical virology. In: The Use of Mass Spectrometry Technology (MALDI-TOF) in Clinical Microbiology, pp. 167–180. Elsevier (2018)
Campuzano, I.D., Nshanian, M., Spahr, C., Lantz, C., Netirojjanakul, C., Li, H., Loo, J.A.: High mass analysis with a fourier transform ion cyclotron resonance mass spectrometer: from inorganic salt clusters to antibody conjugates and beyond. J. Am. Soc. Mass Spectrom. 31(5), 1155–1162 (2020)
Chen, W.-L., Kuo, K.-T., Chou, T.-Y., Chen, C.-L., Wang, C.-H., Wei, Y.-H., Wang, L.-S.: The role of cytochrome c oxidase subunit Va in non-small cell lung carcinoma cells: association with migration, invasion and prediction of distant metastasis. Bmc Cancer 12(1), 1–13 (2012)
Cho, Y.-T., Su, H., Wu, W.-J., Wu, D.-C., Hou, M.-F., Kuo, C.-H., Shiea, J.: Biomarker characterization by MALDI–TOF/MS. Adv. Clin. Chem. 69, 209–254 (2015)
Choi, H., Lee, D., Kim, Y., Nguyen, H.-Q., Han, S., Kim, J.: Effects of matrices and additives on multiple charge formation of proteins in maldi–ms analysis. J. Am. Soc. Mass Spectrom. 30(7), 1174–1178 (2019)
Clark, A.E., Kaleta, E.J., Arora, A., Wolk, D.M.: Matrix-assisted laser desorption ionization–time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology. Clin. Microbiol. Rev. 26(3), 547–603 (2013)
Costa, J.: Glycoconjugates from extracellular vesicles: structures, functions and emerging potential as cancer biomarkers. Biochim. Biophys. Acta 1868(1), 157–166 (2017)
Crossay, T., Antheaume, C., Redecker, D., Bon, L., Chedri, N., Richert, C., Amir, H.: New method for the identification of arbuscular mycorrhizal fungi by proteomic-based biotyping of spores using MALDI-TOF-MS. Sci. Rep. 7(1), 1–16 (2017)
Croxatto, A., Prod’hom, G., Greub, G.: Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol. Rev. 36(2), 380–407 (2012)
Dai, P., Wang, Q., Wang, W., Jing, R., Wang, W., Wang, F., Yan, Z.: Unraveling molecular differences of gastric cancer by label-free quantitative proteomics analysis. Int. J. Mol. Sci. 17(1), 69 (2016)
De Florio, L., Riva, E., Giona, A., Dedej, E., Fogolari, M., Cella, E., Ciccozzi, M.: MALDI-TOF MS identification and clustering applied to Enterobacter species in nosocomial setting. Front. Microbiol. 9, 1885 (2018)
Edwards, J.L., Kennedy, R.T.: Metabolomic analysis of eukaryotic tissue and prokaryotes using negative mode MALDI time-of-flight mass spectrometry. Anal. Chem. 77, 2201–2209 (2005)
de Noo, M.E., Mertens, B.J., Özalp, A., Bladergroen, M.R., van der Werff, M.P., van de Velde, C.J., Tollenaar, R.A.: Detection of colorectal cancer using MALDI-TOF serum protein profiling. Eur. J. Cancer 42(8), 1068–1076 (2006)
Demirev, P.A., Feldman, A.B., Kowalski, P., Lin, J.S: Top-down proteomics for rapid identification of intact microorganisms. Anal Chem 77, 7455 (2005)
Demirev, P., Sandrin, T.R.: Applications of Mass Spectrometry in Microbiology. Springer, New York (2016)
Dieckmann, R., Graeber, I., Kaesler, I., Szewzyk, U., Von Döhren, H.: Rapid screening and dereplication of bacterial isolates from marine sponges of the Sula Ridge by Intact-Cell-MALDI-TOF mass spectrometry (ICM-MS). Appl. Microbiol. Biotechnol. 67(4), 539–548 (2005)
Dinali, R., Ebrahiminezhad, A., Manley-Harris, M., Ghasemi, Y., Berenjian, A.: Iron oxide nanoparticles in modern microbiology and biotechnology. Crit. Rev. Microbiol. 43(4), 493–507 (2017)
Doan, N., Van Hoorde, K., Cnockaert, M., De Brandt, E., Aerts, M., Le Thanh, B., Vandamme, P.: Validation of MALDI-TOF MS for rapid classification and identification of lactic acid bacteria, with a focus on isolates from traditional fermented foods in Northern Vietnam. Lett. Appl. Microbiol. 55(4), 265–273 (2012)
Dong, X., Huang, Y., Cho, B.G., Zhong, J., Gautam, S., Peng, W., Mechref, Y.: Advances in mass spectrometry-based glycomics. Electrophoresis 39(24), 3063–3081 (2018)
Drissner, D., Freimoser, F.M.: MALDI-TOF mass spectroscopy of yeasts and filamentous fungi for research and diagnostics in the agricultural value chain. Chem. Biol. Technol. Agric. 4(1), 1–12 (2017)
Duncan, M.W., Nedelkov, D., Walsh, R., Hattan, S.J.: Applications of MALDI mass spectrometry in clinical chemistry. Clin. Chem. 62(1), 134–143 (2016)
Eddabra, R., Prévost, G., Scheftel, J.-M.: Rapid discrimination of environmental Vibrio by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Microbiol. Res. 167(4), 226–230 (2012)
Frantzi, M., Latosinska, A., Flühe, L., Hupe, M.C., Critselis, E., Kramer, M.W., Vlahou, A.: Developing proteomic biomarkers for bladder cancer: towards clinical application. Nat. Rev. Urol. 12(6), 317–330 (2015)
Franzosa, E.A., Hsu, T., Sirota-Madi, A., Shafquat, A., Abu-Ali, G., Morgan, X.C., Huttenhower, C.: Sequencing and beyond: integrating molecular’omics’ for microbial community profiling. Nat. Rev. Microbiol. 13(6), 360–372 (2015)
Freitas, J., Perestrelo, R., Vaz-Pires, P., Câmara, J.S.: Bacterial diversity analysis of coastal superficial seawaters near aquaculture facilities, using MALDI-TOF approach and Ribopeaks database. Aquaculture 556, 738263 (2022)
Garrett, T.J., Prieto-Conaway, M.C., Kovtoun, V., Bui, H., Izgarian, N., Stafford, G., Yost, R.A.: Imaging of small molecules in tissue sections with a new intermediate-pressure MALDI linear ion trap mass spectrometer. Int. J. Mass Spectrom. 260(2–3), 166–176 (2007)
Gaudreau, A., Labrie, J., Goetz, C., Dufour, S., Jacques, M.: Evaluation of MALDI-TOF mass spectrometry for the identification of bacteria growing as biofilms. J. Microbiol. Methods 145, 79–81 (2018)
Ghyselinck, J., Van Hoorde, K., Hoste, B., Heylen, K., De Vos, P.: Evaluation of MALDI-TOF MS as a tool for high-throughput dereplication. J. Microbiol. Methods 86(3), 327–336 (2011)
Giebel, R., Worden, C., Rust, S., Kleinheinz, G., Robbins, M., Sandrin, T.: Microbial fingerprinting using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS): applications and challenges. Adv. Appl. Microbiol. 71, 149–184 (2010)
Gomes, C., Almeida, A., Ferreira, J.A., Silva, L., Santos-Sousa, H., Pinto-de-Sousa, J.O., Levery, S.B.: Glycoproteomic analysis of serum from patients with gastric precancerous lesions. J. Proteome Res. 12(3), 1454–1466 (2013)
Gonzalo, X., Broda, A., Drobniewski, F., Larrouy-Maumus, G.: Performance of lipid fingerprint-based MALDI-ToF for the diagnosis of mycobacterial infections. Clin. Microbiol. Infect. (2020)
Grenga, L., Pible, O., Armengaud, J.: Pathogen proteotyping: a rapidly developing application of mass spectrometry to address clinical concerns. Clin. Mass Spectrom. 14, 9–17 (2019)
Hagen, F., Khayhan, K., Theelen, B., Kolecka, A., Polacheck, I., Sionov, E., Boekhout, T.: Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 78, 16–48 (2015)
Heim, S., M. Ferrer, H. Heuer, D. Regenhardt, M. Nimtz and K. N. Timmis.: Proteome reference map of Pseudomonas putida strain KT2440 for genome expression profiling: distinct responses of KT2440 and Pseudomonas aeruginosa strain PAO1 to iron deprivation and a new form of superoxide dismutase. Environ. Microbiol. 5(12), 1257–1269 (2003)
Hettick, J.M., Green, B.J., Buskirk, A.D., Kashon, M.L., Slaven, J.E., Janotka, E., Beezhold, D.H.: Discrimination of Aspergillus isolates at the species and strain level by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry fingerprinting. Anal. Biochem. 380(2), 276–281 (2008a)
Hettick, J.M., Green, B.J., Buskirk, A.D., Kashon, M.L., Slaven, J.E., Janotka, E., Beezhold, D.H.: Discrimination of Penicillium isolates by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry fingerprinting. Rapid Commun. Mass Spectrom. 22(16), 2555–2560 (2008b)
Holcapek, M.; Cerven ˇ á, B.; Cífková, E.; Lísa, M.; Chagovets, V.; Vostálová, J.; Bancíˇrová, M.; Galuszka, J.: Hill, M. Lipidomic analysis of plasma, erythrocytes and lipoprotein fractions of cardiovascular disease. patients using UHPLC/MS, MALDI-MS and multivariate data analysis. J. Chromatogr. B Anal. Technol. Biomed Life Sci 990, 52–63 (2015).
Hou, T.-Y., Chiang-Ni, C., Teng, S.-H.: Current status of MALDI-TOF mass spectrometry in clinical microbiology. J. Food Drug Anal. 27(2), 404–414 (2019)
Hu, L., Zhou, H., Li, Y., Sun, S., Guo, L., Ye, M., Zou, H.: Profiling of endogenous serum phosphorylated peptides by titanium (IV) immobilized mesoporous silica particles enrichment and MALDI-TOFMS detection. Anal. Chem. 81(1), 94–104 (2009)
Ichiki, Y., Ishizawa, N., Tamura, H., Teramoto, K., Sato, H., Yoshikawa, H.: Environmental distribution and novel high-throughput screening of APEO-degrading bacteria using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-MS). J. Pest. Sci. 0803100028-0803100028 (2008)
Jang, K.-S., Kim, Y.H.: Rapid and robust MALDI-TOF MS techniques for microbial identification: a brief overview of their diverse applications. J. Microbiol. 56(4), 209–216 (2018)
Jeong, Y.S., Choi, S., Chong, E., Kim, J., Kim, S.J.: Rapid detection of B acillus spore aerosol particles by direct in situ analysis using MALDI-TOF mass spectrometry. Lett. Appl. Microbiol. 59(2), 177–183 (2014)
Jiang, Y., Sun, J., Huang, X., Shi, H., Xiong, C., Nie, Z.: Direct identification of forensic body fluids by MALDI-MS. Analyst 144(23), 7017–7023 (2019)
Karas, M., Krüger, R.: Ion formation in MALDI: the cluster ionization mechanism. Chem. Rev. 103(2), 427–440 (2003)
Knochenmuss, R.: Ion formation mechanisms in UV-MALDI. Analyst 131(9), 966–986 (2006)
Kodana, M., Tarumoto, N., Kawamura, T., Saito, T., Ohno, H., Maesaki, S., Ikebuchi, K.: Utility of the MALDI-TOF MS method to identify nontuberculous mycobacteria. J. Infect. Chemother. 22(1), 32–35 (2016)
Kosová, K., Vítámvás, P., Prášil, I.T.: Proteomics of stress responses in wheat and barley—search for potential protein markers of stress tolerance. Front. Plant Sci. 5, 711 (2014)
Kurian, D., Phadwal, K., Mäenpää, P.: Proteomic characterization of acid stress response in Synechocystis sp. PCC 6803. Proteomics 6(12), 3614–3624 (2006)
Lasch, P., Noda, I.: Two-dimensional correlation spectroscopy for multimodal analysis of FT-IR, Raman, and MALDI-TOF MS hyperspectral images with hamster brain tissue. Anal. Chem. 89(9), 5008–5016 (2017)
Lee, Y.J., Perdian, D.C., Song, Z., Yeung, E.S., Nikolau, B.J.: Use of mass spectrometry for imaging metabolites in plants. Plant J. 70(1), 81–95 (2012)
Lee, Y.-J., Park, J.-G., Rhee, O.-J., Lee, G.-C.: Identification of waterborne microbial pathogens by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry and the biotyper 2.0 databases. J. Pure Appl. Microbiol. 8(5), 3525–3530 (2014)
Lellis, B., Fávaro-Polonio, C.Z., Pamphile, J.A., Polonio, J.C.: Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 3(2), 275–290 (2019)
Leopold, J., Popkova, Y., Engel, K.M., Schiller, J.: Recent developments of useful MALDI matrices for the mass spectrometric characterization of lipids. Biomolecules 8(4), 173 (2018)
Li, Y., Liang, M., Shu, X., Liu, C., Shu, J.: Differentiation of basidiospores by MALDI-TOF lipid profiling. Int. J. Mass Spectrom. 352, 44–50 (2013)
L’Ollivier, C., Ranque, S.: MALDI-TOF-based dermatophyte identification. Mycopathologia 182(1), 183–192 (2017)
Lopes, R.B., Faria, M., Souza, D.A., Bloch, C., Jr., Silva, L.P., Humber, R.A.: MALDI-TOF mass spectrometry applied to identifying species of insect-pathogenic fungi from the Metarhizium anisopliae complex. Mycologia 106(4), 865–878 (2014)
Lotz, A., Ferroni, A., Beretti, J.-L., Dauphin, B., Carbonnelle, E., Guet-Revillet, H., Gaillard, J.-L.: Rapid identification of mycobacterial whole cells in solid and liquid culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 48(12), 4481–4486 (2010)
Lovecka, P., Pacovska, I., Stursa, P., Vrchotova, B., Kochankova, L., Demnerova, K.: Organochlorinated pesticide degrading microorganisms isolated from contaminated soil. New Biotechnol. 32(1), 26–31 (2015)
Lu, I.-C., Chu, K.Y., Lin, C.-Y., Wu, S.-Y., Dyakov, Y.A., Chen, J.-L., Ni, C.-K.: Ion-to-neutral ratios and thermal proton transfer in matrix-assisted laser desorption/ionization. J. Am. Soc. Mass Spectrom. 26(7), 1242–1251 (2015)
Machen, A., Kobayashi, M., Connelly, M.R., Wang, Y.F.W.: Comparison of heat inactivation and cell disruption protocols for identification of mycobacteria from solid culture media by use of Vitek matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbial. 51(12), 4226–4229 (2013)
Marinach-Patrice, C., Lethuillier, A., Marly, A., Brossas, J.-Y., Gene, J., Symoens, F., Hennequin, C.: Use of mass spectrometry to identify clinical Fusarium isolates. Clin. Microbiol. Infect. 15(7), 634–642 (2009)
Martini, M., De Santis, M.C., Braccini, L., Gulluni, F., Hirsch, E.: PI3K/AKT signaling pathway and cancer: an updated review. Ann. Med. 46(6), 372–383 (2014)
May, S., Romberger, D.J., Poole, J.A.: Respiratory health effects of large animal farming environments. J. Toxicol. Environ. Health Part B 15(8), 524–541 (2012)
Meng, Q., Lei, T., Zhang, M., Zhao, J., Zhao, X.-H., Zhang, M.: Identification of proteins differentially expressed in adriamycin-resistant (pumc-91/ADM) and parental (pumc-91) human bladder cancer cell lines by proteome analysis. J. Cancer Res. Clin. Oncol. 139(3), 509–519 (2013)
Morgan-Kiss, R.M., Chan, L.-K., Modla, S., Weber, T.S., Warner, M., Czymmek, K.J., Hanson, T.E.: Chlorobaculum tepidum regulates chlorosome structure and function in response to temperature and electron donor availability. Photosyn. Res. 99(1), 11–21 (2009)
Müller, C.P., Reichel, M., Mühle, C., Rhein, C., Gulbins, E., Kornhuber, J.: Brain membrane lipids in major depression and anxiety disorders. Biochim. Biophys. Acta 1851(8), 1052–1065 (2015)
Munteanu, B., Hopf, C.: Emergence of whole-cell MALDI-MS biotyping for high-throughput bioanalysis of mammalian cells? Bioanalysis 5(8), 885–893 (2013)
Munoz, R., Lopez-Lopez, A., Urdiain, M., Moore, E.R., Rossello-Mora, R.: Evaluation of matrix-assisted laser desorption ionization-time of flight whole cell profiles for assessing the cultivable diversity of aerobic and moderately halophilic prokaryotes thriving in solar saltern sediments. Syst Appl Microbiol. 34(1):69–75 (2011).
Nazir, R., Ganai, B.A., Rahi, P., Rehman, S., Farooq, S., Dar, R., Abd Allah, E.F.: MALDI-TOF-MS and 16S rRNA characterization of lead tolerant metallophile bacteria isolated from saffron soils of Kashmir for their sequestration potential. Saudi J. Biol. Sci. 27(8), 2047–2053 (2020)
Niyompanich, S., Srisanga, K., Jaresitthikunchai, J., Roytrakul, S., Tungpradabkul, S.: Utilization of whole-cell MALDI-TOF mass spectrometry to differentiate Burkholderia pseudomallei wild-type and constructed mutants. PLoS One 10(12), e0144128 (2015)
Nollet, L.M., Toldrá, F.: Food Analysis by HPLC. CRC Press, Boca Raton (2012)
Noumi, E., Merghni, A., Alreshidi, M., Del Campo, R., Adnan, M., Haddad, O., Snoussi, M.: Phenotypic and genotypic characterization with MALDI-TOF-MS based identification of Staphylococcus spp. isolated from Mobile phones with their antibiotic susceptibility, biofilm formation, and adhesion properties. Int. J. Environ. Res. Public Health 17(11), 3761 (2020)
Ojima-Kato, T., Yamamoto, N., Takahashi, H., Tamura, H.: Matrix-assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) can precisely discriminate the lineages of Listeria monocytogenes and species of Listeria. PLoS ONE 11(7), e0159730 (2016)
Oswald-Richter, K.A., Beachboard, D.C., Seeley, E.H., Abraham, S., Shepherd, B.E., Jenkins, C.A., Drake, W.P.: Dual analysis for mycobacteria and propionibacteria in sarcoidosis BAL. J. Clin. Immunol. 32(5), 1129–1140 (2012)
Passarini, M.R., Santos, C., Lima, N., Berlinck, R.G., Sette, L.D.: Filamentous fungi from the Atlantic marine sponge Dragmacidon reticulatum. Arch. Microbiol. 195(2), 99–111 (2013)
Patel, R.: MALDI-TOF MS for the diagnosis of infectious diseases. Clin. Chem. 61(1), 100–111 (2015)
Patil, V.S., Salunkhe, R.C., Patil, R.H., Husseneder, C., Shouche, Y.S., Ramana, V.V.: Enterobacillus tribolii gen. nov., sp. nov., a novel member of the family Enterobacteriaceae, isolated from the gut of a red flour beetle, Tribolium castaneum. Ant. Leeuwen. 107(5), 1207–1216 (2015)
Peng, J., Yang, F., Xiong, Z., Guo, J., Du, J., Hu, Y., Jin, Q.: Sensitive and rapid detection of viruses associated with hand foot and mouth disease using multiplexed MALDI-TOF analysis. J. Clin. Virol. 56(2), 170–174 (2013)
Pessione, E., Giuffrida, M.G., Prunotto, L., Barello, C., Mazzoli, R., Fortunato, D., Giunta, C.: Membrane proteome of Acinetobacter radioresistens S13 during aromatic exposure. Proteomics 3(6), 1070–1076 (2003)
Piao, J., Jiang, J., Xu, B., Wang, X., Guan, Y., Wu, W., Wang, P.: Simultaneous detection and identification of enteric viruses by PCR-mass assay. PLoS ONE 7(8), e42251 (2012)
Pietrowska, M., Polańska, J., Suwiński, R., Wideł, M., Rutkowski, T., Marczyk, M., Polański, A.: Comparison of peptide cancer signatures identified by mass spectrometry in serum of patients with head and neck, lung and colorectal cancers: association with tumor progression. Int. J. Oncol. 40(1), 148–156 (2012)
Pomastowski, P., Złoch, M., Rodzik, A., Ligor, M., Kostrzewa, M., Buszewski, B.: Analysis of bacteria associated with honeys of different geographical and botanical origin using two different identification approaches: MALDI-TOF MS and 16S rDNA PCR technique. PLoS ONE 14(5), e0217078 (2019)
Pristaš, P., Kvasnová, S., Gáperová, S., Gašparcová, T., Gáper, J.: Application of MALDI-TOF mass spectrometry for in vitro identification of wood decay polypores. For. Pathol. 47(5), e12352 (2017)
Reddel, S., Del Chierico, F., Quagliariello, A., Giancristoforo, S., Vernocchi, P., Russo, A., El Hachem, M.: Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep. 9(1), 1–10 (2019)
Reeve, M.A., Bachmann, D., Caine, T.S.: Identification of Penicillium species by MALDI-TOF MS analysis of spores collected by dielectrophoresis. Biol. Methods Protoc. 4(1), bpz018 (2019)
Regoui, S., Hennebique, A., Girard, T., Boisset, S., Caspar, Y., Maurin, M.: Optimized MALDI TOF mass spectrometry identification of Francisella tularensis Subsp. holarctica. Microorganisms 8(8), 1143 (2020)
Rodrigues, P., Santos, C., Venâncio, A., Lima, N.: Species identification of Aspergillus section Flavi isolates from Portuguese almonds using phenotypic, including MALDI-TOF ICMS, and molecular approaches. J. Appl. Microbiol. 111(4), 877–892 (2011)
Rodríguez-Piñeiro, A.M., de la Cadena, M.P., López-Saco, Á., Rodríguez-Berrocal, F.J.: Differential expression of serum clusterin isoforms in colorectal cancer. Mol. Cell. Proteomics 5(9), 1647–1657 (2006)
Sandrin, T.R., Goldstein, J.E., Schumaker, S.: MALDI TOF MS profiling of bacteria at the strain level: a review. Mass Spectrom. Rev. 32(3), 188–217 (2013)
Santos, I.C., Hildenbrand, Z.L., Schug, K.A.: Applications of MALDI-TOF MS in environmental microbiology. Analyst 141(10), 2827–2837 (2016)
Sargent, D.J., Conley, B.A., Allegra, C., Collette, L.: Clinical trial designs for predictive marker validation in cancer treatment trials. J. Clin. Oncol. 23(9), 2020–2027 (2005)
Schirmeister, F., Wieczorek, A., Dieckmann, R., Taureck, K., Strauch, E.: Evaluation of molecular methods to discriminate the closely related species Vibrio fluvialis and Vibrio furnissii. Int. J. Med. Microbiol. 304(7), 851–857 (2014)
Schneider, S.: Inositol transport proteins. FEBS Lett. 589(10), 1049–1058 (2015)
Schulthess, B., Bloemberg, G.V., Zbinden, R., Böttger, E.C., Hombach, M.: Evaluation of the Bruker MALDI Biotyper for identification of Gram-positive rods: development of a diagnostic algorithm for the clinical laboratory. J. Clin. Microbiol. 52(4), 1089–1097 (2014)
Seuylemezian, A., Aronson, H.S., Tan, J., Lin, M., Schubert, W., Vaishampayan, P.: Development of a custom MALDI-TOF MS database for species-level identification of bacterial isolates collected from spacecraft and associated surfaces. Front. Microbiol. 9, 780 (2018)
Sharma, A., Kumar, V., Shahzad, B., Tanveer, M., Sidhu, G.P.S., Handa, N., Parihar, R.D.: Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 1(11), 1–16 (2019)
Singhal, R., Carrigan, J.B., Wei, W., Taniere, P., Hejmadi, R.K., Forde, C., Johnson, P.J.: MALDI profiles of proteins and lipids for the rapid characterisation of upper GI-tract cancers. J. Proteomics 80, 207–215 (2013)
Singhal, N., Kumar, M., Kanaujia, P.K., Virdi, J.S.: MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front. Microbiol. 6, 791 (2015)
Sivoňová, M.K., Tatarková, Z., Jurečeková, J., Kliment, J., Híveš, M., Lichardusová, L., Kaplán, P.: Differential profiling of prostate tumors versus benign prostatic tissues by using a 2DE-MALDI-TOF-based proteomic approach. Neoplasma 68(1), 154–164 (2021)
Smaoui, S., Ben Braïek, O., Ben Hlima, H.: Mycotoxins analysis in cereals and related foodstuffs by liquid chromatography-tandem mass spectrometry techniques. J. Food Qual. (2020)
Souček, K., Kamaid, A., Phung, A.D., Kubala, L., Bulinski, J.C., Harper, R.W., Eiserich, J.P.: Normal and prostate cancer cells display distinct molecular profiles of α-tubulin posttranslational modifications. Prostate 66(9), 954–965 (2006)
Spitaels, F., Wieme, A.D., Janssens, M., Aerts, M., Daniel, H.-M., Van Landschoot, A., Vandamme, P.: The microbial diversity of traditional spontaneously fermented lambic beer. PLoS ONE 9(4), e95384 (2014)
Spitaels, F., Wieme, A.D., Vandamme, P.: MALDI-TOF MS as a novel tool for dereplication and characterization of microbiota in bacterial diversity studies. In: Applications of mass spectrometry in microbiology, pp. 235–256. Springer (2016)
Spitaels, F., Wieme, A.D., Janssens, M., Aerts, M., Van Landschoot, A., De Vuyst, L., Vandamme, P.: The microbial diversity of an industrially produced lambic beer shares members of a traditionally produced one and reveals a core microbiota for lambic beer fermentation. Food Microbiol. 49, 23–32 (2015)
Stafsnes, M.H., Dybwad, M., Brunsvik, A., Bruheim, P.: Large scale MALDI-TOF MS based taxa identification to identify novel pigment producers in a marine bacterial culture collection. Ant. Leeuwen. 103(3), 603–615 (2013)
Stets, M.I., Pinto, A.S., Jr., Huergo, L.F., de Souza, E.M., Guimarães, V.F., Alves, A.C., Cruz, L.M.: Rapid identification of bacterial isolates from wheat roots by high resolution whole cell MALDI-TOF MS analysis. J. Biotechnol. 165(3–4), 167–174 (2013)
Stevenson, L.G., Drake, S.K., Shea, Y.R., Zelazny, A.M., Murray, P.R.: Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of clinically important yeast species. J. Clin. Microbiol. 48(10), 3482–3486 (2010)
Swiatly, A., Horala, A., Hajduk, J., Matysiak, J., Nowak-Markwitz, E., Kokot, Z.J.: MALDI-TOF-MS analysis in discovery and identification of serum proteomic patterns of ovarian cancer. Bmc Cancer 17(1), 1–9 (2017)
Tabandeh, F., Shariati, P., Khodabandeh, M.: Application of two-dimensional gel electrophoresis to microbial systems. Gel Electrophoresis-Principles and Basics (2012)
Tamang, J.P., Watanabe, K., Holzapfel, W.H.: Diversity of microorganisms in global fermented foods and beverages. Front. Microbial. 7, 377 (2016)
Tanigawa, K., Kawabata, H., Watanabe, K.: Identification and typing of Lactococcus lactis by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry. Appl. Environ. Microbiol. 76, 4055–4062 (2020)
Thomas, D., Rathinavel, A.K., Radhakrishnan, P.: Altered glycosylation in cancer: a promising target for biomarkers and therapeutics. Biochim. Biophys. Acta 1875(1), 188464 (2021)
Toby, T.K., Fornelli, L., Kelleher, N.L.: Progress in top-down proteomics and the analysis of proteoforms. Annu. Rev. Anal. Chem. 9, 499–519 (2016)
Tomás-Gallardo, L., Canosa, I., Santero, E., Camafeita, E., Calvo, E., López, J.A., Floriano, B.: Proteomic and transcriptional characterization of aromatic degradation pathways in Rhodoccocus sp. strain TFB. Proteomics 6(S1), S119–S132 (2006)
Tong, S.Y., Schaumburg, F., Ellington, M.J., Corander, J., Pichon, B., Leendertz, F., Peters, G.: Novel staphylococcal species that form part of a Staphylococcus aureus-related complex: the non-pigmented Staphylococcus argenteus sp. nov. and the non-human primate-associated Staphylococcus schweitzeri sp. nov. Int. J. Syst. Evolut. Microbiol. 65(1), 15 (2015)
Tsuchida, S., Umemura, H., Nakayama, T.: Current status of matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF MS) in clinical diagnostic microbiology. Molecules 25(20), 4775 (2020)
Uhlik, O., Strejcek, M., Junkova, P., Sanda, M., Hroudova, M., Vlcek, C., Macek, T.: Matrix-assisted laser desorption ionization (MALDI)-time of flight mass spectrometry-and MALDI biotyper-based identification of cultured biphenyl-metabolizing bacteria from contaminated horseradish rhizosphere soil. Appl. Environ. Microbiol. 77(19), 6858–6866 (2011)
Ullah, A., Heng, S., Munis, M.F.H., Fahad, S., Yang, X.: Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ. Exp. Bot. 117, 28–40 (2015)
van Veen SQClaas, E.C., Kuijper, E.J,: High-throughput identification of bacteria and yeast by matrix-assisted laser desorption ionization-time of flight mass spectrometry in conventional medical microbiology laboratories. J Clin Microbiol 48, 900–907 (2010).
Verstraete, B., Peeters, C., van Wyk, B., Smets, E., Dessein, S., Vandamme, P.: Intraspecific variation in Burkholderia caledonica: Europe vs. Africa and soil vs. endophytic isolates. Syst. Appl. Microbiol. 37(3), 194–199 (2014)
Wang, J., Zhao, J., Nie, S., Xie, M., Li, S.: Rapid profiling strategy for oligosaccharides and polysaccharides by MALDI TOF mass spectrometry. Food Hydrocolloids 124, 107237 (2022)
Wei, Z., Ge, F., Che, Y., Wu, S., Dong, X., Song, D.: Metabolomics coupled with pathway analysis provides insights into sarco-osteoporosis metabolic alterations and estrogen therapeutic effects in mice. Biomolecules 12(1), 41 (2021)
Weigt, D., Sammour, D.A., Ulrich, T., Munteanu, B., Hopf, C.: Automated analysis of lipid drug-response markers by combined fast and high-resolution whole cell MALDI mass spectrometry biotyping. Sci. Rep. 8(1), 1–9 (2018)
Weis, C.V., Jutzeler, C.R., Borgwardt, K.: Machine learning for microbial identification and antimicrobial susceptibility testing on MALDI-TOF mass spectra: a systematic review. Clin. Microbiol. Infect. (2020)
Welham, K.J., Domin, M.A., Johnson, K., Jones, L., Ashton, D.S.: Characterization of fungal spores by laser desorption/ionization time-of-Fight mass spectrometry. Rapid. Commun Mass Spectrom 14, 307–310 (2000)
Wenning, M., Breitenwieser, F., Konrad, R., Huber, I., Busch, U., Scherer, S.: Identification and differentiation of food-related bacteria: a comparison of FTIR spectroscopy and MALDI-TOF mass spectrometry. J. Microbiol. Methods 103, 44–52 (2014)
Wieme, A.D., Spitaels, F., Aerts, M., De Bruyne, K., Van Landschoot, A., Vandamme, P.: Identification of beer-spoilage bacteria using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Int. J. Food Microbiol. 185, 41–50 (2014)
Wieser, A., Schneider, L., Jung, J., Schubert, S.: MALDI-TOF MS in microbiological diagnostics—identification of microorganisms and beyond (mini review). Appl. Microbiol. Biotechnol. 93(3), 965–974 (2012)
Wilmes, P., Wexler, M., Bond, P.L.: Metaproteomics provides functional insight into activated sludge wastewater treatment. PLoS ONE 3(3), e1778 (2008)
Wörner, T.P., Snijder, J., Bennett, A., Agbandje-McKenna, M., Makarov, A.A., Heck, A.J.: Resolving heterogeneous macromolecular assemblies by Orbitrap-based single-particle charge detection mass spectrometry. Nat. Methods 17(4), 395–398 (2020)
Yamazaki, Y., Nakaya, S., Ito, K., Kato, K.: Analysis of high-molecular-weight polyrotaxanes by MALDI-TOF-MS using 3-aminoquinoline-based ionic liquid matrix. J. Am. Soc. Mass Spectrom. 31(6), 1180–1188 (2020)
Yan H, Li Y, Cheng S, Zeng Y.: Advances in analytical technologies for extracellular vesicles. Anal Chem. 93, 4739–4774 (2021)
Zaima, N., Sasaki, T.. Tanaka, H., Cheng, X.W., Onoue, K., Hayasaka, T., Goto-Inoue, N., Enomoto, H., Unno, N., Kuzuya, M. et al. Imaging mass spectrometry-based histopathologic examination of atherosclerotic lesions. Atherosclerosis 217, 427–432 (2011).
Zawada, A., Naskręt, D., Matuszewska, E., Kokot, Z., Grzymisławski, M., Zozulińska-Ziółkiewicz, D., Matysiak, J.: MALDI-TOF protein profiling reflects changes in type 1 diabetes patients depending on the increased amount of adipose tissue, poor control of diabetes and the presence of chronic complications. Int. J. Environ. Res. Public Health 18(5), 2263 (2021)
Zenobi, R.: Single-cell metabolomics: analytical and biological perspectives. Science 342(6163), 1243259 (2013)
Zhang, S.-Y., Shi, W., Cheng, P., Zaworotko, M.J.: A mixed-crystal lanthanide zeolite-like metal–organic framework as a fluorescent indicator for lysophosphatidic acid, a cancer biomarker. J. Am. Chem. Soc. 137(38), 12203–12206 (2015)
Zhu, D., Wang, J., Ren, L., Li, Y., Xu, B., Wei, Y., Xu, J.: Serum proteomic profiling for the early diagnosis of colorectal cancer. J. Cell. Biochem. 114(2), 448–455 (2013)
Zuo, P., Ma, Y., Huang, Y., Ye, F., Wang, P., Wang, X., Xie, X.: High GMFG expression correlates with poor prognosis and promotes cell migration and invasion in epithelial ovarian cancer. Gynecol. Oncol. 132(3), 745–751 (2014)
Funding
The work was not supported by any research grant.
Author information
Authors and Affiliations
Contributions
KUN and NT conceived and designed this review. KUN, NT and QN collected the literature for this review. KUN, NT wrote the manuscript draft. KUN and NT edited this manuscript. All the authors gave final shape to this manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tarfeen, N., Nisa, K.U. & Nisa, Q. MALDI-TOF MS: application in diagnosis, dereplication, biomolecule profiling and microbial ecology. Proc.Indian Natl. Sci. Acad. 88, 277–291 (2022). https://doi.org/10.1007/s43538-022-00085-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43538-022-00085-2