Abstract
The unsatisfactory nutrient slow-release and water-retention performance of traditional biochar-based compound fertilizers (BCF) severely limit their practical application. Herein, a new type of slow-release fertilizer with high water retention was fabricated via the incorporation of hydrotalcite and starch into BCF, named as HS-BCF. The water-retention and nutrient releasing performance of the prepared HS-BCF and related nutrient slow-release mechanism were investigated. The results showed that the incorporation of hydrotalcite and starch into BCF could increase the soil water-retention ratio by 5–10% points. The accumulated N, P, and K leaching amounts of HS-BCF in soil within 30 days were 49.4%, 13.3%, and 87.4% of BCF at most, respectively. Kinetic analysis indicated that the release of nutrients from HS-BCF was attributed to the coupling of the diffusion-controlled and relaxation-controlled mechanism. Moreover, hydrotalcite could bind with P in HS-BCF, contributing to the enhanced durability of P in HS-BCF. Finally, pot experiments showed that the N–P–K utilization efficiencies of HS-BCF were all higher than those of BCF due to a better synchronization between the nutrient release of HS-BCF and the uptake of tomato plants. Overall, the study may provide a promising strategy for simultaneously improving the water-retention and slow-release performance of traditional biochar-based fertilizers.
Graphical Abstract

Article highlights
-
1.
A new biochar fertilizer was developed by incorporating hydrotalcite and starch.
-
2.
HS-BCF exhibited better water-retention and slow-release performance than BCF.
-
3.
The nutrient release of HS-BCF was diffusion and relaxation-controlled mechanism.
-
4.
The enhanced P durability of HS-BCF was partially due to the binding of hydrotalcite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Modern agriculture relies heavily on the extensive use of chemical fertilizers to increase soil fertility and crop yields (Zhang et al. 2018). However, fertilizer use inevitably leads to leaching of nutrients to adjacent water bodies, especially nitrogen (N) and phosphorus (P), causing nutrient pollution and threatening the health of aquatic organisms and humans (Sinha et al. 2022). An important reason for these problems is the low use efficiency of fertilizers (Gao et al. 2022). Recently, increasing attention by researchers around the world has been concentrated on developing slow-release fertilizers to improve the utilization efficiency of nutrients (An et al. 2021b; Bakshi et al. 2021; Salimi et al. 2021). Compared with conventional chemical fertilizers, slow-release fertilizers supply nutrients in a slower manner for a longer duration aiming to better match the requirements of the crop (Sarkar et al. 2021). To date, biochar has been widely considered as one of the most promising fertilizer carriers to delay the release of nutrients, owing to its dense porous structure with abundant functional groups (Lu et al. 2019; Sim et al. 2021). Moreover, biochar is widely regarded as a promising soil amendment, since numerous studies found that the addition of biochar could improve the physical structure of soil (Fungo et al. 2017), increase the cation exchange capacity of soil (Munera-Echeverri et al. 2018), and boost the organic carbon contents of soil (Han et al. 2022; Li et al. 2018). Therefore, biochar-based slow-release fertilizers (BSRFs) have great potential to simultaneously increase the use efficiency of fertilizers and amend the soil.
Incorporating high water-holding materials into BSRFs is expected to improve the applicability of BSRFs in arid and semi-arid regions, which can increase the water retention in soils and thus reduce the frequency of irrigation (An et al. 2021a). Starch is a kind of natural polymer, which is composed of amylose and amylopectin and has multiple hydroxyl groups on its surface (Ali et al. 2011). The abundance and hydrophilicity of starch make it as a suitable candidate to prepare BSRFs with high water retention. Besides, starch possesses other desirable properties, such as biodegradability, non-toxicity, and low cost (Perez and Francois 2016). Notably, starch is an environmentally friendly binding material (Cheng et al. 2022). The presence of starch in BSRFs can effectively enhance the adhesion of nutrients to biochar, which is conducive to improving the slow-release performance of BSRFs, thereby improving the utilization efficiency of nutrients (Dong et al. 2020). However, the excess hydrophilicity and inferior mechanical properties of starch severely hinder the practical application potential of starch-based BSRFs. To tackle these shortcomings, the strategy of coupling starch with some fillers such as cellulose fibers (Franca et al. 2021), clays (Wei et al. 2019), and macromolecular polymers (Perez and Francois 2016; Tian et al. 2017) has been proposed. Of them, clays hold great potential to modify starch for use in BSRFs due to their swellable nature with good water retainability. It has been reported that the incorporation of clays in starch leads to the increased permeation resistance of water molecules (Monteiro et al. 2018, Tan and Thomas 2016) and improved mechanical properties including strength and stiffness (Lendvai et al. 2019). Inspired by these reported studies, we hypothesized that the incorporation of clays and starch into biochar-based fertilizers may be an effective strategy to synthesize promising slow-release fertilizers with high water retention. Given that hydrotalcite is known as an anionic clay with excellent adsorption performance towards nutrients (e.g., phosphate) in aqueous solution (Hsu et al. 2019; Kumari et al. 2022), the presence of hydrotalcite in biochar-based fertilizers may be beneficial to improving the nutrient slow-release performance. Therefore, we studied the incorporation of hydrotalcite and starch into biochar-based fertilizers to improve the nutrient slow-release performance and water-retention capacity.
Our previous study has revealed that a desirable biochar-based P–K slow-release fertilizer could be obtained through the co-pyrolysis of rice straw, nutrients (P and K), and clay. The presence of clay could improve the slow-release performance of nutrients through the improvement of the porous structure of the obtained biochar and the formation of chemical bonds between nutrients and clay (An et al. 2020a). Therefore, the biochar-based P–K slow-release fertilizer was prepared at first in this study by the co-pyrolysis of hydrotalcite, rice straw, calcium superphosphate, and potassium chloride, which was then blended with starch and urea to prepare biochar-based N–P–K slow-release fertilizer. The prepared BSRFs were comprehensively characterized and their water-retention capacities and nutrient slow-release performance were evaluated. Then, the transformation of nutrients during the release process of BSRFs was investigated, together with the analysis of release kinetics and the characterizations of BSRFs before and after the release of nutrients to elucidate the mechanism underlying the release of nutrients from the prepared BSRFs. Finally, pot experiment was performed to verify if the prepared BSRFs could effectively promote the growth of tomato seedlings.
2 Materials and methods
2.1 Chemicals
Urea (CO(NH2)2), superphosphate (Ca(H2PO4)2), ammonium chloride (NH4Cl), ammonium fluoride (NH4F), sodium citrate (C6H5Na3O7), sodium hydrosulfite (Na2S2O4), and starch were purchased from Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Potassium chloride (KCl) and sodium hydroxide (NaOH) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Sulfuric acid (H2SO4) and hydrogen peroxide (H2O2) were purchased from Yonghua Chemical Co., Ltd. (Jiangsu, China). Rice straw and hydrotalcite were produced in Hangzhou, Zhejiang Province, China.
2.2 Synthesis of various BSRFs
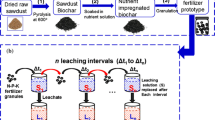
Rice straw was first cut into 2 cm pieces, washed with deionized water, and dried in an oven at 70 °C for 24 h, which was then crushed and sieved to obtain 80-mesh fine powder samples. The obtained samples were transferred to a corundum ark pyrolysis reactor placed in a tubular furnace. The pyrolysis temperature increased from room temperature to 500 °C at the rate of 10 °C min−1, which was then maintained at 500 °C for 2 h to prepare biochar.(Bakshi et al. 2021) Notably, the whole pyrolysis process was carried out under the condition of N2 flow rate of 30 mL min−1. Biochar-based compound fertilizer (BCF) was synthesized by a simple mixture of biochar and N–P–K fertilizers in a fixed mass ratio according to an N:P:K ratio of 16:8:16 (biochar 13.0%, urea 28.6%, Ca(H2PO4)2 32.4%, and KCl 25.9%) (Min et al. 2021). Starch/biochar-based compound fertilizer (S-BCF) was prepared by blending the obtained BCF with H2O2-modified starch at a mass ratio of 70.5:29.5. To further improve the slow-release performance of the starch/biochar-based compound fertilizer, different contents of hydrotalcite (5 wt.%, 10 wt.%, 15 wt.%, and 20 wt.%) were added. As shown in Fig. 1a, hydrotalcite was co-pyrolyzed with rice straw, calcium superphosphate, and potassium chloride at 500 °C for 2 h in a tubular furnace under the condition of N2 flow rate of 30 mL min−1. The obtained pyrolysis product was then blended with starch and urea to prepare hydrotalcite-modified Starch/biochar-based compound fertilizers (HS-BCFs). The HS-BCFs with the hydrotalcite contents of 5 wt.%, 10 wt.%, 15 wt.%, and 20 wt.% were named as H5S-BCF, H10S-BCF, H15S-BCF, and H20S-BCF, respectively. The basic characteristics of the prepared HS-BCFs are shown in Table 1.
2.3 Characterization and analysis
The specific surface area and pore size of the prepared BSRFs were measured by Brunauer–Emmett–Teller (BET, Beckman Coulter, USA). The surface morphologies were examined by scanning electron microscopy (SEM, Hitachi MC1000, Japan) coupled with an energy dispersive spectrometer (EDS, Bruker QUANTAX 400-10, Germany). The used field emission gun was operated at 3 kV. BSRFs samples were mounted on aluminum stubs and sputter-coated with gold. The micrographs were taken at magnifications between 100 and 20,000×. X-ray diffraction (XRD) analysis of the prepared BSRFs was performed on a Rigaku diffractometer with Bragg Brentano geometry and CuKa radiation (λ = 1.5418 Å, 40 kV, 20 mA) in the range of 2θ = 5–50. The scanning rate was 1 min−1 with a scan step of 0.05°. Fourier transform infrared (FTIR) spectra of the prepared BSRFs were recorded on a Nicolet 380 FTIR spectrometer (Thermo Scientific, USA) operating in the range of 4000–400 cm−1 with a resolution of 4 cm−1. BSRFs samples were ground and mixed evenly with KBr at a mass ratio of 1:20. The obtained powder mixture was compressed by a hydraulic press to form KBr discs for FTIR analysis. The chemical compositions of the prepared BSRFs were determined with an X-ray photoelectron spectroscopy (XPS) instrument (Thermo Scientific K-Alpha, USA). The detected peaks were fitted using a XPS 80 Peak 4.1 software.
2.4 Swelling and water-retention capacities of various BSRFs
The procedures for measuring the swelling capacities of the prepared BSRFs were as follows (An et al. 2021c): 0.1 g of each BSRF was wrapped in 400-mesh nylon bag. The obtained nylon bag-wrapped BSRFs were then soaked in 50 mL deionized water for 3 h. Finally, the weights of nylon expanded BSRF samples were measured. To investigate the water-retention capacities of various BSRFs, 1.0 g of each BSRF was mixed with 150 g of dry soil in a square pot. Then, 50 mL of distilled water was poured into the basin and weighed (W0). The square pots were kept at room temperature for 24 days and weighed at regular intervals (Wt). Finally, the soil water-retention ratio (WR%) was calculated by the following equation (Sui et al. 2021):
2.5 Slow-release performance of various BSRFs
To determine the static slow-release performance of the prepared BSRFs in water, 0.1 g of each BSRF sample was immersed in 40 mL of deionized water, as shown in Fig. 1b. Periodically, 1 mL of liquid sample was taken out for the quantitative analysis of N and P released from the prepared BSRFs. Notably, 1 mL of deionized water was added after each test to keep the constant of the total liquid volume. Nitrogen concentration was determined by the indoxyl blue colorimetric method at 655 nm and phosphorus concentration was determined by the molybdenum-blue colorimetric method at 700 nm using an ultraviolet spectrophotometer (UV2600, SDPTOP, Shanghai, China) (An et al. 2021c). Column leaching experiments were carried out to investigate the slow-release performance of the prepared BSRFs in soil. The detailed experimental procedures were as follows (Llive et al. 2019): 150 g of vermiculite (60% humidity) was placed in each column, as shown in Fig. 1c. Then, 1.0 g of each BSRF was buried 5 cm below the vermiculite surface. A 400-mesh nylon bag was wrapped around the bottom of the column to maintain the vermiculite moisture content at 60% by periodic weighing in 30 days. The bottom drench solution was collected to determine the released amounts of N, P, and K. The kinetic models were employed to analyze the slow-release kinetics of the prepared BSRFs with the corresponding equations shown below:
where \(Q\) is the release ratio of P in different time intervals, and \({k}_{1}\) and \({k}_{R}\) are the release rate constants.
2.6 Fractional extraction of phosphorus from various BSRFs
The basic principle of inorganic phosphorus determination method was to use the characteristics of different chemical extracts to separate various forms of inorganic phosphate step by step (Jun et al. 2010). The samples were first extracted with 1 mol L−1 NH4Cl, then shaken and centrifuged. In the second stage, Al–P and Fe–P were basically separated by 0.5 mol L−1 NH4F. In the third stage the samples extracted with 0.1 mol L−1 NaOH, the hydrolysis reaction of Fe and NaOH occurred, and Fe–P was determined. In the fourth stage, the O–P was extracted by 0.3 mol L−1 sodium citrate and sodium hydrosulfite. Finally, Ca–P was extracted by 0.5 mol L−1 H2SO4.
2.7 Pot experiments
Pot experiments were performed to study the effects of BSRFs on plant growth. Tomato seeds were selected for seedling development, which were transplanted into pots containing 1 g of BSRF and 150 g of vermiculite at the seedling stage. Tomato seedlings of similar size were transplanted in each pot (40% humidity) and cultivated in a light-filled environment at 25 °C to 30 °C. These plants were grown for 60 days. The heights of these plants were measured regularly. The roots, stems and leaves of the plants were collected and washed with distilled water, then dried with absorbent paper and the fresh weights of the roots, stems and leaves were measured. Subsequently, they were dried in an oven at 105 °C for 30 min and 70 °C until dry, then their dry weights were measured. Finally, the contents of nitrogen, phosphorus and potassium in the plants were measured and calculated. Each treatment was repeated three times.
2.8 Statistical analysis
Statistical analysis was performed using SPSS, version 17.0 (SPSS Institute, USA) to represent the significant differences in potted outcomes. The statistical technique of ANOVA was used to analyze the differences among different treatments. When different treatments were considered significant, p < 0.05.
3 Results and discussion
3.1 Characterization
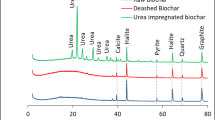
The SEM images of BCF and S-BCF are shown in Fig. 2a. It was observed that nutrient particles and biochar were loosely compounded together, which is a typical morphological characteristic of common biochar-based fertilizers derived from the mixture of biochar and chemical fertilizers (El Sharkawi et al. 2018). After the incorporation of starch, the obtained S-BCF exhibited similar morphology with BCF, but its surface became much smoother (Fig. 2b). This is because the BCF particles in S-BCF could be coated by starch, leading to the decreased roughness of BCF particles. In comparison with BCF and S-BCF, the morphology of the prepared HS-BCF was more compact and the particle size was larger (Fig. 2c), which is likely due to the occurrence of cross-linking reactions among starch, nutrient particles, and hydrotalcite. It has been reported that starch can be cross-linked with clay and nutrients, leading to the decreased porosity of the prepared composite material (Ni et al. 2013). Figure 2d shows a wide and uniform distribution of N, P, and K over the entire structure of HS-BCF, indicating that these nutrients were successfully loaded on HS-BCF. Figure 2e shows the FTIR spectra of BCF, S-BCF, and HS-BCF. The characteristic peaks of N–H were identified at 3332 cm−1 and 1451 cm−1, respectively. The characteristic peaks of \({\text{PO}}_{4}^{3 - }\) and P–O–P were identified at 1074 cm−1 and 866 cm−1 (Zhang et al. 2023). Compared with BCF, the intensities of N–H and P-related peaks of S-BCF and HS-BCF were weaker, which was due to the coating of N–P nutrients by starch and hydrotalcite. The XRD patterns of BCF, S-BCF, and HS-BCF are shown in Fig. 2f. The strong characteristic diffraction peaks of urea, CaHPO4, and KCl were detected in the XRD pattern of BCF. The intensities of these diffraction peaks were weaker in the XRD patterns of S-BCF and HS-BCF, also attributed to the coating of starch and hydrotalcite, which agrees well with the above FTIR analysis. The chemical compositions and states of BCF, S-BCF, and HS-BCF were analyzed by XPS spectra. Several peaks representing C 1 s, O 1 s, N 1 s, P 2p, and K 2p were identified in the XPS survey spectra of BCF, S-BCF, and HS-BCF (Fig. 2g). As shown in Fig. 2h, the N 1 s peak can be resolved into three peaks at 399.3 eV, 400.2 eV, and 401.4 eV, corresponding to N–C, N–C=O, and N–H, respectively (Cheng et al. 2022). As expected, the larger molar percentage of N–C=O of urea was identified in the N 1 s XPS spectra of BCF, S-BCF, and HS-BCF. Compared with N–C and N–C=O, a smaller molar percentage of N–H was identified, which was attributed to the hydrolysis of urea to \({\text{NH}}_{4}^{ + }\) during the preparation processes of these BCF, S-BCF, and HS-BCF (Yao et al. 2015). As shown in Fig. 2i, three peaks of P-related species at the binding energies of 132.8 eV, 133.7 eV, and 134.4 eV were identified in the P 2p XPS spectrum of BCF, which were assigned to \({\text{HPO}}_{4}^{2 - }\), \({\text{H}}_{2} {\text{PO}}_{4}^{2 - }\), and \({\text{PO}}_{4}^{3 - }\), respectively. Compared with BCF and S-BCF, these P-related peaks were blue-shifted due to the formation of chemical bonds between P and hydrotalcite.
3.2 Water-retention and swelling capacities
Water retention is a crucial factor for agricultural soil. Soils with high water-retention capacity help reduce irrigation frequency and promote plant growth by increasing water availability during periods of water scarcity (Sim et al. 2021). As shown in Fig. 3a, the water-retention capacity of soil with the addition of BCF was always the lowest among all tested soils with the addition of different BSRFs, which is due to the hydrophobic nature of biochar (Mao et al. 2019). As expected, the water-retention capacity of soil with the addition of S-BCF was slightly higher than that of soil with the addition of BCF, attributed to the presence of hydrophilic starch in the prepared S-BCF (Mao et al. 2019). Notably, the water-retention capacities of soils with the addition of different HS-BCF were all higher than those of soils with the addition of BCF or S-BCF. This is due to the presence of hydrotalcite in the prepared HS-BCFs, which can efficiently reduce the water repellency of soil (Horoch 2020). It is worthy of note that the best water-retention performance was achieved by HS-BCF with the hydrotalcite content of 10 wt.% in the first 15 days, which was even higher than that of HS-BCFs with the hydrotalcite contents of 15 wt.% and 20 wt.%. A plausible explanation is that a higher content of hydrotalcite leads to the agglomeration of hydrotalcite in HS-BCFs. After 17 days of soil water-retention experiment, all soil samples with the addition of any BSRF reached the water balance. It was found that the water-retention ratio of soil with the addition of H20S-BCF was still as high as 6.92%, which was higher than other tested groups. The result is reasonable, because H20S-BCF had the largest amount of hydrotalcite. With the extension of time, more hydrotalcite in aggregate particles can play the role in reducing the water repellency of soil.
Swelling occurs when the volume of a BSRF increases due to the absorption of a certain amount of solution. Swelling ratio is an important parameter to evaluate the quality of a BSRF (Shen et al. 2021). As shown in Fig. 3b, all HS-BCFs exhibited higher swelling ratios than BCF and S-BCF due to the presence of hydrophilic starch and hydrotalcite in HS-BCFs. However, the measured swelling ratio of S-BCF was lower than that of BCF, which is due to the dissolution of starch in water, leading to the loss of starch from S-BCF. In fact, the dissolution of S-BCF’s starch was observed during the swelling experiments. In addition, it was found the stability of the prepared HS-BCFs in water was much higher than that of S-BCF, and no obvious dissolution of HS-BCFs in water was observed. Since many studies have revealed the occurrence of cross-linking reactions between starch and clays (Anirudhan and Parvathy 2014, Chaudhuri et al. 2020, Peidayesh et al. 2020), the cross-linking reaction of starch with hydrotalcite leads to the enhanced stability for HS-BCFs in water. Based on the above results, it can be concluded that the incorporation of hydrotalcite and starch into biochar-based fertilizers leads to the improved water-retention and swelling capacities of HS-BCFs.
3.3 Slow-release behaviors of nutrients from various BSRFs
The N slow-release behaviors of different BSRFs in water are shown in Fig. 4a. In comparison with BCF, the N release rates of HS-BCFs were significantly reduced, indicating that the co-incorporation of hydrotalcite and starch into BCF led to the improved N slow-release performance of HS-BCFs. As discussed above, the co-presence of starch and hydrotalcite led to the occurrence of cross-linking reactions, which was conducive to reducing the N release rate of HS-BCFs. Besides, the higher content of hydrotalcite in HS-BCFs, the better N slow-release performance of HS-BCFs, further confirming that the presence of hydrotalcite in HS-BCFs is vital to improve the N slow-release performance of HS-BCFs. Similarly, HS-BCFs exhibited the superior P slow-release performance in water when compared with BCF (Fig. 4b). The nutrient release behaviors of different BSRFs in soil are shown in Fig. 4d–f. The accumulated leaching amounts of N from BCF, H5S-BCF, H10S-BCF, H15S-BCF, and H20S-BCF in soil within 30 days reached 100%, 20.82%, 29.06%, 26.53%, and 44.51%, respectively. The accumulated leaching amounts of P from these BSRFs in soil within 30 days reached 100%, 13.27%, 11.15%, 3.13%, and 6.75%, respectively. Compared the nutrients release performance of each BSRF in water, it was found that the N-P slow-release performance of each BSRF in soil was much better than that in water. The direct contact of nutrients with water should be the main explanation for a faster release rate of BSRFs in water (El Assimi et al. 2020).
During the 30-day soil column leaching experiment shown in Fig. 4d–f, the accumulated leaching amounts of nutrients from BCF were all higher than those from HS-BCFs, which agrees very well with the results of leaching in water shown in Fig. 4a, b. Of note, the P slow-release of HS-BCFs was improved the most among these three nutrients, which should be attributed to the presence of chemical interactions between phosphate and the hydrotalcite in HS-BCFs, as confirmed by the XPS analysis of HS-BCFs shown in Fig. 2i. In contrast, the enhanced slow-release of N and K from HS-BCFs is mainly due to the improved porous structure after the co-incorporation of starch and hydrotalcite into BCF. As confirmed by the BET results that the specific surface area of HS-BCF was larger than that of BCF, and the pore volume and pore size were much smaller than those of BCF (Fig. 4c). Our previous study also revealed that the presence of clay in BSRFs can block the pores of biochar to some extents, which is beneficial for improving the nutrients slow-release performance of clay-modified BSRFs (An et al. 2020a). Given that many reported studies have found that clay-modified biochars exhibit excellent adsorption performance towards ammonia and phosphate (An et al. 2020b; Chen et al. 2017; Huang et al. 2020; Xu et al. 2021), it can be reasonably inferred that the presence of hydrotalcite in HS-BCFs promotes the adsorption of N and P by biochar in HS-BCFs, contributing to the enhanced N-P slow-release performance of HS-BCFs.
3.4 Slow-release mechanism of HS-BCF
To explore the slow-release mechanism of the prepared HS-BCF, the release kinetics of nutrients from HS-BCF was studied at first. Two common mathematical models (the first-order kinetic model and the Ritger-Peppas model) were used to describe the slow-release kinetics of nutrients from BCF and HS-BCF. It was found that the Ritger-Peppas model can be used to well describe the release of N and P from HS-BCF in soil and water with the correlation coefficient (R2) of > 0.92 (Fig. 5a, b). The release exponent values for the N release from HS-BCF in soil and water were calculated to be 0.27 and 0.07, respectively, which are less than 0.45, indicating that the N release of HS-BCF in soil and water follows the Fickian diffusion (Shen et al. 2021). Besides, this result further reveals that the release of N from HS-BCF is closely related to the swelling degree of the prepared HS-BCF, and N can diffuse through a swollen matrix and water filled pores in the HS-BCF (Siepmann and Siepmann 2008). Like N, the exponent value for P release from HS-BCF in water was 0.17, which is also less than 0.50, corresponding to the mechanism of Fickian diffusion as well. Notably, the release exponent value for the P release from HS-BCF in soil was calculated to be 3.60, which is greater than 1.00, suggesting that the mechanism for the release of P from HS-BCF in soil is attributed to Case-II transport (Siepmann and Peppas 2011). In contrast to HS-BCF, the first order model can be used to well describe the release of N-P from BCF in soil with R2 of > 0.94 (the insets of Fig. 5a, b). Therefore, the release of N–P from BCF follows a decreasing exponential release curve, representing quick N–P release behavior of BCF. It has been reported that the release of chemical fertilizers such as urea follows the first-order kinetic model (Shen et al. 2021). The presence of biochar in BCF only alters the release rate of nutrients without changing the release mechanism. Like N and P, the release of K from HS-BCF in soil followed the Ritger-Peppas model with the release exponent value of 0.83 corresponding to the mechanism of anomalous transport, and the release of K from BCF in soil follows the first-order kinetic model, as shown in (Fig. 5c) (Shen et al. 2021). Based on the kinetic analysis, it is clear for us to know that the superior slow-release performance of the prepared HS-BCF is attributed to the coupling of the diffusion-controlled and relaxation-controlled mechanism.
The release kinetics of (a) N, (b) P, and (c) K from BCF and HS-BCF. The XPS spectra of (d) N 1 s, (e) P 2p, and (f) K 2p for S-BCF before and after the release of nutrients; (g) FTIR spectra and (h) XRD patterns of HS-BCF before and after the release of nutrients; and (i) The fractions of different P species in BCF and HS-BCF
Subsequently, XPS was used to characterize HS-BCF before and after the release of nutrients. Figure 5d shows that the peak intensity of N–H in the N 1 s XPS spectra of HS-BCF was greatly reduced after 72 h of nutrient release, indicating that N was released from HS-BCF in the form of \({\text{NH}}_{4}^{ + }\). Figure 5e shows that the intensities of the peaks representing \({\text{HPO}}_{4}^{2 - }\) and \({\text{H}}_{2} {\text{PO}}_{4}^{2 - }\) were obviously reduced after 72 h of nutrient release, indicating that P was mainly released from HS-BCF in the form of \({\text{HPO}}_{4}^{2 - }\) and \({\text{H}}_{2} {\text{PO}}_{4}^{2 - }\). As expected, a significant decrease of the peak intensities of K 2p1/2 and K 2p3/2 after 72 h of nutrient release was observed (Fig. 5f). The FTIR spectra of HS-BCF before and after the release of nutrients are shown in Fig. 5g. It was found that the intensities of the peaks representing N–H and P–O–P were significantly reduced or even disappeared after the release of nutrients, indicating that urea and superphosphate in HS-BCF were released in the form of ammonia and phosphate ions. As can be seen from Fig. 5h, the XRD patterns of HS-BCF before and after nutrient release did not show any obvious changes, indicating that no new crystalline substance was formed during the nutrient release process. Figure 5i shows the sequential extraction results of BCF and HS-BCF. The main species of P in HS-BCF were Ca2-P, Ca8-P, and Al-P, which accounted for 16.7%, 32.1%, and 28.6% of the total P in BCF, respectively. Compared with BCF, the content of Ca2-P in HS-BCF decreased by 65%, and the content of Al-P was much higher than BCF. The results showed that the soluble P content in BCF was higher than that in HS-BCF, leading to the inferior P slow-release performance of BCF. Compared with BCF, the content of soluble P in HS-BCF was reduced and the content of P species with higher stability in HS-BCF was increased. This is because the addition of hydrotalcite makes the metallic covalent bond in hydrotalcite stably bind with P in HS-BCF, contributing to the enhanced durability of P in HS-BCF. Notably, the fractional extraction results of P are consistent with the slow-release results in Fig. 5, which further proves that the presence of hydrotalcite in HS-BCF improved the sustained P release performance of HS-BCF. Moreover, the forms of N species existed in BCF and HS-BCF were also studied. It was found that only ammonia nitrogen was identified in BCF and HS-BCF, indicating that the presence of hydrotalcite in HS-BCF did not lead to the formation of stable N-related species. The enhanced N slow-release performance of HS-BCF should be attributed to its improved porous structure and swelling property compared to BCF.
3.5 Pot experiments
To verify if the application of the prepared HS-BCF could effectively promote the growth of plants and improve the utilization efficiency of nutrients, pot experiments using tomato plant as a model plant were performed. As shown in Fig. 6a, there was no significant difference for the heights of tomato plants fertilized by BCF and HS-BCF in the first 15 days. which belongs to the seedling stage of tomato plant. This is because that both BCF and HS-BCF can supply adequate nutrients to meet the need for the growth of tomato seedlings. However, the tomato plants treated by HS-BCF began to grow better than that treated by BCF. The average heights of tomato plants fertilized by BCF and HS-BCF reached 40.7 cm and 45.5 cm, respectively. Based on the nutrients slow-release results shown in Fig. 4, it can be reasonably inferred that the prepared HS-BCF has a better slow-release performance of nutrients, which could provide more adequate nutrients for the growth of tomato plants. Figure 6b shows the average fresh weights and dry weights of tomato plants fertilized by BCF and HS-BCF after 60 days’ cultivation. It was found that the average dry weight and fresh weight of tomato plants fertilized by HS-BCF were 6.34% and 4.57% higher than that fertilized by BCF, respectively, also confirming that the application of HS-BCF can promote the growth of tomato plants more than BCF. Finally, the N, P, and K use efficiencies of tomato plants fertilized by BCF and HS-BCF were also measured, as shown in Fig. 6c. The use efficiencies of N, P, and K for tomato plants fertilized by HS-BCF were 95.41%, 93.67%, and 24.87%, respectively. In contrast, the use efficiencies of N, P, and K for tomato plants fertilized by BCF were only 88.93%, 59.28%, and 21.92%, respectively, which were lower than those fertilized by HS-BCF. This trend agrees very well with their slow-release performance in water or soil shown in Fig. 4, further confirming that a better slow-release performance of nutrients for HS-BCF led to a better synchronization with the uptake of tomato plants in this study. For the practical application of HS-BCF, the coordination between the nutrient release rate of HS-BCF and the absorption rate of plants is crucial. The digital photos of tomato plants fertilized by BCF and HS-BCF in the pot experiments are shown in Fig. 6d. A similar growth for tomato plants fertilized by BCF and HS-BCF during the seedling stage was clearly observed, and the tomato plants fertilized by HS-BCF began to grow better than those fertilized by BCF after 30 days’ cultivation. Finally, the result of economic evaluation showed that the production cost of the HS-BCFs was lower than that of commercial slow-release fertilizers (Table 2) (Tanan et al. 2021).
a The heights of tomato plants fertilized by BCF and HS-BCF; b The fresh weights and dry weights of tomato plants fertilized by BCF and HS-BCF; c The N, P, and K utilization efficiencies of tomato plants fertilized by BCF and HS-BCF; and d The digital photos of tomato plants fertilized by BCF and HS-BCF
4 Conclusions
In this study, a new type of biochar-based slow-release fertilizer with high water retention was developed via the co-incorporation of hydrotalcite and starch into the traditional biochar-based compound fertilizer. The results showed that the presence of hydrotalcite and starch in the prepared HS-BCF increased the water-retaining ability of soil and durability of nutrients in HS-BCF. The incorporation of hydrotalcite and starch into BCF could increase the soil water-retention ratio by 5–10% points. The maximum leaching ratio of N, P, and K from HS-BCF during the 30-day soil column leaching experiment were 44.51%, 13.27%, and 87.38%, respectively, which were all less than that of BCF. The superior slow-release performance of the prepared HS-BCF was attributed to the coupling of the diffusion-controlled and relaxation-controlled mechanism. Admittedly, the HS-BCF developed here may fall short in terms of the high production cost. Particularly, the synthetic process of the HS-BCF involves the co-pyrolysis and blending, which is much more complicated than that of common BCFs. However, the strategy of incorporating hydrotalcite and starch into the traditional BCFs has been demonstrated here to be a feasible way to improve their nutrient slow-release performance. Further improvements of the prepared HS-BCFs’ application prospects may be expected in the near future from optimized synthetic processes and reduced production cost.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ali SS, Tang X, Alavi S, Faubion J (2011) Structure and physical properties of starch/poly vinyl alcohol/sodium montmorillonite nanocomposite films. J Agric Food Chem 59(23):12384–12395. https://doi.org/10.1021/jf201119v
An X, Wu Z, Yu J, Cravotto G, Liu X, Li Q, Yu B (2020a) Copyrolysis of biomass, bentonite, and nutrients as a new strategy for the synthesis of improved biochar-based slow-release fertilizers. ACS Sustain Chem Eng 8(8):3181–3190. https://doi.org/10.1021/acssuschemeng.9b06483
An X, Wu Z, Yu J, Ge L, Li T, Liu X, Yu B (2020b) High-efficiency reclaiming phosphate from an aqueous solution by bentonite modified biochars: a slow release fertilizer with a precise rate regulation. ACS Sustain Chem Eng 8(15):6090–6099. https://doi.org/10.1021/acssuschemeng.0c01112
An X, Wu Z, Liu X, Shi W, Tian F, Yu B (2021a) A new class of biochar-based slow-release phosphorus fertilizers with high water retention based on integrated co-pyrolysis and co-polymerization. Chemosphere 285:131481. https://doi.org/10.1016/j.chemosphere.2021.131481
An X, Wu Z, Qin H, Liu X, He Y, Xu X, Li T, Yu B (2021b) Integrated co-pyrolysis and coating for the synthesis of a new coated biochar-based fertilizer with enhanced slow-release performance. J Clean Prod 283:124642. https://doi.org/10.1016/j.jclepro.2020.124642
An X, Wu Z, Shi W, Qi H, Zhang L, Xu X, Yu B (2021c) Biochar for simultaneously enhancing the slow-release performance of fertilizers and minimizing the pollution of pesticides. J Hazard Mater 407:124865. https://doi.org/10.1016/j.jhazmat.2020.124865
Anirudhan T, Parvathy J (2014) Novel semi-IPN based on crosslinked carboxymethyl starch and clay for the in vitro release of theophylline. Int J Biol Macromol 67:238–245. https://doi.org/10.1016/j.ijbiomac.2014.03.041
Bakshi S, Banik C, Laird DA, Smith R, Brown RC (2021) Enhancing biochar as scaffolding for slow release of nitrogen fertilizer. ACS Sustain Chem Eng 9(24):8222–8231. https://doi.org/10.1021/acssuschemeng.1c02267
Chaudhuri SD, Mandal A, Dey A, Chakrabarty D (2020) Tuning the swelling and rheological attributes of bentonite clay modified starch grafted polyacrylic acid based hydrogel. Appl Clay Sci 185:105405. https://doi.org/10.1016/j.clay.2019.105405
Chen L, Chen XL, Zhou CH, Yang HM, Ji SF, Tong DS, Zhong ZK, Yu WH, Chu MQ (2017) Environmental-friendly montmorillonite-biochar composites: facile production and tunable adsorption-release of ammonium and phosphate. J Clean Prod 156:648–659. https://doi.org/10.1016/j.jclepro.2017.04.050
Cheng J, Liao Z, Hu S-C, Geng Z-C, Zhu M-Q, Xu W-Z (2022) Synthesis of an environmentally friendly binding material using pyrolysis by-products and modified starch binder for slow-release fertilizers. Sci Total Environ 819:153146. https://doi.org/10.1016/j.scitotenv.2022.153146
Dong D, Wang C, Van Zwieten L, Wang H, Jiang P, Zhou M, Wu W (2020) An effective biochar-based slow-release fertilizer for reducing nitrogen loss in paddy fields. J Soils Sediments 20(8):3027–3040. https://doi.org/10.1007/s11368-019-02401-8
El Assimi T, Lakbita O, El Meziane A, Khouloud M, Dahchour A, Beniazza R, Boulif R, Raihane M, Lahcini M (2020) Sustainable coating material based on chitosan-clay composite and paraffin wax for slow-release DAP fertilizer. Int J Biol Macromol 161:492–502. https://doi.org/10.1016/j.ijbiomac.2020.06.074
El Sharkawi HM, Tojo S, Chosa T, Malhat FM, Youssef AM (2018) Biochar-ammonium phosphate as an uncoated-slow release fertilizer in sandy soil. Biomass Bioenerg 117:154–160. https://doi.org/10.1016/j.biombioe.2018.07.007
Franca D, Angelo LM, Souza CF, Faez R (2021) Biobased poly (3-hydroxybutyrate)/starch/cellulose nanofibrils for nutrients coatings. ACS Appl Polym Mater 3(6):3227–3237. https://doi.org/10.1021/acsapm.1c00418
Fungo B, Lehmann J, Kalbitz K, Thionģo M, Okeyo I, Tenywa M, Neufeldt H (2017) Aggregate size distribution in a biochar-amended tropical Ultisol under conventional hand-hoe tillage. Soil Tillage Res 165:190–197. https://doi.org/10.1016/j.still.2016.08.012
Gao Y, Fang Z, Van Zwieten L, Bolan N, Dong D, Quin BF, Meng J, Li F, Wu F, Wang H (2022) A critical review of biochar-based nitrogen fertilizers and their effects on crop production and the environment. Biochar 4(1):1–19. https://doi.org/10.1007/s42773-022-00160-3
Han J, Zhang A, Kang Y, Han J, Yang B, Hussain Q, Wang X, Zhang M, Khan MA (2022) Biochar promotes soil organic carbon sequestration and reduces net global warming potential in apple orchard: a two-year study in the Loess Plateau of China. Sci Total Environ 803:150035. https://doi.org/10.1016/j.scitotenv.2021.150035
Horoch O (2020) The effect of hydrotalcite, analogues and biological materials on soil water repellency and remediation, Murdoch University. http://researchrepository.murdoch.edu.au/id/eprint/58918
Hsu L-C, Tzou Y-M, Chiang P-N, Fu W-M, Wang M-K, Teah HY, Liu Y-T (2019) Adsorption mechanisms of chromate and phosphate on hydrotalcite: a combination of macroscopic and spectroscopic studies. Environ Pollut 247:180–187. https://doi.org/10.1016/j.envpol.2019.01.012
Huang X, Bai J, Li K, Zhao Y, Tian W, Hu C (2020) Preparation of clay/biochar composite adsorption particle and performance for ammonia nitrogen removal from aqueous solution. J Ocean Univ China 19(3):729–739. https://doi.org/10.1007/s11802-020-4150-9
Jun W, Wen-Zhao L, Han-Feng M, Ting-Hui D (2010) Inorganic phosphorus fractions and phosphorus availability in a calcareous soil receiving 21-year superphosphate application. Pedosphere 20(3):304–310. https://doi.org/10.1016/s1002-0160(10)60018-5
Kumari S, Sharma A, Kumar S, Thakur A, Thakur R, Bhatia SK, Sharma AK (2022) Multifaceted potential applicability of hydrotalcite-type anionic clays from green chemistry to environmental sustainability. Chemosphere 306:135464. https://doi.org/10.1016/j.chemosphere.2022.135464
Lendvai L, Sajó I, Karger-Kocsis J (2019) Effect of storage time on the structure and mechanical properties of starch/bentonite nanocomposites. Starch-Stärke 71(1–2):1800123. https://doi.org/10.1002/star.201800123
Li Y, Li Y, Chang S, Yang Y, Fu S, Jiang P, Luo Y, Yang M, Chen Z, Hu S (2018) Biochar reduces soil heterotrophic respiration in a subtropical plantation through increasing soil organic carbon recalcitrancy and decreasing carbon-degrading microbial activity. Soil Biol Biochem 122:173–185. https://doi.org/10.1016/j.soilbio.2018.04.019
Llive L, Bruno E, Molina-García AD, Schneider-Teixeira A, Deladino L (2019) Biodegradation of yerba mate waste based fertilizer capsules. Effect of temperature. J Polym Environ 27(6):1302–1316. https://doi.org/10.1007/s10924-019-01433-y
Lu X, Li Y, Wang H, Singh B, Hu S, Luo Y, Li J, Xiao Y, Cai X, Li Y (2019) Responses of soil greenhouse gas emissions to different application rates of biochar in a subtropical Chinese chestnut plantation. Agric for Meteorol 271:168–179. https://doi.org/10.1016/j.agrformet.2019.03.001
Mao J, Zhang K, Chen B (2019) Linking hydrophobicity of biochar to the water repellency and water holding capacity of biochar-amended soil. Environ Pollut 253:779–789. https://doi.org/10.1016/j.envpol.2019.07.051
Min J, Sun H, Wang Y, Pan Y, Kronzucker H, Zhao D, Shi W (2021) Mechanical side-deep fertilization mitigates ammonia volatilization and nitrogen runoff and increases profitability in rice production independent of fertilizer type and split ratio. J Clean Prod 316:128370. https://doi.org/10.1016/j.jclepro.2021.128370
Monteiro MKS, Oliveira VRLd, Santos FKGd, Neto EB, Leite RHdL, Aroucha EMM, Silva RR, Silva KNdO (2018) Incorporation of bentonite clay in cassava starch films for the reduction of water vapor permeability. Food Res Int 105:637–644. https://doi.org/10.1016/j.foodres.2017.11.030
Munera-Echeverri JL, Martinsen V, Strand LT, Zivanovic V, Cornelissen G, Mulder J (2018) Cation exchange capacity of biochar: an urgent method modification. Sci Total Environ 642:190–197. https://doi.org/10.1016/j.scitotenv.2018.06.017
Ni X, Wu Y, Wu Z, Wu L, Qiu G, Yu L (2013) A novel slow-release urea fertiliser: physical and chemical analysis of its structure and study of its release mechanism. Biosys Eng 115(3):274–282. https://doi.org/10.1016/j.biosystemseng.2013.04.001
Peidayesh H, Ahmadi Z, Khonakdar HA, Abdouss M, Chodák I (2020) Fabrication and properties of thermoplastic starch/montmorillonite composite using dialdehyde starch as a crosslinker. Polym Int 69(3):317–327. https://doi.org/10.1002/pi.5955
Perez JJ, Francois NJ (2016) Chitosan-starch beads prepared by ionotropic gelation as potential matrices for controlled release of fertilizers. Carbohyd Polym 148:134–142. https://doi.org/10.1016/j.carbpol.2016.04.054
Salimi M, Motamedi E, Safari M, Motesharezadeh B (2021) Synthesis of urea slow-release fertilizer using a novel starch-g-poly (styrene-co-butylacrylate) nanocomposite latex and its impact on a model crop production in greenhouse. J Cleaner Prod 322:129082. https://doi.org/10.1016/j.jclepro.2021.129082
Sarkar A, Biswas DR, Datta SC, Dwivedi BS, Bhattacharyya R, Kumar R, Bandyopadhyay KK, Saha M, Chawla G, Saha JK (2021) Preparation of novel biodegradable starch/poly (vinyl alcohol)/bentonite grafted polymeric films for fertilizer encapsulation. Carbohyd Polym 259:117679. https://doi.org/10.1016/j.carbpol.2021.117679
Shen Y, Wang H, Liu Z, Li W, Liu Y, Li J, Wei H, Han H (2021) Fabrication of a water-retaining, slow-release fertilizer based on nanocomposite double-network hydrogels via ion-crosslinking and free radical polymerization. J Ind Eng Chem 93:375–382. https://doi.org/10.1016/j.jiec.2020.10.014
Siepmann J, Peppas NA (2011) Higuchi equation: derivation, applications, use and misuse. Int J Pharm 418(1):6–12. https://doi.org/10.1016/j.ijpharm.2011.03.051
Siepmann J, Siepmann F (2008) Mathematical modeling of drug delivery. Int J Pharm 364(2):328–343. https://doi.org/10.1016/j.ijpharm.2008.09.004
Sim D, Tan I, Lim L, Hameed B (2021) Encapsulated biochar-based sustained release fertilizer for precision agriculture: a review. J Clean Prod 303:127018. https://doi.org/10.1016/j.jclepro.2021.127018
Sinha E, Calvin KV, Kyle PG, Hejazi MI, Waldhoff ST, Huang M, Vishwakarma S, Zhang X (2022) Implication of imposing fertilizer limitations on energy, agriculture, and land systems. J Environ Manage 305:114391. https://doi.org/10.1016/j.jenvman.2021.114391
Sui X, Guo H, Cai C, Li Q, Wen C, Zhang X, Wang X, Yang J, Zhang L (2021) Ionic conductive hydrogels with long-lasting antifreezing, water retention and self-regeneration abilities. Chem Eng J 419:129478. https://doi.org/10.1016/j.cej.2021.129478
Tan B, Thomas NL (2016) A review of the water barrier properties of polymer/clay and polymer/graphene nanocomposites. J Membr Sci 514:595–612. https://doi.org/10.1016/j.memsci.2016.05.026
Tanan W, Panichpakdee J, Suwanakood P, Saengsuwan S (2021) Biodegradable hydrogels of cassava starch-g-polyacrylic acid/natural rubber/polyvinyl alcohol as environmentally friendly and highly efficient coating material for slow-release urea fertilizers. J Ind Eng Chem 101:237–252. https://doi.org/10.1016/j.jiec.2021.06.008
Tian H, Wang K, Liu D, Yan J, Xiang A, Rajulu AV (2017) Enhanced mechanical and thermal properties of poly (vinyl alcohol)/corn starch blends by nanoclay intercalation. Int J Biol Macromol 101:314–320. https://doi.org/10.1016/j.ijbiomac.2017.03.111
Wei H, Wang H, Chu H, Li J (2019) Preparation and characterization of slow-release and water-retention fertilizer based on starch and halloysite. Int J Biol Macromol 133:1210–1218. https://doi.org/10.1016/j.ijbiomac.2019.04.183
Xu Q, Liu T, Li L, Liu B, Wang X, Zhang S, Li L, Wang B, Zimmerman AR, Gao B (2021) Hydrothermal carbonization of distillers grains with clay minerals for enhanced adsorption of phosphate and methylene blue. Bioresour Technol 340:125725. https://doi.org/10.1016/j.biortech.2021.125725
Yao C, Stephen J, Li L, Pan G, Lin Y, Paul M, Pace B, Sarasadat T, Lukas VZ, Torsten T (2015) Developing more effective enhanced biochar fertilisers for improvement of pepper yield and quality. Pedosphere 25(5):703–712. https://doi.org/10.1016/s1002-0160(15)30051-5
Zhang J, Balkovič J, Azevedo LB, Skalský R, Bouwman AF, Xu G, Wang J, Xu M, Yu C (2018) Analyzing and modelling the effect of long-term fertilizer management on crop yield and soil organic carbon in China. Sci Total Environ 627:361–372. https://doi.org/10.1016/j.scitotenv.2018.01.090
Zhang H, Yang H, Shao J, Chen Y, Zhang S, Chen H (2023) Multifunctional carboxymethyl cellulose sodium encapsulated phosphorus-enriched biochar composites: multistage adsorption of heavy metals and controllable release of soil fertilization. Chem Eng J 453:139809. https://doi.org/10.1016/j.cej.2022.139809
Acknowledgements
Not applicable.
Funding
This work was financially supported by the Key Research and Development Project of Science and Technology Department of Zhejiang Province (2023C02019), the National Key Research and Development Program of China (2022YFE0127800), the National Natural Science Foundation of China (22006018), the talent starting-up project of research development fund of Zhejiang A&F University (2034020103), and the Overseas Expertise Introduction Project for Discipline Innovation (111 Project D18008).
Author information
Authors and Affiliations
Contributions
JL: Investigation, Methodology, Formal analysis, Writing—original draft. YL: Investigation, Formal analysis, YC: Investigation, Formal analysis. PJ: Writing—review and editing. BY: Conceptualization, Supervision, Funding acquisition, Writing—original draft, Project administration.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Bin Gao.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, J., Li, Y., Cai, Y. et al. Co-incorporation of hydrotalcite and starch into biochar-based fertilizers for the synthesis of slow-release fertilizers with improved water retention. Biochar 5, 44 (2023). https://doi.org/10.1007/s42773-023-00242-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00242-w