Abstract
Listening to, and acting on, the voices of children and families during clinical research and innovation is fundamental to ensuring enhanced pediatric health care, medicines development, and technological advances. While this is often discussed as an important step in ensuring patient-centered care, involving children and families across the life cycle of clinical research is not currently routine. The pediatric research community needs to address how to meaningfully involve children and families if they are to succeed in designing clinical research that suits the needs of pediatric patients and their families. This paper describes how an international community working under the umbrella International Children’s Advisory Network (iCAN) and European Young Person’s Advisory Group Network (eYPAGnet) has involved children and families in the design and delivery of pediatric clinical research. It offers practical solutions through various case studies assessed against seven patient engagement quality criteria within the Patient Engagement Quality Guidance (PEQG) tool, highlighting some of the lessons learnt from involving and engaging with children and families across different stages of clinical research, including pediatric trials for drug development programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Undertaking pediatric clinical research presents unique challenges. Barriers to successful study completion include parental and patient expectations, motivations, and attitudes regarding the benefit and burden of study participation; a higher rate of early patient drop-out; the inherent characteristics of the pediatric population; and the intensity of study procedures and associated demands on patients and families. These barriers all contribute to reduced patient participation or retention, resulting in far fewer therapeutic advances for children and young people [1]. One way to overcome some of these barriers is to involve children and families throughout the research and development process, from identifying unmet needs and patient priorities through to the dissemination of study findings. This involvement also respects the rights of children and young people.
The potential influence and impact that patients and the public (including children and young people) have on the design and conduct of clinical research are increasingly being recognized by researchers, funding bodies, and journal editors. In health and social care research, this is often badged under the umbrella term ‘Patient and Public Involvement’ (PPI), which has been defined as “research…carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them”. [2]
While policy and evidence strongly advocate for the active and meaningful PPI in clinical research, it is more difficult to define how this is embodied in practice, especially where children are concerned. Most of the published literature focuses on PPI work with adults and the reporting of children and family involvement in clinical research being scarce [3,4,5]. As a result, understanding of how involvement works for children and families, what the key challenges are, and what needs to be in place to make it meaningful for those involved is limited [6].
Those who work with children and families understand that involving them meaningfully requires tailoring practices and methods to suit their needs and requirements [7]. Involving children, in particular, is complex and multi-dimensional, requiring consideration of four key areas:
-
Level of participation (degrees of power-sharing between adults and children),
-
Focus of decision-making (individual or collective),
-
Model of participation (consultation, collaboration, child-led), and
-
Clarity of the term ‘children’ which covers a diverse group who are not only different in their personal circumstances (age, sex, ethnicity, culture, disability, social and economic circumstances) but their changing interests and capacities as they grow older [8].
We write this paper as an international community of advocates for children and young people (hereon children) and family involvement in all stages of the research process. The goal of this paper is to share our lessons learned and practical tips to enhance meaningful PPI with children and families. All authors are linked in various capacities to the International Children’s Advisory Network, Inc. (iCAN) http://icanresearch.org/ and the European Young Person’s Advisory Group Network (eYPAGnet) http://eypagnet.eu/ who work alongside pharmaceutical companies, academic researchers, regulatory agencies, ethics committees, and others to make sure children and families are involved in the decision-making processes [9]. The networks are renowned for working in partnership with children through the forum of a Young Person’s Advisory Group (YPAG), which first emerged in 2006 in the UK [10]. Both networks are led by the principle that YPAG activities should transform children from research subjects into research partners. Members of eYPAGnet and iCAN YPAGs normally consist of a mix of children between the ages of 8–18 years old with either experience of having a chronic condition, hospitalization, clinical trial participation, or a general interest in science and research [11]. YPAGs are stable organizations that provide opportunities for members to encounter clinical research and learn about some key features of research that they can influence. The networks also have experience of working with other, ad hoc, groups of children and families (who are not members of YPAGs) from various backgrounds, interests, and experiences of childhood illnesses as and when required [12].
Published principles or quality PPI standards [13] may tell us what we should aspire to. However, they often lack practical details on implementing PPI in practice, especially with children and families [14]. To share our experience with the practicalities of involving children and families throughout the research process, the authors have used a Patient Engagement Quality Guidance (PEQG) tool [15] as a guide to reflect on the lessons learned from PPI with children and families. The PEQG tool was developed by the Patient Focused Medicines Development initiative, a not-for-profit collaborative organization to benefit patients and health stakeholders by encouraging patient-centered healthcare systems [16]. The tool was developed with over 70 experts in 51 organizations as a practical guide to planning, developing, and assessing the quality of patient involvement activities and projects. The tool contains seven quality measures, which include the following: (1) Shared Purpose, (2) Respect and accessibility, (3) Representativeness of stakeholders, (4) Roles and responsibilities, (5) Capacity and capability for engagement, (6) Transparency and communications and documentation, and (7) Continuity and sustainability. A brief description and rationale for each measure can be found in Table 1.
Accordingly, the aims of this paper are as follows:
-
1.
Summarize pertinent case studies
-
2.
Identify lessons learnt and common themes from the case studies
-
3.
Review the quality of the case studies in the light of the PEQG tool
Methods
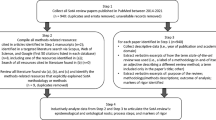
Eleven PPI activities with children and families were identified by the authors and assessed using the PEQG tool. The activities were purposively chosen to provide a selection of requests received to partner with children and families from regulatory bodies, life science and biotech companies, and academic institutions (hereon referred to as researchers). Six of the authors (4 from eYPAGnet, and 2 from iCAN Inc) responsible for coordinating and facilitating children and family involvement completed 14 case study templates (see Table 2) and shared them with the wider authorship team before agreeing on the final eleven examples. Three case studies were excluded as they focused on engagement activities (e.g., inviting children to conferences or producing a video to tell their story or to promote the importance of involving children in clinical research) as opposed to focusing on the actual involvement activity itself. Attention was paid to selecting cases that could represent a variety of activities across different stages of the research process, working with different populations. Each case study was reviewed by the team to ensure that the guidance linked to the PEQG tool was systematically followed to ensure an open and transparent reporting process that highlighted both positive and negative lessons learned.
Results
Of the eleven activities, five activities fell under the category of research priority setting. Three involved children and/or families throughout all stages of the research process and three activities fell under the category of dissemination, communication, and post-approval phases. The types of involvement included virtual panel meetings, Delphi surveys, focus groups, study management membership, consensus meetings, and workshops. Eight of the eleven case studies involved children only: one involved parents only, and two involved both parents and young adults. Table 3 summarizes the eleven activities including who was involved, outcomes, and gaps in practice. A link to a full description of the case studies is included in Table 3.
We describe the overall lessons learned according to the PEQG criteria.
Shared Purpose
The development of a shared purpose is fundamental to optimal outcomes of PPI. Researchers come with an intention which often needs to be shaped by mediators before it can become a purpose that is shared by the researcher and the participants in PPI.
All eleven activities describe some form of recruitment process prior to the PPI activity commencing to identify the most relevant candidates for the tasks. These processes start the process of establishing a shared purpose. The recruitment process was simpler for activities targeting members of an existing YPAG, entailing sharing the opportunity with the group/s, consenting for the activity to take place, and then supporting members throughout the activity. Most often, this entailed a considerable amount of correspondence and meetings between the researcher or research team and a facilitator who would then relay information to group members prior to the activity taking place. This iterative process allowed opportunity for facilitators and group members to shape a shared purpose before the activity started. If activities that were more ad hoc and/or required working with children and families with a particular expertise (i.e., the experience of living with a rare condition) this required additional steps in the process, such as production of flyers on how to get involved, expression of interest forms, terms of reference documents, or consent documentation that clearly explained the roles required. The terms of reference or consent forms detailed the aims of the activity, the remit and membership of the group, and other information (including payment and expenses, accountability, and confidentiality). The terms of reference or consent forms served to induct both children and parents into an activity. These forms were also used as a resource to fully inform participants about the activity and their role and to manage expectations regarding the activity throughout its course.
Lessons learned:
-
Managing expectations of PPI for all parties requires a considerable amount of time prior to any activity taking place. Developing a shared purpose is fundamental for optimal outcomes.
Respect and Accessibility
Regardless of the type of involvement chosen (i.e., focus group, consensus meeting, etc.), it was important that such activities were planned around the children and families’ schedules, either after work/school hours or during weekends, and not around the needs of research professionals’ availability (whenever possible). Voting polls circulated in advance of activities are helpful to identify convenient dates and times for children and parents to meet. Opportunities to attend via video teleconference/zoom should also be offered as alternatives to attending face to face, which became essential during the COVID-19 pandemic. Additional factors included making sure there was an allocated budget for meetings, reimbursement of travel, refreshments, and payment for children and parent contributions (although iCAN and some YPAGs do not pay their members but are reimbursed by the research team to pay the running costs of the networks). Those who did pay children and parents for their time found this quite complicated due to issues such as the requirement of having honorary contracts (for those aged 18 + and parents), tax and benefits issues. Paying children was particularly difficult so most YPAGs chose to offer gifts of appreciation in the form of ‘vouchers’ as a thank you for their contributions as opposed to cash or bank transfer (as not all children have bank accounts depending on their age). It is important to be open and transparent about payments or gifts of appreciation before involvement begins, and this can be achieved by having a payments policy in place that is agreed by all members.
Lesson learned:
-
Being flexible around the timing of activities was seen to be the biggest factor in recruiting and retaining children and families throughout the activities.
-
Realistic resources (including money, staff, time) should be allocated for PPI.
Representativeness of Stakeholders
Diversity and representativeness of children and families is an issue not just for PPI, but for clinical research participation in general. It is essential that researchers consider who their target for participation is, which in turn will aid the decision on who needs to be involved. PPI facilitators are best placed to have a conversation with researchers and to organize the most suitable activity with the relevant stakeholders. Facilitators also support the selection of participants that meet the diversity profile agreed upon with the researcher. As the eleven case studies highlight, not all contributing children were members of a YPAG. When the case study facilitators opted for a different involvement model, they did this because of the condition being studied, which required specific input from children living with the disease to avoid tokenism. Under these circumstances, direct involvement of children living with a disease can have more impact on both the research design and on those who get involved.
Lessons learned:
-
Organizing activities that involve those affected by a certain disease or condition requires more planning, time, and resources to ensure representativeness in terms of gender, disability, age, country diversity, and inclusion of children and families from disadvantaged socio-demographic backgrounds. This requires working in partnership with patient organisations and with clinicians working directly with patients and families affected by the disease.
-
Involving both children and their parents in the activity provides a holistic view of the impact of the disease on the child and the family, but also requires additional planning to avoid parents dominating the conversations. A solution to this approach is to hold separate meetings for the children and adults, which requires not only more planning but also additional facilitators to manage and record the discussions.
Roles and Responsibilities
The researcher is responsible for defining their requirements and identifying the resources needed to conduct the PPI activity. Ideally, the researcher can use feedback from facilitators and groups to make adaptions to activities before they take place. It is essential that the researcher sends timely feedback to participants involved in PPI activities about how their input has influenced the research or activity.
In some of the case study activities, researchers, or clinicians with expertise in the disease area or methodology were invited to join activity sessions to explain the study/project in detail and answer any questions that children and families had. This required many conversations between the facilitators and researchers before the activity took place so that everyone had a clear understanding of their roles during and after the sessions.
The facilitator’s role is to guide and support the researcher to make sure the planned activity is fit for their needs. Specific facilitator responsibilities may include activity design (i.e., selecting the best methodology for involvement), logistics (organizing meetings, facilitating discussions, etc.), and evaluation (reporting feedback to researchers, evaluating activities). More importantly, the facilitator’s role is to support children and families throughout the process so that their experience of involvement is a positive one and productive for all parties.
Lessons learned:
-
A critical success factor is having skilled facilitators with experience working with children and families.
-
Regardless of role, all participants in a PPI activity need to cooperate in the activity respectfully by complying to agreed role descriptions and terms of references. Having a clear memorandum between all parties, especially for long-term involvement activities is extremely helpful.
Capacity and Capability for Engagement
Capacity and capability can be managed directly by skilled facilitators. Regardless of the activity, none of the case studies offered any formal (structured or accredited) training for children or families. That is not to say that formal training is not offered to children and families, but for the case studies a more flexible approach to learning was chosen and driven by individual needs and preferences. This included brief presentations and educational videos about a particular disease or research methodology during regular meetings, organizing topic-specific workshops (i.e., core outcome setting methods, etc.), and group discussions that generated a culture of learning and collaboration.
Lessons learned:
-
Skilled, experienced facilitators offer a direct contact point of support to those who want to be involved in activities and to research teams with little experience of PPI.
-
Flexible approaches to learning opportunities for children and families depends on individual needs and preferences.
Transparency, Communications, and Documentation
Regardless of the PPI activity and type of involvement, it is clear from our experiences that tailoring communications to suit the needs of children (e.g., age and ability appropriate information) and families is essential. In some cases, the facilitators highlighted that recording in-depth notes for each PPI activity is important to capture what children and parents expressed. This required gaining permission for sessions to be recorded and transcribed for the purposes of publication (whether a report to the researcher or journal article), which is quite time-consuming. Another important consideration is to give participants feedback in a timely manner on how the study team acted upon their insights. Without this feedback, those who take part are left wondering about the value of their input and ultimately what impact it had on the activity.
Lessons learned:
-
Tailored communication equipped children to get involved in activities and, more importantly, to stay involved and engaged throughout the process.
Continuity and sustainability
Regardless of the length and type of activity, building meaningful relationships with children and families before, during, and after the activity is key. Children and families want to know that their time is valued, and their opinions are listened to and acted upon. Self-reflection, evaluation, and feedback mechanisms on the processes and value of the PPI activity are elements that need to be built into the activity from the very beginning. Sometimes these are an afterthought, resulting in missed opportunities to gather children, families, and researchers’ views of the strengths, weaknesses, and areas for improvement. At the very least, those who take part in activities should be provided with some written feedback about their contributions and thanked for their time and efforts. One of the biggest challenges for PPI facilitators is obtaining feedback in a timely manner. Realistic financial resources are also key to sustain PPI activities. Each PPI activity expands the experience of all participants and reduces the costs to future PPI activities.
Lessons learned:
-
Self-reflection, evaluation and feedback mechanisms on the processes and the value of PPI need to be embedded into practice.
-
Sustainability requires adequate financial resources.
Discussion
There is an ongoing need to share examples of best practice PPI with children and families in the clinical research process to ensure that approaches are robust and meaningful to those who get involved. This paper reports on experiences of involving children and families at various stages of the clinical research process using a Patient Engagement Quality Guidance (PEQG) tool to guide reflections. The paper also links to a substantial corpus of projects that provide worked examples for PPI practitioners and people who commission PPI. The process of using the PEQG tool was an informative way of critiquing our experiences and practice of PPI with children and families. Systematic reflection on these experiences unearthed some important lessons and led to a comprehensive synthesis of lessons learned. These lessons contribute to the existing evidence base [6, 17, 18] by providing practical examples of how children, families, and researchers can work together, the difficulties encountered, and what is needed for meaningful PPI. The lessons learned about the process of involvement have shown that meaningful PPI requires support from skilled facilitators with experience of working with children and families and who can offer a direct point of contact to those who want to be involved in activities as well as research teams with little experience of PPI with children and families. Skilled facilitators can advise on the most suitable involvement approach and advise on planning child-appropriate activities, which saves a lot of time for researchers. This aligns to recently published findings [6] which highlighted the need for approaches adapted to each PPI activity. Furthermore, having these conversations early in the process helps to plan accordingly and review regularly how often children, and families will meet (i.e., around school/work and family commitments) and being flexible to the needs of those who want to be involved. This is particularly important for those affected by long-term chronic conditions who may not participate as frequently due to illness, medical emergencies, or caring responsibilities.
The lack of diversity within PPI is a well-recognized issue [19, 20] Therefore, it is essential during the planning process to consider the target group for a specific PPI activity. For some activities, the stability and expertise of a YPAG is useful. On the other hand, some of the case studies in this paper highlight that asking members of an existing YPAG is not always the best approach to gain children’s views, especially those living with certain chronic or rare conditions. As highlighted in previous literature [21, 22] this then requires looking at other approaches to involve those affected by the disease that is being studied, which in turn requires more planning, time, resources, and established links with key stakeholders (e.g., parent organisations, clinicians, charities, etc.). Another consideration is whether activities should include both parent’s and children’s views. If so, this requires careful planning and management of the activities to ensure that parents do not dominate the conversations. Regardless of the type or stage of involvement time and resources need to be invested to keep children and families motivated and engaged.
Skilled development training (i.e., in research methods, child rights/advocacy, communication skills, etc.) is one way to keep children and families motivated, especially for long-term projects/activities such as YPAG membership. However, similar to other published research findings [18, 23, 24] we found in some of the case studies that this level of training was viewed as unnecessary when children and families preferred informal conversational approaches to help them understand their roles. Thus, adopting a more flexible, informal induction into the activity with clear terms of reference, consent documentation and support from the PPI facilitator was felt to be sufficient.
Reimbursements are also a valuable and tangible demonstration of appreciation for children and families. No one should incur out of pocket expenses when taking part in PPI activities; at a minimum, travel and subsistence costs should be covered. This especially has implications for low-resource organisations with minimal budgets and impacts their ability to meaningfully involve children and families. One way to overcome this is to build in suitable budgets as part of grant applications to specifically support PPI activities, including budgeting for a skilled facilitator with experience working with children and families. Another way to keep children and families motivated is to provide feedback on the outcomes and impact of their input (both the impact on the study design and on the young people themselves). Van Schelven and colleagues (2020) also highlighted feedback as a motivational factor [17]. However, providing such feedback requires time to evaluate the activities, and incorporation of a clear tool or process for collecting and analyzing the feedback from participants, and researchers.
Limitations
We note some limitations of this paper. The cases were gathered as a sample of convenience and not as a systematic survey. Each of the case studies had included an evaluation of children and families’ experiences of taking part in activities. However, the thematic reflections within the case studies were undertaken by the facilitators who had led the PPI activities. The timelines, effectiveness, impact, and the magnitude of the costs of PPI activities were not addressed in our analysis. Nevertheless, we believe that this paper provides useful insight into how to conduct PPI for industry and academic clinical researchers, and introduces a useful quality assessment for PPI with children and families.
Conclusion
We no longer have to defend the view that involving children and families in the design and conduct of clinical research benefits both research and those who get involved. However, we must find ways to meaningfully involve children and families in these processes. Using the PEQF tool was helpful to self-reflect, capture, and share our learnings to guide future PPI projects with children and families. We suggest that the planning of future PPI projects will benefit from addressing the PEQF criterion to identify potential gaps prior to starting any PPI work with children and families. The lessons provided here provide a baseline for continuous improvement of the processes of PPI with children and families. High-quality PPI requires resources in time and money, skilled facilitators, and timely feedback from researchers.
References
Children and clinical research: ethical issues. 2015, Nuffield Council on Bioethics
INVOLVE. Briefing notes for researchers: public involvement in NHS, public health and social care research. 2012, NIHR INVOLVE.: Eastleigh
Brady LM, Preston J. How do we know what works? Evaluating data on the extent and impact of young people’s involvement in English health research. Res All. 2020;4(2):194–206.
Moore T, Noble-Carr D, McArthur M. Changing things for the better: the use of children and young people’s reference groups in social research. Int J Soc Res Methodol. 2016;19(2):241–56.
Bird D, Culley L, Lakhanpaul M. Why collaborate with children in health research: an analysis of the risks and benefits of collaboration with children. Arch Dis Child. 2013;98(2):42–8.
Flynn R, Walton S, Scott SD. Engaging children and families in pediatric Health Research: a scoping review. Res Involv Engagem. 2019;5:32–32.
Mitchell SJ, et al. An evaluation of the experiences of young people in Patient and Public Involvement for palliative care research. Palliat Med. 2021;35(4):793–8.
Sinclair R. Participation in practice: making it meaningful, effective and sustainable. Child Soc. 2004;18(2):106–18.
Tsang VWL, et al. A novel way to engage youth in research: evaluation of a participatory health research project by the international children’s advisory network youth council. Int J Adolesc Youth. 2020;25(1):676–86.
Newman J, et al. Medicines for chidren: Reflecting on how young people improve research, in Involving Children and Young People in Health and Social Care Research. Online access with subscription: Proquest Ebook Central, ed. J. Fleming and T. Boeck. 2012: Routledge.
Tsang VWL, et al. Role of patients and parents in pediatric drug development. Ther Innov Regul Sci. 2019;53(5):601–8.
Gaillard S, et al. Involving children and young people in clinical research through the forum of a European Young Persons’ Advisory Group: needs and challenges. Fundam Clin Pharmacol. 2018;32(4):357–62.
National Standards for Public Involvement. 2019; Available from: https://sites.google.com/nihr.ac.uk/pi-standards/home. Accessed 21 Sept 2021
Wilson O, Daxenberger L, Dieudonne, et al. A rapid evidence review of young peoples involvement in health research. 2020, Wellcome: London.
Patient Engagement Quality Guidance. 2020; https://synapse.pfmd.org/initiatives/the-patient-engagement-quality-guidance-peqg. Accessed 21 Sept 2021
Deane K, et al. Co-creation of patient engagement quality guidance for medicines development: an international multistakeholder initiative. BMJ Innov. 2019;5(1):43–55.
van Schelven F, et al. Patient and Public Involvement of young people with a chronic condition in projects in health and social care: a scoping review. Health Expect. 2020;23(4):789–801.
Flotten KJO, et al. Adolescent and young adult patients as co-researchers: a scoping review. Health Expect. 2021;24(4):1044–55.
Fran B. Developing user involvement in Social Work Education (No. 29, Workforce Development Report. 2009, SCIE, London
Brett J, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. 2014;5:637.
Guerreiro A, Fløtten K. Article 12: The Translation into Practice of Children’s Right to Participation in Health Care. The United Nations Convention on the Rights of the Child. 2017, Brill | Nijhoff: Leiden, The Netherlands. p. 681–707
Lightfoot J, Sloper P. Having a say in health: involving young people with a chronic illness or physical disability in local health services development. Child Soc. 2003;17(4):277–90.
Postma L, et al. The attitudes of healthy children and researchers towards the challenges of involving children in research: an exploratory study. Res Involv Engagem. 2021;7(1):30.
Dudley L, et al. A little more conversation please? Qualitative study of researchers’ and patients’ interview accounts of training for patient and public involvement in clinical trials. Trials. 2015;16(1):190.
Funding
This project was supported by funding from the National Institute for Health Research (NIHR) Alder Hey Clinical Research Facility (CRF).
Author information
Authors and Affiliations
Contributions
All authors were responsible for the conception and design of the paper. All authors were involved in the acquisition, analysis, and/or interpretation of the data captured in the case studies. JP drafted the paper and subsequent re-drafts. SG and MT critically revised the paper for important intellectual content. All authors approved the final version of the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this paper have no conflict of interest. This publication reflects the author's view and neither the NIHR or any Associated Partners are responsible for any use that may be made of the information contained therein.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Preston, J., Nafria, B., Ohmer, A. et al. Developing a More Tailored Approach to Patient and Public Involvement with Children and Families in Pediatric Clinical Research: Lessons Learned. Ther Innov Regul Sci 56, 948–963 (2022). https://doi.org/10.1007/s43441-022-00382-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-022-00382-4