Abstract
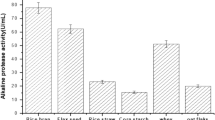
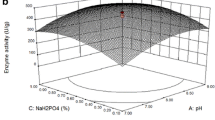
The aim of the study was to optimize fermentation parameters for coproduction of protease and cellulase from Bacillus subtilis M-11 in solid state fermentation by a statistical approach and to evaluate the stability and compatibility of enzymes for detergent formulation. Eight different substrates were investigated to find the best medium supporting maximum enzyme productions. Box–Behnken design (BBD) was employed to optimize culture conditions for the coproduction of proteases and cellulose. The effect of pH and temperature, surfactants and commercial detergents on stability of the enzymes was evaluated. Oat flour was found to be the best substrate for production of both enzymes. The results indicate that BBD is an effective tool for optimizing the medium for coproduction of enzymes and the fermentation parameters that affect production of the enzymes. Furthermore, the enzymes showed excellent compatibility with detergents, as well as high stability in wide ranges of temperature (25–60 °C) and pH (7.0–9.5). In conclusion, the present study offers a cost-effective method to coproduce protease and cellulase from B. subtilis M-11. Also, it can be suggested that both the enzymes have strong potential for usage in the detergent industry.



Similar content being viewed by others
Change history
20 June 2020
The authors found a mistake in Figs. 1 and 2 of this published paper.
References
Beg QK, Gupta R (2003) Purification and characterization of an oxidation-stable, thiol-dependent serine alkaline protease from Bacillus mojavensis. Enzyme Microb Technol 32(2):294–304
Cupp-Enyard C (2008) Sigma’s non-specific protease activity assay-casein as a substrate. J Vis Exp 19:899. https://doi.org/10.3791/899
Garai D, Kumar VA (2013) Box–Behnken design approach for the production of xylanase by Aspergillus candidus under solid state fermentation and its application in saccharification of agro residues and Parthenium hysterophorus L.. Ind Crops Prod 44:352–363
Gaur R, Tiwari S (2015) Isolation, production, purification and characterization of an organic-solvent-thermostable alkalophilic cellulase from Bacillus vallismortis RG-07. BMC Biotechnol 15(19):1–12
George N, Sondhi S, Soni SK, Gupta N (2014) Lime and sulphide-free dehairing of animal skin using collagenase-free alkaline protease from Vibrio metschnikovii NG155. Indian J Microbiol 54(2):139–142
Ghorbel S, Kammoun M, Soltana H, Nasri M, Hmidet N (2014) Streptomyces flavogriseus HS1: isolation and characterization of extracellular proteases and their compatibility with laundry detergents. BioMed Res Int. https://doi.org/10.1155/2014/345980
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268
Govarthanan M, Park SH, Kim JW, Lee KJ, Cho M, Kamala-Kannan S, Oh BT (2014) Statistical optimization of alkaline protease production from brackish environment Bacillus sp. SKK11 by SSF using horse gram husk. Prep Biochem Biotechnol 44(2):119–131
Hmidet N, Ali NEH, Haddar A, Kanoun S, Alya SK, Nasri M (2009) Alkaline proteases and thermostable α-amylase co-produced by Bacillus licheniformis NH1: characterization and potential application as detergent additive. Biochem Eng J 47(1–3):71–79
Imran M, Zahid Anwar M, Zafar M, Ali A, Arif M (2018) Production and characterization of commercial cellulase produced through Aspergillus niger IMMIS1 after screening fungal species. Pak J Bot 50(4):1563–1570
Irfan M, Mushtaq Q, Tabssum F, Shakir HA, Qazi JI (2017) Carboxymethyl cellulase production optimization from newly isolated thermophilic Bacillus subtilis K-18 for saccharification using response surface methodology. AMB Express 7(29):1–9
Kalaiyarasi M, Vijayaraghavan P, Raj SRF, Vincent SGP (2017) Statistical approach for the production of protease and cellulase from Bacillus cereus KA3. Bioprocess Eng 1(4):93–103
Krishna C (2005) Solid-state fermentation systems—an overview. Crit Rev Biotechnol 25(1–2):1–30
Kumar D, Chauhan PS, Puri N, Gupta N (2014) Production of alkaline thermostable protease by immobilized cells of alkalophilic Bacillus sp. NB 34. Int J Curr Microbiol Appl Sci 3(10):1063–1080
Ladeira SA, Cruz E, Delatorre AB, Barbosa JB, Leal Martins ML (2015) Cellulase production by thermophilic Bacillus sp.: SMIA-2 and its detergent compatibility. Electron J Biotechnol 18(2):110–115
Lam MQ, Mut NNN, Thevarajoo S, Chen SJ, Selvaratnam C (2018) Characterization of detergent compatible protease from halophilic Virgibacillus sp. CD6. 3 Biotech 8(2):104
Lee YJ, Kim BK, Lee BH, Jo KI, Lee NK, Chung CH et al (2008) Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Biores Technol 99(2):378–386
Li W, Zhao LC, Wang Z, Zheng YN, Liang J, Wang H (2012) Response surface methodology to optimize enzymatic preparation of deapio-platycodin D and platycodin D from radix platycodi. Int J Mol Sci 13(4):4089–4100
Mhamdi S, Haddar A, Mnif IH, Frikha F, Nasri M, Kamoun AS (2014) Optimization of protease production by Bacillus mojavensis A21 on chickpea and faba bean. Adv Biosci Biotechnol 5(14):1049–1059
Niyonzima FN (2019) Detergent-compatible bacterial cellulases. J Basic Microbiol 59(2):134–147
Niyonzima FN, More SS (2015a) Coproduction of detergent compatible bacterial enzymes and stain removal evaluation. J Basic Microbiol 55(10):1149–1158
Niyonzima FN, More S (2015b) Detergent-compatible proteases: microbial production, properties, and stain removal analysis. Prep Biochem Biotechnol 45(3):233–258
Pandey A, Selvakumar P, Soccol CR, Nigam P (1999) Solid state fermentation for the production of industrial enzymes. Curr Sci 77(1):149–162
Pawar SV, Rathod VK (2018) Optimization of novel and greener approach for the coproduction of uricase and alkaline protease in Bacillus licheniformis by Box–Behnken model. Prep Biochem Biotechnol 48(1):24–33
Petlamul W, Boukaew S (2019) Optimisation and stabilisation of cellulase and xylanase production by Beauveria bassiana. Environ Asia 12(1):11–19
Pouryafar F, Najafpour GD, Noshadi N, Jahanshahi M (2015) Thermostable alkaline protease production via solid state fermentation in a tray bioreactor using Bacillus licheniformis ATCC 21424. Int J Environ Res 9(4):1127–1134
Prakasham RS, Rao CS, Sarma PN (2006) Green gram husk-an inexpensive substrate for alkaline protease production by Bacillus sp. in solid-state fermentation. Bioresour Technol 97(13):1449–1454
Rekik H, Jaouadi NZ, Gargouri F, Bejar W, Frikha F, Jmal N (2019) Production, purification and biochemical characterization of a novel detergent-stable serine alkaline protease from Bacillus safensis strain RH12. Int J Biol Macromol 121:1227–1239
Sadhu S, Saha P, Sen SK, Mayilraj S, Maiti TK (2013) Production, purification and characterization of a novel thermotolerant endoglucanase (CMCase) from Bacillus strain isolated from cow dung. Springer Plus 2:10–20
Sahin S, Ozmen I, Kir E (2015) Purification immobilization and characterization of protease from local Bacillus subtilis M-11. Asia Pac J Chem Eng 10(2):241–247
Sellami-Kamoun A, Haddar A, Ali NEH, Ghorbel-Frikha B, Kanoun S, Nasri M (2008) Stability of thermostable alkaline protease from Bacillus licheniformis RP1 in commercial solid laundry detergent formulations. Microbiol Res 163(3):299–306
Shahid ZH, Irfan M, Nadeem M, Syed Q, Qazi JI (2016) Production purification and characterization of carboxymethyl cellulase from novel strain Bacillus megaterium. Environ Prog Sustain Energy 35:1741–1749
Shajahan S, Moorthy IG, Sivakumar N, Selvakumar G (2017) Statistical modeling and optimization of cellulase production by Bacillus licheniformis NCIM 5556 isolated from the hot spring Maharashtra. India J King Saud Univ Sci 29(3):302–310
Singh S, Bajaj BK (2015) Medium optimization for enhanced production of protease with industrially desirable attributes from Bacillus subtilis K-1. Chem Eng Commun 202(8):1051–1060
Singh S, Bajaj BK (2016) Bioprocess optimization for production of thermoalkali-stable protease from Bacillus subtilis K-1 under solid-state fermentation. Prep Biochem Biotechnol 46(7):717–724
Soccol CR, da Costa ESF, Letti LAJ, Karp SG, Woiciechowski AL, de Souza Vandenberghe LP (2017) Recent developments and innovations in solid state fermentation. Biotechnol Res Inn 1(1):52–71
Sreena CP, Sebastian D (2018) Augmented cellulase production by Bacillus subtilis strain MU S1 using different statistical experimental designs. J Genet Eng Biotechnol 16(1):9–16
Verma V, Verma A, Kushwaha A (2012) Isolation and production of cellulase enzyme from bacteria isolated from agricultural fields in district Hardoi, Uttar Pradesh, India. Adv Appl Sci Res 3:171–174
Zhang Y, Zhang X, Qi W, Xu J, Yuan Z, Wang Z (2018) ANN and RSM based optimization of cellulase production by Hypocrea sp. Z28 by submerged fermentation. Cell Chem Technol 52(3–4):259–264
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özbek Yazıcı, S., Özmen, I. Optimization for coproduction of protease and cellulase from Bacillus subtilis M-11 by the Box–Behnken design and their detergent compatibility. Braz. J. Chem. Eng. 37, 49–59 (2020). https://doi.org/10.1007/s43153-020-00025-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43153-020-00025-x