Abstract
Purpose
Sarcoidosis is a non-caseating granulomatous disease for which a role for infectious antigens continues to strengthen. Recent studies have reported molecular evidence of mycobacteria or propionibacteria. We assessed for immune responses against mycobacterial and propionibacterial antigens in sarcoidosis bronchoalveolar lavage (BAL) using flow cytometry, and localized signals consistent with microbial antigens with sarcoidosis specimens, using matrix-assisted laser desorption ionization imaging mass spectrometry (MALDI-IMS).
Methods
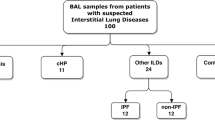
BAL cells from 27 sarcoidosis, 14 PPD- controls, and 9 subjects with nontuberculosis mycobacterial (NTM) infections were analyzed for production of IFN-γ after stimulation with mycobacterial ESAT-6 and Propionibacterium acnes proteins. To complement the immunological data, MALDI-IMS was performed to localize ESAT-6 and Propionibacterium acnes signals within sarcoidosis and control specimens.
Results
CD4+ immunologic analysis for mycobacteria was positive in 17/27 sarcoidosis subjects, compared to 2/14 PPD- subjects, and 5/9 NTM subjects (p = 0.008 and p = 0.71 respectively, Fisher’s exact test). There was no significant difference for recognition of P. acnes, which occurred only in sarcoidosis subjects that also recognized ESAT-6. Similar results were also observed for the CD8+ immunologic analysis. MALDI-IMS localized signals consistent with ESAT-6 only within sites of granulomatous inflammation, whereas P. acnes signals were distributed throughout the specimen.
Conclusions
MALDI-IMS localizes signals consistent with ESAT-6 to sarcoidosis granulomas, whereas no specific localization of P. acnes signals is detected. Immune responses against both mycobacterial and P. acnes are present within sarcoidosis BAL, but only mycobacterial signals are distinct from disease controls. These immunologic and molecular investigations support further investigation of the microbial community within sarcoidosis granulomas.





Similar content being viewed by others
References
M’Koma AE, Blum DL, Norris JL, Koyama T, Billheimer D, Motley S, et al. Detection of pre-neoplastic and neoplastic prostate disease by MALDI profiling of urine. Biochem Biophys Res Commun. 2007;353(3):829–34.
Yanagisawa K, Shyr Y, Xu BJ, Massion PP, Larsen PH, White BC, et al. Proteomic patterns of tumour subsets in non-small-cell lung cancer. Lancet. 2003;362(9382):433–9.
Demirkok SS, Basaranoglu M, Coker E, Karayel T. Seasonality of the onset of symptoms, tuberculin test anergy and Kveim positive reaction in a large cohort of patients with sarcoidosis. Respirology. 2007;12(4):591–3.
Grunewald J, Wahlstrom J, Berlin M, Wigzell H, Eklund A, Olerup O. Lung restricted T cell receptor AV2S3+ CD4+ T cell expansions in sarcoidosis patients with a shared HLA-DRbeta chain conformation. Thorax. 2002;57(4):348–52.
Kajdasz DK, Judson MA, Mohr Jr LC, Lackland DT. Geographic variation in sarcoidosis in South Carolina: its relation to socioeconomic status and health care indicators. Am J Epidemiol. 1999;150(3):271–8.
Morimoto T, Azuma A, Abe S, Usuki J, Kudoh S, Sugisaki K, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J 2007 Oct 24.
Wilsher ML. Seasonal clustering of sarcoidosis presenting with erythema nodosum. Eur Respir J. 1998;12(5):1197–9.
Sharma OP. Murray Kornfeld, American College Of Chest Physician, and sarcoidosis: a historical footnote: 2004 Murray Kornfeld Memorial Founders Lecture. Chest. 2005;128(3):1830–5.
Drake WP, Pei Z, Pride DT, Collins RD, Cover TL, Blaser MJ. Molecular analysis of sarcoidosis tissues for mycobacterium species DNA. Emerg Infect Dis. 2002;8(11):1334–41.
Dubaniewicz A, Dubaniewicz-Wybieralska M, Sternau A, Zwolska Z, Izycka-Swieszewska E, Augustynowicz-Kopec E, et al. Mycobacterium tuberculosis complex and mycobacterial heat shock proteins in lymph node tissue from patients with pulmonary sarcoidosis. J Clin Microbiol. 2006;44(9):3448–51.
Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201(5):755–67.
Chen ES, Wahlstrom J, Song Z, Willett MH, Wiken M, Yung RC, et al. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol. 2008;181(12):8784–96.
Allen SS, Evans W, Carlisle J, Hajizadeh R, Nadaf M, Shepherd BE, et al. Superoxide dismutase A antigens derived from molecular analysis of sarcoidosis granulomas elicit systemic Th-1 immune responses. Respir Res. 2008;9:36.
Carlisle J, Evans W, Hajizadeh R, Nadaf M, Shepherd B, Ott RD, et al. Multiple Mycobacterium antigens induce interferon-gamma production from sarcoidosis peripheral blood mononuclear cells. Clin Exp Immunol. 2007;150(3):460–8.
Drake WP, Dhason MS, Nadaf M, Shepherd BE, Vadivelu S, Hajizadeh R, et al. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun. 2007;75(1):527–30.
Hajizadeh R, Sato H, Carlisle J, Nadaf MT, Evans W, Shepherd BE, et al. Mycobacterium tuberculosis Antigen 85A induces Th-1 immune responses in systemic sarcoidosis. J Clin Immunol. 2007;27(4):445–54.
Oswald-Richter KA, Culver DA, Hawkins C, Hajizadeh R, Abraham S, Shepherd BE, et al. Cellular responses to mycobacterial antigens are present in sarcoidosis diagnostic bronchoalveolar lavage. Infect Immun. 2009;77(9):3740–8.
Dubaniewicz A, Trzonkowski P, Dubaniewicz-Wybieralska M, Dubaniewicz A, Singh M, Mysliwski A. Mycobacterial heat shock protein-induced blood T lymphocytes subsets and cytokine pattern: comparison of sarcoidosis with tuberculosis and healthy controls. Respirology. 2007;12(3):346–54.
Ishige I, Usui Y, Takemura T, Eishi Y. Quantitative PCR of mycobacterial and propionibacterial DNA in lymph nodes of Japanese patients with sarcoidosis. Lancet. 1999;354(9173):120–3.
Abe C, Iwai K, Mikami R, Hosoda Y. Frequent isolation of Propionibacterium acnes from sarcoidosis lymph nodes. Zentralbl Bakteriol Mikrobiol Hyg A. 1984;256(4):541–7.
Eishi Y, Suga M, Ishige I, Kobayashi D, Yamada T, Takemura T, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40(1):198–204.
Yamada T, Eishi Y, Ikeda S, Ishige I, Suzuki T, Takemura T, et al. In situ localization of Propionibacterium acnes DNA in lymph nodes from sarcoidosis patients by signal amplification with catalysed reporter deposition. J Pathol. 2002;198(4):541–7.
Ebe Y, Ikushima S, Yamaguchi T, Kohno K, Azuma A, Sato K, et al. Proliferative response of peripheral blood mononuclear cells and levels of antibody to recombinant protein from Propionibacterium acnes DNA expression library in Japanese patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17(3):256–65.
Seeley EH, Caprioli RM. Molecular imaging of proteins in tissues by mass spectrometry. Proc Natl Acad Sci U S A. 2008;105(47):18126–31.
Pathan AA, Wilkinson KA, Klenerman P, McShane H, Davidson RN, Pasvol G, et al. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol. 2001;167(9):5217–25.
Oswald-Richter KA, Beachboard DC, Zhan X, Gaskill CF, Abraham S, Jenkins C, et al. Multiple mycobacterial antigens are targets of the adaptive immune response in pulmonary sarcoidosis. Respir Res. 2010;11:161.
Breen RA, Hardy GA, Perrin FM, Lear S, Kinloch S, Smith CJ, et al. Rapid diagnosis of smear-negative tuberculosis using immunology and microbiology with induced sputum in HIV-infected and uninfected individuals. PLoS One. 2007;2(12):e1335.
Fuhrmann S, Streitz M, Kern F. How flow cytometry is changing the study of TB immunology and clinical diagnosis. Cytometry A. 2008;73(11):1100–6.
Jafari C, Ernst M, Kalsdorf B, Greinert U, Diel R, Kirsten D, et al. Rapid diagnosis of smear-negative tuberculosis by bronchoalveolar lavage enzyme-linked immunospot. Am J Respir Crit Care Med. 2006;174(9):1048–54.
Cornett DS, Mobley JA, Dias EC, Andersson M, Arteaga CL, Sanders ME, et al. A novel histology-directed strategy for MALDI-MS tissue profiling that improves throughput and cellular specificity in human breast cancer. Mol Cell Proteomics. 2006;5(10):1975–83.
Hiramatsu J, Kataoka M, Nakata Y, Okazaki K, Tada S, Tanimoto M, et al. Propionibacterium acnes DNA detected in bronchoalveolar lavage cells from patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20(3):197–203.
Smith J, Manoranjan J, Pan M, Bohsali A, Xu J, Liu J, et al. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun. 2008;76(12):5478–87.
van Ingen J, De Zwaan R, Dekhuijzen R, Boeree M, van Soolingen D. Region of difference 1 in nontuberculous Mycobacterium species adds a phylogenetic and taxonomical character. J Bacteriol. 2009;191(18):5865–7.
Barksdale L, Kim KS. Propionibacterium, Corynebacterium, Mycobacterium and Lepra bacilli. Acta Leprol. 1984;2(2–4):153–74.
Sahiratmadja E, Alisjahbana B, de Boer T, Adnan I, Maya A, Danusantoso H, et al. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect Immun. 2007;75(2):820–9.
Furukawa A, Uchida K, Ishige Y, Ishige I, Kobayashi I, Takemura T, et al. Characterization of Propionibacterium acnes isolates from sarcoid and non-sarcoid tissues with special reference to cell invasiveness, serotype, and trigger factor gene polymorphism. Microb Pathog. 2009;46(2):80–7.
Atarashi R, Moore RA, Sim VL, Hughson AG, Dorward DW, Onwubiko HA, et al. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods. 2007;4(8):645–50.
Chaurand P, Schwartz SA, Reyzer ML, Caprioli RM. Imaging mass spectrometry: principles and potentials. Toxicol Pathol. 2005;33(1):92–101.
Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327(5964):466–9.
Acknowledgements
We thank Joan Isom, L.P.N. for her assistance with data collection and coordination and Jamie Allen for sample preparation for MALDI analysis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funding
This work was supported by National Institutes of Health grants (RO1-HL83839; R01-AI65744; MO1 RR-00095, The Eliassen Foundation and Vanderbilt CTSA grant 1 UL1 RR024975 to W.P.D., T32 HL069765 to K.R., 5RO1-GM058008-11 to R.M.C.), funding from NFCR Center for Proteomics and Drug Action to R.M.C., and Vanderbilt Ingram Cancer Center Core Support Grant 5P30-CA068485-13 to E.H.S.
Rights and permissions
About this article
Cite this article
Oswald-Richter, K.A., Beachboard, D.C., Seeley, E.H. et al. Dual Analysis for Mycobacteria and Propionibacteria in Sarcoidosis BAL. J Clin Immunol 32, 1129–1140 (2012). https://doi.org/10.1007/s10875-012-9700-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-012-9700-5