Abstract
The evaluation of dolutegravir based on available preclinical and clinical studies reveals a risk of central nervous system (CNS) disorders associated with long-term use of the drug. The available literature on the pharmacokinetics of the drug, including its penetration of the blood–brain barrier, was reviewed, as well as clinical trials assessing the incidence of adverse effects in the CNS and the frequency of its discontinuation. This paper also summarizes the impact of factors affecting the occurrence of CNS disorders and indicates the key role of pharmacovigilance in the process of supplementing knowledge on the safety of drugs, especially those that are newly registered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a result of damage to the blood–brain barrier (BBB) in human immunodeficiency virus (HIV)-infected patients, the virus invades the central nervous system (CNS) in the first few days, causing the development of a long-term inflammatory process and damage to the nerve cells. This leads to a variety of CNS disorders. Before the era of combined antiretroviral therapy (cART), HIV-associated dementia (HAD) and distal sensory polyneuropathy were experienced by as many as 35% of patients [1]. HAD now occurs in 2–5% of patients, and polyneuropathy has been almost completely eliminated [1]. Other neurological disorders include meningoencephalitis, Guillain-Barré syndrome, polymyositis, transverse myelitis, cranial or peripheral nerve damage, and psychiatric disorders, including anxiety and restlessness, depressive and psychotic disorders, and sleep disorders [2].
Drugs that penetrate well into the CNS are used to reduce the risk of central symptoms in HIV patients, leading to a reduction in HIV RNA in the cerebrospinal fluid (CSF). However, the use of increasingly effective antiretroviral drugs (ATRs) may also affect the CNS and cause adverse effects; the occurrence of neuropsychiatric disorders may lead to discontinuation of treatment [3].
The problem of neurotoxicity may be linked to many groups of ATRs. Such reports have appeared in the case of dolutegravir (DTG), one of the most commonly used drugs belonging to a new class of ATRs prescribed to patients infected with HIV-1. DTG is a second-generation integrase chain transfer inhibitor (INSTI), interrupting an enzyme involved in the reproduction of HIV. DTG works by blocking the transport and incorporation of proviral DNA into the host T-cell genome, inhibiting further steps in the replication process. This slows the multiplication and spread of the virus [4]. DTG was registered for the first time on August 12, 2013, by the Food and Drug Administration (FDA), and it was approved for marketing by the European Medicines Agency (EMA) on January 16, 2014, for both previously untreated and treated patients, including patients with resistance to integrase inhibitors [5].
The INSTI group also includes raltegravir (RAL), elvitegravir (EVG), bictegravir (BIC), and cabotegravir (CAB). Clinical trials in which oral INSTIs were used showed high efficiency with a rapid decrease in HIV RNA already in the fourth week from the start of treatment. In a study that compared the effectiveness of DTG with BIC, 76–80% of ATR-naïve HIV-positive patients presented with virological suppression as early as 4 weeks after starting [6,7,8,9,10]. The INSTIs, including DTG, are well tolerated and considered safe and recommended as first-line drugs in HIV-positive patients as part of combination therapy with other ATRs [10,11,12].
Selected pharmacokinetic parameters of dolutegravir in preclinical studies
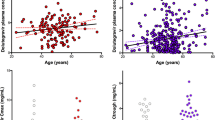
A few preclinical studies have evaluated the neurotoxicity of DTG. Moss et al. [13] revealed the presence of DTG in the brain up to 10 h after administration of a single dose of 50 mg/kg [14C] DTG to male rats. However, DTG radioactivity was low (< 2% of blood radioactivity). A recent mouse pharmacokinetics study of tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and DTG showed that each of the drugs had low brain exposure, with only TDF achieving concentrations above the 90% inhibitory concentration (IC90) [14]. According to the authors, the low concentration of DTG in the brain may be the result of a low degree of non-binding (FUB) to plasma proteins, limiting diffusion through the BBB (Fig. 1).
The effectiveness of a given drug depends on the state of equilibrium between the unbound and bound state of the drug in plasma. The more of the free drug present in the serum, the more effectively it penetrates or diffuses the cell membranes [15]. Studies conducted in pregnant mice have shown that DTG easily reaches the CNS and inhibits the activity of matrix metalloproteinases [16]. In another study, Hinckley et al. [17] showed that DTG has a small but significant effect on neuronal growth.
Bioavailability
Focusing on issues other than pharmacodynamic efficacy, numerous therapeutic failures correspond directly to poor bioavailability of the drug. Absorption in the gastrointestinal tract and passage through the BBB are two pharmacokinetic processes that should be taken into consideration during pharmacotherapy. Though there are various routes of drug administration, oral administration is highly preferred due to comfort and patient compliance. Early estimation of oral bioavailability (i.e., the fraction of the dose that reaches the bloodstream) following oral administration is a key criterion for making therapeutic decisions. Bioavailability depends on many factors, including absorption in the gastrointestinal tract.
Egan et al. [18] developed a routinely used prediction tool to discriminate between well and poorly absorbed molecules based on the 2D plane of drug physicochemical parameters. By analyzing lipophilicity [described by the n-octanol/water partition coefficient (log P)] and polarity [determined by the polar surface area index (PSA)], the proposed model estimates the probability of molecule absorption. Due to the most likely area of absorption being elliptical in shape, it has been called the BOILED-Egg (Brain Or IntestinaL Estimate D permeation method) [19, 20]. Figure 2 shows the Egan egg graph comparing the tested ATRs and selected commonly used drugs. Colored areas represent the optimal prediction range (above 93%) for the brain (BBB, yellow) and gastrointestinal [human intestinal absorption (HIA), white] penetration. Gray represents the area where brain and gastrointestinal penetration can occur, but the probability is significantly below the optimal value.
The Egan egg chart compares the study drugs and selected commonly used drugs. Drugs studied: 3TC lamivudine, ABC abacavir, BIC bictegravir, CAB cabotegavir, COBI cobicistat, DRV darunavir, DTG dolutegravir, EVG elvitegravir, FTC emtricitabine, RAL raltegravir, TDF tenofovir, ATV atazanavir. The colored areas represent the optimal prediction range (above 93%) of penetration for the brain (BBB, yellow) and gastrointestinal tract (HIA, white). Gray indicates the area where penetration into the brain and gastrointestinal tract may occur, but its probability is below the optimal value. A molecule predicted to be effluated from the CNS by P-glycoprotein (PGP + , blue dots) or not (PGP-, red dots). The names of the compounds are indicated in different font colors, i.e., black corresponds to the drugs tested, and purple corresponds to the selected drugs for comparison. Prognostic data were obtained using pkCSM pharmacokinetics [15, 18]
DTG (similar to EVG, BIC, CAB, ABC, FTC, 3TC; Fig. 2) is characterized by a high probability of absorption from the gastrointestinal tract. However, none of the analyzed drugs penetrate the BBB, confirming their low penetration of the CNS. Labarthe et al. showed that DTG is a substrate for the efflux transporters ABCB1 and ABCG2 [mainly P-glycoprotein (PGP)] present in the BBB [14]. However, Tisseraud et al. [21] showed a very low positron emission tomography (PET) signal in the brains of macaques in a PET imaging study using [18F]DTG, which also suggested a low penetration of DTG into the CNS. PGP acts as a biological barrier; therefore, its substrates (toxins and xenobiotics) are excreted from cells, and inhibitors imply specific adverse effects. The presented analysis (Fig. 2) indicates whether a given compound can be a substrate for PGP. Notably, DTG is cleared from the CNS by PGP, which provides another explanation as to why DTG has poor brain penetration in animal studies [21].
DTG penetration of the brain barrier
Biological barriers provide protection against invasion by pathogens and diseases, but also complicate drug delivery [22]. The adult human brain has five barrier interfaces that regulate molecular traffic into the brain parenchyma: the BBB [23, 24], blood–cerebrospinal fluid barrier [25], blood–arachnoid barrier [26], the circumventricular organs [27], and ependyma [28]. The barriers between the blood and extracellular matrix of the brain form tight endothelial cell (EC) structures joined together by protein junctions. The BBB is formed by the ECs lining the cerebral microvessels and separates the blood from the interstitial fluid of the brain [29]. The choroid plexus epithelium sits between the blood and the ventricular CSF and forms the blood–CSF barrier. The arachnoid barriers are formed by the epithelium sandwiched between the blood and the subarachnoid CSF. These three barrier layers participate in limiting and regulating molecular exchange at the interface between the blood and nervous tissue or its fluid spaces [30]. The biological barriers’ intrinsic functions affect both drug delivery and uptake, hindering effective therapeutic outcomes. In addition to hindering treatment options, they also reduce the bioavailability of drugs in areas protected by the barriers, which can ultimately lead to increased drug resistance. On the other hand, inappropriate intervention at these barriers can disrupt their natural functions, increasing the risk of infection or opening channels for pathogens [19]. The PGP protein co-creates cell barriers and is a protein membrane transporter that actively removes harmful substances from cells. PGP localizes inside important organs, such as the brain, placenta, liver, intestine, and kidneys, where it plays a role in the distribution and elimination of drugs from the body. PGP is also found in capillary ECs that function as blood–brain, blood–testis, and blood–placental barriers [31]. The protein forms a transmembrane one-way efflux pump utilizing ATP in active transport of substances from cells against their concentration gradients. PGP has also been shown to be strongly involved in multidrug resistant diseases [32]. This seems to be important in the treatment of HIV infection [34]. In most tissues, PGP is present on the cell’s free surface, facing the lumen of the vessels. This location indicates its most important function, preventing the penetration of xenobiotics (mainly drugs) into the nervous tissue in the brain via removal of xenobiotics from the ECs back into the blood. Thus, PGP influences the pharmacological profile of numerous substances and their metabolites, as it alters their oral bioavailability, absorption in certain tissues, and elimination from the body [33]. As a multidrug transporter, PGP is characterized by broad substrate specificity, as it recognizes a very large number of compounds of various chemical structures and molecular weights (from 330 to 4000 Da). PGP transports hydrophobic or neutral substances or cations, but not anions. The log P ≈ 2.2 for DTG [34] means that it is a medium hydrophobic substance that is only partially bioaccumulating [35]. Numerous pharmacological studies have shown that DTG is immediate pumped back into the blood by PGP when it enters the ECs as a substrate [14]. Disruption of the BBB barrier by HIV causes PGP dysfunction, which contributes to easier penetration of drugs, including DTG, into brain tissues [36]. The resulting increase in the concentration of DTG in the brain results in the intensification of undesirable effects, such as insomnia and headache [37]. Taking into account the presence of PGP in tissues performing efflux function (small intestine, liver, and kidneys), dysfunction of the protein will increase pathological symptoms. Recent research suggests that PGP initiates the production of T effector cells after viral infection, whereas PGP has a protective function against T memory cells in the case of bacterial invasion [38].
DTG penetration of the blood–brain barrier in clinical trials
Studies conducted in a group of 13 HIV-infected patients showed that the concentration of DTG was lower in the CSF (median 9.6 ng/mL; range 3.6–22.8 ng/mL) than serum (median 1675 ng/mL; range 3137–5091 ng/mL) and may be comparable to the concentration of non-protein-bound DTG (median 9.2 ng/mL; range: 0.8–34.5 ng/mL) [39]. In all patients, the concentration of DTG in CSF was above the IC50 (0.2 ng/mL) assessed in vitro [40] and above the therapeutic concentration (~ 2.4 ng/mL) [41]. However, the concentration of DTG in the CSF did not correlate with the total concentration of DTG in serum and the concentration of DTG unbound to proteins. The transfer of DTG to the CSF positively correlated (r = 0.6396, p = 0.0186) with the quotient of serum albumin concentration to CSF concentration. The authors suggest that DTG enters the CSF by diffusion, and DTG diffusion into the CSF increases with increased permeability of the BBB [39].
The concentration of DTG in CSF was also assessed by Letendre et al. [42]. The authors showed that the median concentration of DTG in CSF was 18 ng/mL (range 4–23 ng/mL) in week 2 of treatment and 13 ng/mL (4–18 ng/mL) in week 16. The concentration of DTG in CSF was comparable to unbound DTG in plasma. In week 2 of treatment, the median CSF concentration of DTG was more than 90-times higher, and in week 16 more than 66-times the IC50. At the same time, after 16 weeks, the number of HIV RNA copies was < 50 copies/mL, which indicates high antiretroviral activity. Calgagno et al. showed that people > 50 years of age had higher concentrations of DTG in CSF and a higher CSF-to-serum ratios [43].
Yagura et al. studied the concentration of DTG in the CSF of 162 Japanese patients. In 41 of the examined patients, CNS disorders (e.g., dizziness, headache, restlessness, and anxiety) occurred and the concentration of DTG in CSF was higher than the concentration of the drug in patients without neuropsychiatric adverse events (NPSAEs) [44].
The serum and CSF concentrations of DTG and selected ATRs are presented in Table 1.
Neuropsychiatric disorders in clinical observations
The most common adverse effects observed in patients who started DTG therapy were nausea (13%), diarrhea (18%), and headache (13%). Other common (≥ 1/100 to < 1/10) effects include insomnia, abnormal dreams, depression, anxiety, dizziness, vomiting, flatulence, abdominal pain/upper abdominal pain, abdominal discomfort, rash, pruritus, feeling fatigue, and increased enzymes [alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST), creatine phosphokinase (CPK)] [68].
It should be emphasized that DTG is characterized by high antiviral efficacy. One of the factors limiting its use is the occurrence of neuropsychiatric adverse events (NPSAEs), which is associated with a reduction in the effectiveness of treatment and, in extreme cases, discontinuation of therapy [69].
In 2017, Hoffman et al. [70] estimated the frequency of NPSAEs leading to discontinuation of therapy among patients treated with INSTIs in two German outpatient clinics in 2007–2016. Discontinuation rates due to adverse events occurring within 2 years of starting treatment with DTG, RAL, or EVG (with cobicistat—COBI, TDF—tenofovir, and FTC—emtricitabine) were compared. Factors affecting the discontinuation of DTG were also analyzed. The following neuropsychiatric disorders were assessed in the study: insomnia, sleep disturbances, dizziness, nervousness, anxiety, depression, decreased concentration, slow thinking, and unexplained pain or paresthesia. The rates of NPSAEs leading to discontinuation at 12 and 24 months were 5.6% and 6.7% for DTG, 0.7% and 1.5% for EVG, and 1.9% and 2.3% for RAL, respectively (i.e., more often related to DTG than other drugs in this class). NPSAEs leading to DTG discontinuation were observed more frequently in female patients, in patients over 60 years of age, and in HLA-B*5701-negative patients who started abacavir treatment at the same time. The NPSAEs (DTG vs. EVG/COBI/TDF/FTC vs. RAL) included insomnia and sleep disorders (36 vs. 2 vs. 4), attention deficit disorder (8 vs. 0 vs. 0), dizziness (13 vs. 1 vs. 3) headaches and paresthesia (16 vs. 1 vs. 6), and depression (7 vs. 0 vs. 1) [70].
Since then, many reports have been published on the safety of DTG. They showed that DTG has a favorable profile, but neurological and mental disorders may occur in patients a few months after the start of therapy, leading to discontinuation of DTG treatment. In the vast majority of cases, the severity of an NPSAE did not pose a threat to the patient’s life and did not require hospitalization. The NPSAEs resolved rapidly after discontinuation of DTG [71]. The discontinuation rate of DTG therapy due to neurotoxicity ranged on average from 2 to 10% [71, 72]. An example of the frequency of NPSAEs associated with the use of various ATRs is presented in Table 2.
In 2017, Fettiplace et al. conducted a large-scale study assessing the incidence of NPSAEs in HIV-infected patients during treatment with DTG or other classes of antiretrovirals, including protease inhibitors (PIs) atazanavir (ATV) and darunavir (DRV), non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (EFV), and INSTI raltegravir. The analysis was based on data from five randomized phase III clinical trials (SPRING-2, FLAMINGO, SINGLE, ARIA, and SAILING) in which patients received DTG at a dose of at least 50 mg daily, the Observational Pharmaco-Epidemiology Research & Analysis (OPERA) cohort, and among cases spontaneously reported to the drug manufacturer. The assessed psychiatric disorders included different types of insomnia (insomnia, initial insomnia, terminal insomnia, and intermediate insomnia), anxiety (anxiety, anxiety disorder), depression (reported as depression, major depression, depressed mood, depressive symptoms, and bipolar disorder), and suicidal behavior (defined as suicide attempt, suicidal ideation, completed suicide, intentional self-harm, and self-injurious behavior). A low incidence of NPSAEs was observed in all five clinical trials but were most frequently reported by SINGLE (DTG: 17%), followed by SPRING-2 (DTG: 6%), SAILING (DTG: 3%), FLAMINGO (DTG: 8%), and ARIA (DTG: 4%). The severity of most symptoms was rated as mild or moderate [69].
A low rate of NPSAEs was also observed in the OPERA cohort study. The assessment included NPSAE diagnoses that occurred after treatment initiation, regardless of whether the patient had a prior diagnosis of a psychiatric disorder, and the rate of new NPSAEs that occurred in patients with no history of neuropsychiatric disorder at baseline or earlier. The follow-up period was similar to the other studies approximately 15 months. In the group of patients with a history of neuropsychiatric disorders, symptoms of anxiety, depression, or insomnia were most common in patients treated with DTG and least common in patients treated with EFV. In patients with no history of neuropsychiatric disorders, the rates of anxiety, depression, and insomnia were similar for all four comparators. In both the clinical trials and the OPERA cohort, NPSAEs were rare in patients treated with DTG. Furthermore, the rate of spontaneous reporting was low (clinical trials N = 3353; DTG, n = 1672; comparator therapies, n = 1681) in terms of estimated patient-years exposure time and not significantly different from clinical trial data [69].
Gallant et al. presented the results of a phase III randomized clinical trial comparing the efficacy and safety of a 48-week combination therapy containing DTG/abacavir (ABC)/lamivudine (3TC) or BIC/FTC/tenofovir (TAF) used in one tablet. Adverse reactions were reported more frequently in patients taking DTG/ABC/3TC, with the most common being nausea (BIC/FTC/TAF vs. DTG/ABC/3TC: 10.2% vs. 22.9%), headache (11.5% vs. 13.7%), and sleep disorders (4.5% vs. 6.3%) [77]. Another study involved HIV-infected patients not yet taking ATRs. During the 48-week therapy, patients were assigned to two receive one of two treatments: BIC/FTC/TAF or receiving FTC/TAF. The results of the above study were slightly different and did not indicate a higher incidence of neuropsychiatric disorders associated with DTG use. The most common adverse reactions (BIC/FTC/TAF vs. DTG/FTC/TAF) were headache (12.5% vs. 12.3%), diarrhea (11.8% vs. 12.0%), and nausea (7.8% vs. 9.5%). Adverse reactions leading to discontinuation of the study drug were rare and occurred in only 1 of 325 patients (< 1%) receiving DTG/FTC/TAF and as many as 5 of 320 patients (~ 1.5%) receiving BIC/FTC/TAF [73].
Llibre et al. presented the results of two phase III clinical trials, SWORD 1 and 2, which evaluated switching from triple or quadruple antiretroviral therapy to DTG (50 mg) plus RPV (25 mg) once daily in adults infected with HIV-1. Changing the once-daily regimen of DTG + RPV was highly effective and the outcome not worse than continuing triple or quadruple therapy. Although slightly more adverse events leading to treatment discontinuation occurred with DTG + RPV than with continued three- or four-drug therapy, they still occurred in a small percentage of patients; 2% of those taking DTG + RPV had discontinuation due to NPSAEs, such as anxiety, depression, depressed mood, insomnia, suicidal thoughts, and headaches. No increased risk of virologic failure was observed when switching to DTG from RPV once daily [78].
Cuzin et al. performed another analysis of data from 18 centers in France participating in the Dat’AIDS cohort study in which HIV-positive patients initiated INSTI treatment. All reasons for discontinuation of an INSTI-containing regimen were tracked, and the characteristics of patients discontinuing due to NPSAEs were described. Among the drugs used were DTG, as well as EVG administered together with COBI or RAL. The rate of NPSAEs leading to discontinuation was 2.7% for DTG, 1.3% for EVG, and 1.7% for RAL. Based on the analysis, DTG led to fewer virological failures (< 1%) than other INSTIs, was well tolerated, and showed high virological efficacy. On the other hand, discontinuation due to NPSAEs was reported in 2.7% of patients receiving DTG. Patients treated with DTG were at higher risk of developing NPSAEs than those treated with EVG or RAL in combination with COBI [79].
Peñafiel et al. conducted a retrospective analysis of a prospectively followed cohort of all antiretroviral-naïve and all virologically suppressed antiretroviral patients prescribed a first regimen of RAL, EVG, or DTG and had at least one follow-up visit. Early discontinuation for any reason was 271 per 1000 patient-years for RAL, 168 per 1000 patient-years for EVG, and 264 per 1000 patient-years for DTG (p = 0.0821). Adverse reactions leading to treatment discontinuation were mainly neuropsychiatric, musculoskeletal, or gastrointestinal disorders, and the most commonly reported neuropsychiatric symptoms were insomnia, dizziness, headache, and anxiety, the incidence of which was not significantly different between INSTI-treated patients. Particular NPSAEs leading to early treatment discontinuation were insomnia, dizziness, and headache. Discontinuation due to NPSAEs was more common with DTG than with RAL or EVG (p = 0.0046) [80].
In 2014–2016, a retrospective analysis was carried out of records of HIV-infected patients in the Netherlands. They checked the cause and time of discontinuation of DTG, which was included for both previously untreated and already treated patients. The average duration of DTG therapy was 225 days. Discontinuation of DTG therapy was observed in 85 patients (15.3%), and in 76 patients (13.7%), the reason for discontinuation was drug intolerance. Insomnia and sleep disorders were reported in 5.6% of patients, and neuropsychiatric disorders (e.g., anxiety, psychosis, and depression) occurred in 4.3% of patients. In patients receiving DTG in combination with ABC, discontinuation of DTG was reported even more often, with 58 patients (16.3%) discontinuing treatment, and the reason for discontinuation was of adverse effects [72].
In a prospective cohort study, Elzi et al. showed that DTG (1.7%) caused more neurotoxic effects than RAL (0.6%) and was more often the cause of treatment discontinuation. The authors attributed the slightly lower rates of CNS disorders compared to other studies to the smaller number of patients included in the study [74].
The frequency and reasons for discontinuation of treatment were also assessed by Fernández-Bargiela et al. in patients receiving DTG and EFV. Patients receiving EFV (35.8%) discontinued therapy more often than patients receiving DTG (12.1%), and the most common cause was NPSAEs (DTG, 8.2%; EFV, 25%). Women and those with documented psychiatric events were more likely to discontinue treatment. Furthermore, patients treated with DTG were less likely to be prescribed benzodiazepines. Both groups of patients required consultation and observation in psychiatric wards (DTG, 8.9%; EFV, 16.9%) [75].
In a cohort study by Mendes et al., DTG was found to have a better safety profile than EFV. Overall, 16.9% of CNS-related adverse events occurred in the group of patients receiving DTG/TDF/3TC, and NPSAEs were more than twice as frequent (37.5% of patients) in the group receiving EFV/TDF/3TC. Unexpectedly, alcohol consumption was associated with a lower risk of adverse events. The authors explained this phenomenon by the possibility of alcohol competing with cytochrome enzymes, a change in the metabolism of ATRs, a decrease in their plasma concentration, and a subsequently lower risk of adverse effects. Acute intoxication of the body may also occur after drinking alcohol, which manifests nausea and headaches, among other symptoms. These symptoms are similar to the adverse effects of medications. People who consume alcohol may attribute their symptoms to the effects of alcohol and, consequently, the number of adverse reactions reported was lower than in real life [76].
A recent cohort study conducted in a group of previously untreated and treated patients showed that the majority of patients (84%) discontinued DTG within the first 12 months of treatment, and the most common reason for discontinuation of therapy (92.2%) was CNS disorders. The probability of maintaining DTG treatment was 75.1% after 3 years and 67.2% after 5 years. A higher risk of treatment discontinuation was found in previously untreated patients. Patients who had a longer duration of virological suppression and were at risk of prior virological failure had a lower risk of treatment discontinuation [81].
In 2022, Taramasso et al. published the results of the prospective, observational SCOLTA cohort, which assessed the incidence of CNS adverse events after the administration of DTG and DTG-free ARTs. A total of 4939 HIV-infected subjects were enrolled in the study, of which 1179 were in the DTG group and 3760 in the non-DTG group [lopinavir/ritonavir (LPV/r, n = 731), atazanavir/ritonavir (ATV/r, n = 616), DRV/ritonavir or DRV/cobicistat (DRV/r or DRV/c, n = 721), RPV (n = 481), RAL (n = 514), EVG (n = 339), and BIC (n = 358)]. However, 834 (16.9%) had not received prior ART, whereas the remaining 4,105 had previously used ART, 2289 (55.8%) of which had < 50 copies HIV RNA/mL at the time of initiating the study drug. There was no significant difference in the incidence of neuropsychiatric disorders between the two cohorts at baseline, with 66 NPSAEs reported to lead to ART discontinuation, 39 (of 1179; 3.3%) in the DTG cohort and 27 (of 3760; 0.7%) in the non-DTG. HIV-infected, ART-naïve individuals with higher CD4 + T-cell counts and psychiatric disorders were more likely to develop CNS adverse events; non-NPSAEs were reported in 35/39 patients on DTG and 23/24 on non-DTG therapy. However, most NPSAEs were reversible and resolved when the ART was switched to a drug of the same or different class. At the same time, a lower event resolution rate was found in HIV-infected patients older than 50 years of age (p = 0.017). Thus, NPSAEs leading to discontinuation of ART occurred more frequently in patients treated with DTG than in those not treated with DTG. Most NPSAEs resolved after switching drugs, for both the DTG and non-DTG cohorts [82].
The impact of initial mental conditions on the occurrence of neuropsychiatric disorders after DTG use is unclear due to divergent information among HIV/AIDS specialists. Chan et al. showed that approximately 37 weeks of DTG use may be associated with an increased risk of moderate, but not severe, depressive symptoms [83]. However, the diagnosis of depression before the start of DTG therapy was not associated with the severity of disease symptoms. Povar-Echeverría et al. reported that patients treated with DTG who had a history of psychiatric disorders more often reported NPSAEs (62% vs. 41%) and more often discontinued treatment (62% vs. 41%) than patients without previous psychiatric disorders [84]. Similarly, Fernández-Bargiela et al. [75] tried to prove that a higher risk of discontinuation of DTG treatment is present in patients with mental disorders. Similar observations were made by Cusato et al. [85].
Risk factors for the occurrence of neuropsychiatric disorders
In 2022, Cusato et al. revealed that patients receiving DTG, which inhibits the renally and neuronally expressed organic anion transporter 2 (encoded by SLC22A2), had neuropsychiatric symptoms. The effect of the SLC22A2 808C > A genetic variant in patients receiving DTG was evaluated and analyzed by real-time PCR. Among the 627 participants in the study, CA/AA carriers had a higher frequency of comorbid psychiatric illness and antidepressant use. Following 27.9 months of therapy, 108 participants discontinued DTG, with 64 having done so due to neuropsychiatric symptoms. Patients with a history of psychiatric comorbidities were more likely to discontinue DTG, whereas patients with the SLC22A2 CA/AA genotype were not. Within 30 days, most participants were completely symptom-free (61.8%). Discontinuation of DTG due to NPSAEs was not uncommon, and it was more common in participants with pre-existing psychiatric disorders. An interaction was observed between the SLC22A2 genetic variant and psychiatric comorbidities. Complete recovery from neuropsychiatric symptoms was not observed in 38.2% of patients after discontinuation of DTG, suggesting the involvement of additional factors [85].
Some researchers have looked for risk factors for the occurrence of central adverse events. DTG is metabolized by uridine diphosphate (UDP)-glucuronosyl transferase 1A1 (UGT1A1), and to a lesser extent by cytochrome P450 3A (CYP3A). Yagura et al. studied the relationship between a UGT1A1 gene polymorphism and the risk of neuropsychiatric adverse effects (i.e., dizziness and headache, insomnia, restlessness, and anxiety) in 107 HIV-infected Japanese patients receiving DTG. Patients with one or two UGT1A1*6 and UGT1A1*28 alleles had a higher incidence of adverse events than those with normal alleles. Patients with abnormal alleles who were over 40 years of age had higher serum concentrations of DTG [86]. Other researchers have suggested that the increased risk of adverse effects with DTG use may be related to impaired mitochondrial function and cellular metabolic disorders [87].
As mentioned earlier, risk factors for neuropsychiatric disorders may also include older age, female gender, a history of neuropsychiatric disorders, and a negative human leukocyte antigen (HLA)-B*5701 test result. These factors have not been confirmed by all authors, and their relationship with the occurrence of treatment complications requires further observation [88].
A recent meta-analysis showed that there may be an increased risk of depression when DTG is co-administered with rilpivirine (RLP) compared to a single administration of either drug. The neurotoxic effect of DTG is explained by the ability of the drug to penetrate the BBB by passive diffusion, the possibility of changing the tight connections in the BBB, and secondary neuritis [89]. The authors refer to animal studies showing that DTG accumulating in the CSF can lead to oxidative stress and changes in neuronal hemostasis [42, 90]. According to other authors, the neurotoxic effect of DTG may also be associated with an increased concentration of DTG in the CSF, because, as already mentioned, the concentration of DTG positively correlates with the degree of neurotoxicity [44]. A potential mechanism for the increased toxicity of DTG when co-administered with RLP may be explained by drug–drug interactions with breast cancer resistance protein (BCRP), which is the main efflux transporter protein that removes drugs from inside cells. DTG is a BCRP substrate inhibited by RLP. Thus, increased DTG levels may potentially result from BCRP inhibition by RLP [91,92,93,94].
Neurotoxicity may also occur when DTG is co-administered with sertraline. Ma et al. showed that the interaction of these two drugs increases the permeability of the BBB and risk of NPSAEs [95].
Changes in the summary of product characteristics
The Summary of Product Characteristics (SmPC) for Tivicay® (ViiV Healthcare BV) has been updated several times as new information has become available. Initial results from animal studies show that single oral doses of DTG up to 500 mg/kg body weight in rats and 1000 mg/kg body weight in monkeys have minor effects on the nervous, respiratory, and cardiovascular systems. Already in the first characterization of the Tivicay® medicinal product (13 November 2013) based on the SPRING-2, SAILING, and SINLGE studies, NPSAEs included headache, insomnia, fatigue, depression, and abnormal dreams [5]. The 12 November 2020 SmPC update was based on single case reports of NPSAEs, such as abnormal behavior, affective disorders, depression, insomnia, suicidal ideation, suicide attempts, and overdose, that occurred during study P1093 [96]. The current version of the SmPC is dated 18 November 2022. According to its provisions, central adverse reactions that are very common (≥ 1/10) and common (≥ 1/100 to < 1/10) include headache and insomnia, unusual dreams, depression, anxiety, and dizziness; uncommon (≥ 1/1000 to < 1/100) include panic attacks, suicidal thoughts, and suicide attempts (especially in those with a history of depression or mental illness), and rare (≥ 1/10,000 to < 1/1000) include suicide (especially in people with a history of depression or mental illness). Taking into account the number of HIV patients treated in Poland (16,000) and the fact that therapeutic regimens containing DTG are among the more widely recommended, there is a statistically low chance to observe NPSAEs, though they are reported by patients. It is important that doctors pay attention to the symptoms reported by patients and take a detailed history of insomnia or depressed mood and, discreetly, if possible, adjust the therapy to the patient’s needs [97].
Clinical significance of observed CNS adverse effects
The publications cited above indicate that DTG is associated with CNS disorders that may lead to a discontinuation or change of treatment. In most cases, the factors predisposing to their occurrence and affecting their severity are unknown. Prospective studies that will enable the identification of a larger group of patients with neuropsychiatric disorders are justified and could identify factors predisposing to their occurrence. There are no data in the literature on the therapeutic interventions used, assessment of their effectiveness, impact on the frequency and severity of neuropsychiatric disorders, and the risk of treatment discontinuation. Such studies are necessary to precisely determine the need for treatment of neuropsychiatric disorders and indicate the optimal treatment. At the same time, new information on the safety of a drug, in this case, DTG, is a natural element associated with the presence of a new drug on the market. Clinical trials, although conducted very meticulously according to restrictive rules, are not able to provide full and exhaustive knowledge about the drug, especially in the context of its safety. One should keep in mind the limitations related to the number of patients participating in clinical trials, limitations of their duration, and strictly defined groups of patients who qualify for the trials in accordance with the inclusion and exclusion criteria. All this means that rare, distant adverse effects not directly related to the mechanism of action can be identified only at the post-registration stage. Therefore, the role of pharmacovigilance is essential in this matter. Importantly, the central adverse events identified for DTG do not preclude its use in clinical practice. The drug belongs to a basic group of antiretroviral compounds currently used in the treatment regimens of patients with HIV. The effectiveness of DTG has been proven in pre- and post-registration studies. The research is only to draw attention to the mechanism of selected adverse effects, explain their causes and possible risks, indicate the need to monitor patients for selected symptoms, and allow practitioners to use the drug safely and effectively.
Conclusions
DTG is a first-line drug in HIV-positive patients used as part of combination therapy with other ATRs. It is recommended in two-drug regimens of similar efficacy and tolerability to three-drug regimens. The choice of DTG as a first-line drug is due to its rapid and effective reduction of viral titers in the blood. DTG has been shown to be a more effective drug, easier to take, and to have fewer adverse effects than current alternative drugs. DTG also has a high genetic barrier to the development of drug resistance, which is particularly important in patients who have developed resistance to other types of ARTs. The benefit of DTG therapy is the relatively good tolerability of the drug and the low risk of drug interactions, and it can be used in patients with tuberculosis which might be concomitant to HIV infection. DTG is used once daily, and such drug regimens improve patient compliance.
Undoubtedly, a limitation of treatment is the possibility of neuropsychiatric disorders, which may even lead to treatment withdrawal. The risk of adverse CNS effects is slightly higher for DTG than other INSTIs, but the intensity is generally mild to moderate.
Data availability
Data supporting Fig. 1 are publicly available as a part of a few preclinical studies evaluating the neurotoxicity of DTG: DOIs https://doi.org/10.3109/00498254.2014.942409 and https://doi.org/10.1093/jac/dkab501. Image data supporting Fig. 2, the Egan egg chart comparing the study drugs and selected commonly used drugs, are available as a part of the following publications: DOIs https://doi.org/10.1021/acs.jmedchem.5b00104, https://doi.org/10.1002/cmdc.201600182, https://doi.org/10.1093/jac/dkab501, and https://doi.org/10.3390/ph15050587. Data presented in Table 1 are available as a part of: DOIs https://doi.org/10.1093/ofid/ofz174, https://doi.org/10.1093/cid/ciu477, https://doi.org/10.1371/journal.pone.0006877, https://doi.org/10.1093/jac/dkt339, https://doi.org/10.1128/AAC.00507-10, https://doi.org/10.1089/aid.2015.0337, https://doi.org/10.1016/j.jpba.2020.113250, https://doi.org/10.1093/infdis/jiz624, https://doi.org/10.1093/jac/dkab334, https://doi.org/10.3947/ic.2021.0136, https://doi.org/10.1128/AAC.49.6.2504-2506.2005, https://doi.org/10.1124/dmd.108.020974, https://doi.org/10.1111/bcp.13552, https://doi.org/10.1097/QAD.0b013e3283489cb1, https://doi.org/10.1002/jcph.612, https://doi.org/10.1007/s13365-018-0626-4, https://doi.org/10.1128/AAC.06311-11, https://doi.org/10.1128/AAC.02329-12, https://doi.org/10.1093/jac/dkz504, https://doi.org/10.1097/QAD.0b013e328317a702, https://doi.org/10.1097/QAD.0b013e32814e6b1c, https://doi.org/10.1002/jcph.298, and https://doi.org/10.2174/1381612822666160726113001, as well as https://www.researchgate.net/publication/320879372_Cerebrospinal_Fluid_CSF_Concentrations_Efficacy_and_Neurocognitive_Effects_of_the_Combination_Of_Darunavir_Cobicistat_and_Rilpivirine_in_HIV-1_Naive_Adults. Data presented in Table 2 are available as a part of the following publications: DOIs https://doi.org/10.1111/hiv.12468, https://doi.org/10.1097/QAD.0000000000001590, https://doi.org/10.1136/ejhpharm-2020-002374, and https://doi.org/10.33448/rsd-v11i4.26250, and https://www.ema.europa.eu/en/documents/procedural-steps-after/tivicay-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf, https://www.natap.org/2017/IAS/IAS_39.htm (Late Breaker Poster Abstract TUPDB0201LB).
Abbreviations
- CNS:
-
Central nervous system
- HIV:
-
Human immunodeficiency virus
- BBB:
-
Blood–brain barrier
- cART:
-
Combined antiretroviral therapy
- HAD:
-
HIV-associated dementia
- RNA:
-
Ribonucleic acid
- ATR:
-
Antiretroviral drugs
- DTG:
-
Dolutegravir
- INSTI:
-
Second-generation integrase chain transfer inhibitor
- FDA:
-
Food and drug administration
- DNA:
-
Deoxyribonucleic acid
- EMA:
-
European medicines agency
- RAL:
-
Raltegravir
- EVG:
-
Elvitegravir
- BIC:
-
Bictegravir
- CAB:
-
Cabotegravir
- TDF:
-
Tenofovir disoproxil fumarate
- FTC:
-
Emtricitabine
- IC90 :
-
90% Inhibitory concentration
- PSA:
-
Polar surface area
- BOILED-Egg:
-
Brain or IntestinaL Estimate D permeation method
- HIA:
-
Human intestinal absorption
- ABC:
-
Abacavir
- 3TC:
-
Lamivudine
- ABCB1:
-
ATP-binding cassette transporter protein P-glycoprotein
- ABCG2:
-
ATP-binding cassette transporter
- PGP:
-
P-glycoprotein PET: positron emission tomography
- EC:
-
Endothelial cell
- CSF:
-
Cerebrospinal fluid
- NPSAE:
-
Neuropsychiatric adverse events
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CPK:
-
Creatine phosphokinase
- COBI:
-
Cobicistat
- TDF:
-
Tenofovir disoproxil fumarate
- ATV:
-
Atazanavir
- DRV:
-
Darunavir
- PI:
-
Protease inhibitor
- EFV:
-
Efavirenz
- NNRTI:
-
Non-nucleoside reverse transcriptase inhibitor
- LPV/r:
-
Lopinavir/ritonavir
- ATV/r:
-
Atazanavir/ritonavir
- DRV/r:
-
Darunavir/ritonavir
- DRV/c:
-
Darunavir/cobicistat
- PCR:
-
Polymerase chain reaction
- UDP:
-
Uridine diphosphate
- UGT1A1:
-
Glucuronosyl transferase 1A1
- CYP3A:
-
Cytochrome P450 3A
- (HLA)-B*5701:
-
Human leukocyte antigen B*5701
- RLP:
-
Rilpivirine
- BCRP:
-
Breast cancer resistance protein
- SmPC:
-
Summary of Product Characteristics
References
Vivithanaporn P, Gill MJ, Power Ch. Impact of current antiretroviral therapies on neuroAIDS. Exp Rev of Anti Infect Ther. 2011;9(4):371–4.
Watkins CC, Treisman GJ. Cognitive impairment in patients with AIDS—prevalence and severity. HIV AIDS (Auckl). 2015;29(7):35–47.
Upton ChT, Taiwo B, Robertson KR. Neurotoxicity of antiretroviral therapy. Futur Virol. 2013;8(5):469–75.
Dow DE, Bartlett JA. Dolutegravir. The second-generation of integrase strand transfer inhibitors (INSTIs) for the treatment of HIV. Infect Dis Ther. 2014;3(2):83–102.
EMA 2020. Assessment report TIVICAY EMA/CHMP/540603/2020.
Blanco JL, Whitlock G, Milinkovic A, Moyle G. HIV integrase inhibitors: a new era in the treatment of HIV. Expert Opin Pharmacother. 2015;16(9):1313–24.
Acosta RK, Willkom M, Martin R, Chang S, Wei X, Garner W, et al. Resistance analysis of bictegravir-emtricitabine-tenovir alafenamide in HIV-1 treatment-naïve patients through 48 weeks. Antimicrob Agents Chemother. 2019;63(5):e02533-e2618.
Kintu K, Malaba TR, Nakibuka J, Papamichael C, Colbers A, Byrne K, et al. Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial. Lancet HIV. 2020;7(5):e332–9.
Jacobson K, Ogbuagu O. Integrase inhibitor-based regimens result in more rapid virologic suppression rates among treatment-naïve human immunodeficiency virus-infected patients compared to non-nucleoside and protease inhibitor-based regimens in a real-world clinical setting: a retrospective cohort study. Medicine (Baltimore). 2018;97(43): e13016.
Scarsi KK, Havens JP, Podany AT, Avedissian SN, Fletcher CV. Integrase inhibitors: a comparative review of efficacy and safety. Drugs. 2020;80(16):1649–76.
Günthard HF, Aberg JA, Eron JJ, Hoy JF, Telenti A, Benson CA, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the international antiviral society-USA panel. JAMA. 2014;312(4):410–25.
EACS Guidelines version 11.1, Oct 2022
Moss L, Wagner D, Kanaoka E, Olson K, Yueh YL, Bowers GD. The comparative disposition and metabolism of dolutegravir, a potent HIV-1 integrase inhibitor, in mice, rats, and monkeys. Xenobiotica. 2015;45(1):60–70.
Labarthe L, Gelé T, Gouget H, Benzemrane MS, Le Calvez P, Legrand N, et al. Pharmacokinetics and tissue distribution of tenofovir, emtricitabine and dolutegravir in mice. J Antimicrob Chemother. 2022;77(4):1094–101.
Pires DE, Blundell TL, Ascher DB. pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem. 2015;58(9):4066–72.
Bade AN, McMillan JM, Liu Y, Edagwa BJ, Gendelman HE. Dolutegravir inhibition of matrix metalloproteinases afects mouse neurodevelopment. Mol Neurobiol. 2021;58(11):5703–21.
Hinckley S, Sherman S, Best BM, Momper J, Ma Q, Letendre SR, et al. Neurotoxicity screening of antiretroviral drugs with human iPSC-derived neurons. Conference on Retroviruses and Opportunistic Infections. Boston, Massachusetts; 2016
Egan WJ, Merz KM Jr, Baldwin JJ. Prediction of drug absorption using multivariate statistics. J Med Chem. 2000;43(21):3867–77.
Kadry H, Noorani B, Cucullo L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17(1):69.
Daina A, Zoete V. A boiled-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016;11(11):1117–21.
Tisseraud M, Goutal S, Bonasera T, Goislard M, Desjardins D, Le Grand R, et al. Isotopic radiolabeling of the antiretroviral drug [18 F] dolutegravir for pharmacokinetic PET imaging. Pharmaceuticals (Basel). 2022;15(5):587.
Elliott RO, He M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics. 2021;13(1):122.
Profaci CP, Munji RN, Pulido RS, Daneman R. The blood-brain barrier in health and disease: important unanswered questions. J Exp Med. 2020;217(4): e20190062.
Hajal C, Le Roi B, Kamm RD, Maoz BM. Biology and models of the blood-brain barrier. Annu Rev Biomed Eng. 2021;23:359–84.
Kratzer I, Ek J, Stolp H. The molecular anatomy and functions of the choroid plexus in healthy and diseased brain. Biochim Biophys Acta Biomembr. 2020;1862(11): 183430.
Uchida Y, Goto R, Usui T, Tachikawa M, Terasaki T. Blood-arachnoid barrier as a dynamic physiological and pharmacological interface between cerebrospinal fluid and blood. AAPS Adv Pharm Sci Ser. 2022;33:93–121.
Kiecker C. The origins of the circumventricular organs. J Anat. 2018;232(4):540–53.
Pandit R, Chen L, Götz J. The blood-brain barrier: physiology and strategies for drug delivery. Adv Drug Deliv Rev. 2020;165–166:1–14.
Villaseñor R, Lampe J, Schwaninger M, Collin L. Intracellular transport and regulation of transcytosis across the blood-brain barier. Cell Mol Life Sci. 2019;76(6):1081–92.
Yazdani S, Jaldin-Fincati JR, Pereira RVS, Klip A. Endothelial cell barriers: transport of molecules between blood and tissues. Traffic. 2019;20(6):390–403.
Leopoldo M, Nardulli P, Contino M, Leonetti F, Luurtsema G, Colabufo NA. An updated patent review on P-glycoprotein inhibitors (2011–2018). Expert Opin Ther Pat. 2019;29(6):455–61.
Ahmed Juvale II, Abdul Hamid AA, Abd Halim KB, Che Has AT. P-glycoprotein: new insights into structure, physiological function, regulation and alterations in disease. Heliyon. 2022;8(6): e09777.
Minuesa G, Arimany-Nardi C, Erkizia I, Cedeño S, Moltó J, Clotet B, et al. G. P-glycoprotein (ABCB1) activity decreases raltegravir disposition in primary CD4þ P-gphigh cells and correlates with HIV-1 viral load. J Antimicrob Chemother. 2016;71(10):2782–92.
Product information for AusPAR Tivicay ViiV Healthcare Pty Ltd Pty Ltd PM2012-04124-1-2 Final 19 May 2014. This Product Information was approved at the time this AusPAR was published
Shultz MD. Two decades under the influence of the rule of five and the changing properties of approved oral drugs: miniperspective. J Med Chem. 2019;62(4):1701–14.
Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog Neurobiol. 2005;76(1):22–76.
U.S. FDA approves GlaxoSmithKline’s HIV drug Tivicay. Available online: https://www.reuters.com/article/us-glaxosmithkline-hivdrug-idUSBRE97B0WU20130812. Accessed 05 July 2023
Chen ML, Sun A, Cao W, Eliason A, Mendez KM, Getzler AJ, et al. Physiological expression and function of the MDR1 transporter in cytotoxic T lymphocytes. J Exp Med. 2020;217(5): e20191388.
Gelé T, Furlan V, Taburet AM, Pallier C, Becker PH, Goujard C, et al. Dolutegravir cerebrospinal fluid diffusion in HIV-1–infected patients with central nervous system impairment. Open Forum Infect Dis. 2019;6(6):ofz174.
Kobayashi M, Yoshinaga T, Seki T, Wakasa-Morimoto C, Brown KW, Ferris R, et al. In Vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011;55:813–21.
Min S, Sloan L, DeJesus E, Hawkins T, McCurdy L, Song I, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25:1737–45.
Letendre SL, Mills AM, Tashima KT, Thomas DA, Min SS, Chen S, et al. ING116070: a study of the pharmacokinetics and antiviral activity of dolutegravir in cerebrospinal fluid in HIV-1-infected, antiretroviral therapy-naive subjects. Clin Infect Dis. 2014;59:1032–7.
Calcagno A, Moltó J, Borghetti A, Gervasoni C, Milesi M, Valle M, et al. Older age is associated with higher dolutegravir exposure in plasma and cerebrospinal fluid of people living with HIV. Clin Pharmacokinet. 2021;60(1):103–9.
Yagura H, Watanabe D, Nakauchi T. Effect of dolutegravir plasma concentration on central nervous system side effects. Poster present at: Conference on retroviruses and opportunistic infections, February 13–16, 2017, Seattle WA
Yilmaz A, Gisslén M, Spudich S, Lee E, Jayewardene A, Aweeka F, et al. Raltegravir cerebrospinal fluid concentrations in HIV-1 infection. PLoS One. 2009;4(9): e6877.
Calcagno A, Cusato J, Simiele M, Motta I, Audagnotto S, Bracchi M, et al. High interpatient variability of raltegravir CSF concentrations in HIV-positive patients: a pharmacogenetic analysis. J Antimicrob Chemother. 2014;69(1):241–5.
Croteau D, Letendre S, Best BM, Ellis RJ, Breidinger S, Clifford D, et al. Total raltegravir concentrations in cerebrospinal fluid exceed the 50-percent inhibitory concentration for wild-type HIV-1. Antimicrob Agents Chemother. 2010;54(12):5156–60.
Calcagno A, Simiele M, Motta I, Mornese Pinna S, Bertucci R, et al. Elvitegravir/cobicistat/tenofovir/emtricitabine penetration in the cerebrospinal fluid of three HIV-positive patients. AIDS Res Hum Retroviruses. 2016;32(5):409–11.
Rigo-Bonnin R, Tiraboschi JM, Álvarez-Álvarez M, Pérez-Fernández GA, Sanjuás-Iglesias M, Scévola S, et al. Measurement of total and unbound bictegravir concentrations in plasma and cerebrospinal fluid by UHPLC-MS/MS. J Pharm Biomed Anal. 2020;185: 113250.
Tiraboschi J, Imaz A, Khoo S, Niubo J, Prieto P, Saumoy M, et al. Total and unbound bictegravir concentrations and viral suppression in cerebrospinal fluid of human immunodeficiency virus-infected patients (Spanish HIV/AIDS research network, PreEC/RIS 56). J Infect Dis. 2020;221(9):1425–8.
Gelé T, Chéret A, Castro Gordon A, Nkam L, Furlan V, Pallier C, et al. Cerebrospinal fluid exposure to bictegravir/emtricitabine/tenofovir in HIV-1-infected patients with CNS impairment. J Antimicrob Chemother. 2021;76(12):3280–5.
Letendre SL, Mills A, Hagins D, Swindells S, Felizarta F, Devente J, et al. Pharmacokinetics and antiviral activity of cabotegravir and rilpivirine in cerebrospinal fluid following long-acting injectable administration in HIV-infected adults. J Antimicrob Chemother. 2020;75(3):648–55.
Capparelli EV, Letendre SL, Ellis RJ, Patel P, Holland D, McCutchan JA. Population pharmacokinetics of abacavir in plasma and cerebrospinal fluid. Antimicrob Agents Chemother. 2005;49(6):2504–6.
Giri N, Shaik N, Pan G, Terasaki T, Mukai C, Kitagaki S, et al. Investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) on pharmacokinetics and central nervous system penetration of abacavir and zidovudine in the mouse. Drug Metab Dispos. 2008;36(8):1476–84.
Calcagno A, Pinnetti C, De Nicolò A, Scarvaglieri E, Gisslen M, Tempestilli M, et al. Cerebrospinal fluid abacavir concentrations in HIV-positive patients following once-daily administration. Br J Clin Pharmacol. 2018;84(6):1380–3.
Calcagno A, Bonora S, Simiele M, Rostagno R, Tettoni MC, Bonasso M, et al. Tenofovir and emtricitabine cerebrospinal fluid-to-plasma ratios correlate to the extent of blood-brain barrier damage. AIDS. 2011;25(11):1437–9.
Lahiri CD, Reed-Walker K, Sheth AN, Acosta EP, Vunnava A, Ofotokun I. Cerebrospinal fluid concentrations of tenofovir and emtricitabine in the setting of HIV-1 protease inhibitor-based regimens. J Clin Pharmacol. 2016;56(4):492–6.
Imaz A, Niubó J, Amara A, Khoo S, Ferrer E, Tiraboschi JM, et al. Cerebrospinal fluid drug concentrations and viral suppression in HIV-1-infected patients receiving ritonavir-boosted atazanavir plus lamivudine dual antiretroviral therapy (Spanish HIV/AIDS research network, PreEC/RIS 39). J Neurovirol. 2018;24(4):391–7.
Yilmaz A, Watson V, Dickinson L, Back D. Efavirenz pharmacokinetics in cerebrospinal fluid and plasma over a 24-hour dosing interval. Antimicrob Agents Chemother. 2012;56(9):4583–5.
Avery LB, Sacktor N, McArthur JC, Hendrix CW. Protein-free efavirenz concentrations in cerebrospinal fluid and blood plasma are equivalent: applying the law of mass action to predict protein-free drug concentration. Antimicrob Agents Chemother. 2013;57(3):1409–14.
Best BM, Koopmans PP, Letendre SL, Capparelli EV, Rossi SS, Clifford DB, et al. Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J Antimicrob Chemother. 2011;66(2):354–7.
Ma Q, Letendre SL, Woods SP, Fletcher CV, Mayberry CC, Davis V et al. Cerebrospinal fluid (CSF) concentrations, efficacy, and neurocognitive effects of the combination of darunavir /cobicistat and rilpivirine in HIV-1 naive adults. 16th European AIDS conference (EACS), Milan, Italy, October 25–27, 2017
Best BM, Letendre SL, Brigid E, Clifford DB, Collier AC, Gelman BB, et al. Low atazanavir concentrations in cerebrospinal fluid. AIDS. 2009;23(1):83–7.
Vernazza P, Daneel S, Schiffer V, Decosterd L, Fierz W, Klimkait T, et al. The role of compartment penetration in PI-monotherapy: the atazanavir-ritonavir monomaintenance (ATARITMO) trial. AIDS. 2007;21(10):1309–15.
Delille CA, Pruett ST, Marconi VC, Lennox JL, Armstrong WS, Arrendale RF, et al. Effect of protein binding on unbound atazanavir and darunavir cerebrospinal fluid concentrations. J Clin Pharmacol. 2014;54(9):1063–71.
Yilmaz A, Izadkhashti A, Price RW, Mallon PW, De Meulder M, Timmerman P, et al. Darunavir concentrations in cerebrospinal fluid and blood in HIV-1-infected individuals. AIDS Res Hum Retroviruses. 2009;25(4):457–61.
Croteau D, Rossi SS, Best BM, Capparelli E, Ellis RJ, Clifford DB, et al. Darunavir is predominantly unbound to protein in cerebrospinal fluid and concentrations exceed the wild-type HIV-1 median 90% inhibitory concentration. J Antimicrob Chemother. 2013;68(3):684–9.
Fettiplace A, Stainsby C, Winston A, Givens N, Puccini S, Vannappagari V, et al. Psychiatric symptoms in patients receiving dolutegravir. J Acquir Immune Defic Syndr. 2017;74(4):423–31.
Hoffmann C, Welz T, Sabranski M, Kolb M, Wolf E, Stellbrink H-J, et al. Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med. 2017;18(1):56–63.
Menard A, Montagnac C, Solas C, Meddeb L, Dhiver C, Tomei C, et al. Neuropsychiatric adverse effects on dolutegravir: an emerging concern in Europe. AIDS. 2017;31(8):1201–3.
de Boer MGJ, van den Berk GEL, van Holten N, et al. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS. 2016;30(18):2831–4.
Sax P, Pozniak A, Arribas J, Koenig E, DeJesus E, Stellbrink G-J, et al. Phase 3 randomized, controlled clinical trial of bictegravir coformulated with FTC/TAF in a fixed-dose combination (B/F/TAF) vs dolutegravir (DTG) + F/TAF in treatment naïve HIV-1 positive adults: week 48 results. Paris: IAS, Late Breaker Poster Abstract TUPDB0201LB; 2017.
Elzi L, Erb S, Furrer H, Cavassini M, Calmy A, Vernazza P, et al. Adverse events of raltegravir and dolutegravir. AIDS. 2017;31(13):1853–8.
Fernández-Bargiela N, Rotea-Salvo S, Margusino-Framiñán L, Balboa-Barreiro V, Martín-Herranz I, Castro-Iglesias Á, et al. Discontinuation due to neuropsychiatric adverse events with efavirenz- and dolutegravir-based antiretroviral therapy: a comparative real-life study. Eur J Hosp Pharm. 2022;29(4):207–11.
Mendes JC, Braga MG, Reis AMM, Silveira MR. Incidence and factors associated with adverse drug reactions in a cohort of individuals starting dolutegravir or efavirenz. Res Soc Dev. 2022;1(4): e0811426250.
Gallant J, Lazzarin A, Mills A, Orkin C, Podzamczer D, Tebas P, et al. A Phase 3 Randomized Controlled Clinical Trial of Bictegravir in a Fixed Dose Combination, B/F/TAF, vs ABC/DTG/3TC in Treatment-naïve Adults at Week 48. Paris: IAS, Late Breaker Oral Abstract MOAB0105LB; 2017.
Llibre JM, Hung CC, Brinson C, Castelli F, Girard PM, Kahl L et al. Phase III SWORD 1 and 2: switch to DTG + RPV Maintains Virologic Suppression Thorugh 48 wks. CROI, O-4 Abstract 44LB; 2017;13–6.
Cuzin L, Pugliese P, Katlama C, Bani-Sadr F, Ferry T, Rey D, et al. Integrase strand transfer inhibitors and neuropsychiatric adverse events in a large prospective cohort. J Antimicrob Chemother. 2019;74(3):754–60.
Peñafiel J, de Lazzari E, Padilla M, Rojas J, Gonzalez-Cordon A, Blanco JL, et al. Tolerability of integrase inhibitors in a real-life setting. J Antimicrob Chemother. 2017;72(6):1752–9.
Ciccullo A, Baldin G, Borghi V, Lagi F, Latini A, d’Ettorre G, et al. Real-life impact of drug toxicity on dolutegravir tolerability: clinical practice data from a multicenter Italian cohort. Viruses. 2022;14(1):163.
Taramasso L, Orofino G, Ricci E, Menzaghi B, De Socio GV, Squillace N, et al. Reversibility of central nervous system adverse events in course of art. Viruses. 2022;14(5):1028.
Chan P, Goh O, Kroon E, Colby D, Sacdalan C, Pinyakorn S, et al. Neuropsychiatric outcomes before and after switching to dolutegravir-based therapy in an acute HIV cohort. AIDS Res Ther. 2020;17(1):1.
Povar-Echeverría M, Comet-Bernad M, Gasso-Sánchez A, Ger-Buil A, Navarro-Aznarez H, Martínez-Álvarez R, et al. Neuropsychiatric adverse effects of dolutegravir in real-life clinical practice. Enferm Infecc Microbiol Clin (Engl Ed). 2021;39(2):78–82.
Cusato J, Borghetti A, Teti E, Milesi M, Tettoni MC, Bonora S, et al. Dolutegravir discontinuation for neuroosychiatric symptoms in people living with HIV and their outcomes after treatment change: a pharmacogenetic study. Metabolites. 2022;12(12):1202.
Yagura H, Watanabe D, Kushida H, Tomishima K, Togami H, Hirano A, et al. Impact of UGT1A1 gene polymorphisms on plasma dolutegravir trough concentrations and neuropsychiatric adverse events in Japanese individuals infected with HIV-1. BMC Infect Dis. 2017;17(1):622.
George JW, Mattingly JE, Roland NJ, Small CM, Lamberty BG, Fox HS, et al. Physiologically relevant concentrations of dolutegravir, emtricitabine, and efavirenz induce distinct metabolic alterations in HeLa epithelial and BV2 microglial cells. Front Immunol. 2021;12: 639378.
Yombi JC. Dolutegravir neuropsychiatric adverse events: specific drug effect or class effect. AIDS Rev. 2018;20(1):14–26.
Allen Reeves A, Fuentes AV, Caballero J, Thomas JE, Mosley Ii JF, Harrington C. Neurotoxicities in the treatment of HIV between dolutegravir, rilpivirine and dolutegravir/rilpivirine: a meta-analysis. Sex Transm Infect. 2021;97(4):261–7.
Montenegro-Burke JR, Woldstad CJ, Fang M, Bade AN, McMillan J, Edagwa B, et al. Nanoformulated antiretroviral therapy attenuates brain metabolic oxidative stress. Mol Neurobiol. 2019;56:2896–907.
Lee CA, O’Connor MA, Ritchie TK, Galetin A, Cook JA, Ragueneau-Majlessi I, et al. Breast cancer resistance protein (ABCG2) in clinical pharmacokinetics and drug interactions: practical recommendations for clinical victim and perpetrator drug-drug interaction study design. Drug Metab Dispos. 2015;43:490–509.
Moss DM, Liptrott NJ, Curley P, Siccardi M, Back DJ, Owen A. Rilpivirine inhibits drug transporters ABCB1, SLC22A1, and SLC22A2 in vitro. Antimicrob Agents Chemother. 2013;57:5612–8.
Reese MJ, Savina PM, Generaux GT, Tracey H, Humphreys JE, Kanaoka E, et al. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos. 2013;41:353–61.
Cottrell ML, Hadzic T, Kashuba ADM. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet. 2013;52:981–94.
Ma Q, Schifitto G, Venuto C, Ocque A, Dewhurst S, Morse GD, et al. Effect of dolutegravir and sertraline on the blood brain barrier (BBB). J Neuroimmune Pharmacol. 2020;15(1):7–9.
EMA 2013. Assessment Report TIVICAY EMA/CHMP/772068/2013
Funding
This work was supported by statutory grant of Warszawski Uniwersytet Medyczny, 1M9/N/2023.
Author information
Authors and Affiliations
Contributions
AJ—collecting data, interpreting the data, and drafting the manuscript, including Table 1 and Fig. 1. AP—conception of the work, collecting data, drafting the manuscript, interpretation of the data, and corrections. AWD—interpreting the data, critical opinion on clinical part of the manuscript, and approval of final version. AN—interpreting the data, preparing part of the manuscript focused on pharmacokinetics, preparing Fig. 2, corrections, and approval of final version. DMG—conception of the work, interpreting the data, corrections, and approval of final version.
Corresponding author
Ethics declarations
Conflict of interest
AJ, AP, AN, and DMG declare no conflict of interest in the presented area. AWD received consulting fees or honoraria for lectures from GSK, Gilead Sc, MSD, and travel grants from Gilead Sc.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jakimiuk, A., Piechal, A., Wiercińska-Drapało, A. et al. Central nervous system disorders after use of dolutegravir: evidence from preclinical and clinical studies. Pharmacol. Rep 75, 1138–1151 (2023). https://doi.org/10.1007/s43440-023-00515-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00515-y