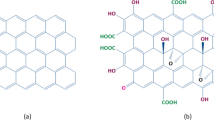
Abstract
In this study, graphene oxide (GO) was synthesized by the improved Hummers’ method. The degree of oxidation from graphite (Gi) to GO was determined through interlayer spacing calculated from X–ray diffraction. Besides, the effect of KMnO4:Gi ratios (X1), H2SO4 volume (X2), oxidation temperature (X3), oxidation time of stage 1 (X4), and oxidation time of stage 2 (X5) was screened by the Plackett–Burman model. The simultaneous impact of three factors that influenced the degree of oxidation (X1, X2, and X3) was studied by the Box–Behnken experimental model of response surface methodology to achieve suitable conditions for the GO synthesis process. The characterization of GO product was investigated via the modern analytical methods: X-ray diffraction, Raman spectroscopy, Fourier transform infrared spectroscopy, UV–Vis spectroscopy, field emission scanning electron microscopy, transmission electron microscopy, and atomic force microscopy. In addition, the study was also carried out on a pilot scale for orientation in industrial application with the yield of 14 g/batch.










Similar content being viewed by others
Data availability statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Ray S (2015) Applications of graphene and graphene-oxide based nanomaterials. Elsevier, Amsterdam
Khan ZU, Kausar A, Ullah H, Badshah A, Khan WU (2016) A review of graphene oxide, graphene buckypaper, and polymer/graphene composites: properties and fabrication techniques. J Plast Film Sheeting 32:336–379
Qureshi TS, Panesar DK (2019) A comparison of graphene oxide, reduced graphene oxide and pure graphene: early age properties of cement composites. In: 2nd RILEM Spring Convention & International Conference on Sustainable Materials, Systems and Structures. Rovinj, Croatia.
Pendolino F, Armata N (2017) Synthesis, characterization and models of graphene oxide. Graphene oxide in environmental remediation process. Springer, Berlin, pp 5–21
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Dimiev AM, Eigler S (2016) Mechanism of formation and chemical structure of graphene oxide. Graphene oxide fundamentals and applications. Wiley, Chichester, pp 36–84
Sun L, Fugetsu B (2013) Mass production of graphene oxide from expanded graphite. Mater Lett 109:207–210
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Chen J, Yao B, Li C, Shi G (2013) An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 64:225–229. https://doi.org/10.1016/j.carbon.2013.07.055
Bennett K, Chen Y (2019) A two-level Plackett-Burman non-geometric experimental design for main and two factor interaction sensitivity analysis of zigzag-channel PCHEs. Therm Sci Eng Prog 11:167–194
Ranade SS, Thiagarajan P (2017) Selection of a design for response surface. IOP Conf. Ser. Mater. Sci. Eng. 263:22043
Zribi M, Samet B, Baklouti S (2020) Screening of factors influencing phosphate-based geopolymers consolidation time, using Plackett-Burman design. Advances in materials, mechanics and manufacturing: Proceedings of the Second International Conference on Advanced Materials, Mechanics and Manufacturing (A3M’2018), December 17–19, 2018 Hammamet, Tunisia. Springer, Berlin, pp 115–122
Paulchamy B, Arthi G, Lignesh BD (2015) A simple approach to stepwise synthesis of graphene oxide nanomaterial. J Nanomed Nanotechnol 6:1
Hou Y, Lv S, Liu L, Liu X (2020) High-quality preparation of graphene oxide via the Hummers’ method: understanding the roles of the intercalator, oxidant, and graphite particle size. Ceram Int 46:2392–2402
Kang JH, Kim T, Choi J, Park J, Kim YS, Chang MS, Jung H, Park KT, Yang SJ, Park CR (2016) Hidden second oxidation step of Hummers method. Chem Mater 28:756–764
Emiru TF, Ayele DW (2017) Controlled synthesis, characterization and reduction of graphene oxide: a convenient method for large scale production. Egypt J Basic Appl Sci 4:74–79
Zhu Y, Kong G, Pan Y, Liu Y, Yang B, Zhang S, Lai D, Che C (2022) An improved Hummers method to synthesize graphene oxide using much less concentrated sulfuric acid. Chin Chem Lett 33:4541–4544
Luo D, Zhang F, Ren Z, Ren W, Yu L, Jiang L, Ren B, Wang L, Wang Z, Yu Y (2019) An improved method to synthesize nanoscale graphene oxide using much less acid. Mater Today Phys 9:100097
Li C, Shi Y, Chen X, He D, Shen L, Bao N (2018) Controlled synthesis of graphite oxide: formation process, oxidation kinetics, and optimized conditions. Chem Eng Sci 176:319–328
An S, Zeng Q, Li W, Fortner J (2021) A graphene oxide Cookbook: exploring chemical and colloidal properties as a function of synthesis parameters. J Colloid Interface Sci 588:725–736
Yuwen C, Liu B, Zhou B, Tian S, Zhang L (2021) Structure and properties of graphene oxide during the synthesis process at fixed temperatures. Ceram Int 47:17487–17493
Khalili D (2016) Graphene oxide: a promising carbocatalyst for the regioselective thiocyanation of aromatic amines, phenols, anisols and enolizable ketones by hydrogen peroxide/KSCN in water. New J Chem 40:2547–2553
Kumar PS, Pavithra KG, Naushad M (2019) Characterization techniques for nanomaterials. Characterization techniques for nanomaterials. Elsevier, Amsterdam, pp 97–124
Krishnamoorthy K, Veerapandian M, Yun K, Kim S-J (2013) The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 53:38–49
Kim S-G, Park O-K, Lee JH, Ku B-C (2013) Layer-by-layer assembled graphene oxide films and barrier properties of thermally reduced graphene oxide membranes. Carbon Lett 14:247–250
Wang J, Wang Y, Wang T, Li G, Lou R, Cheng G, Bai J (2019) Nonlinear optical response of graphene oxide langmuir-blodgett film as saturable absorbers. Nanomaterials 9:640
Acknowledgements
We acknowledge the support of time and facilities from Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest to disclose
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Phuc, N.T., Giang, N.T.H., An, V.N.T.T. et al. Optimization of the eco-friendly synthesis of graphene oxide from graphite using Plackett–Burman and Box–Behnken models for industrial production orientation. Carbon Lett. 33, 489–500 (2023). https://doi.org/10.1007/s42823-022-00439-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42823-022-00439-2