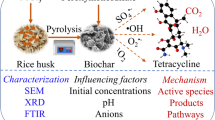
Abstract
Metal-free photocatalysts have attracted growing concern recently. Herein, the composites combining g-C3N4 with wood pulp cellulose biochar (WPBC/g-C3N4) were synthesized to effectively activate peroxymonosulfate (PMS) under visible light for the degradation of diclofenac (DCF). The incorporation of WPBC endowed g-C3N4 with enhanced visible light absorption, improved charge separation capability, reduced electrical conductivity, and increased photocatalytic and PMS activation capability. Based on quenching tests, electron paramagnetic resonance (EPR), electrochemical analysis and solvent exchange experiments, both radical and nonradical mechanisms were proposed. Radical species including ·OH, h+, ·O2– were identified to contribute to DCF degradation. The 1O2 and electron transfer were the dominant nonradical pathways for DCF degradation. Moreover, the common influencing factors were examined, and DCF concentration was the most influential factor based on principal component analysis. Generally, the composites exhibited good reusability during consecutive runs. Based on HPLC/MS analysis, four intermediates were detected and the possible DCF degradation pathway was proposed. This work provided a potential strategy based on metal-free WPBC/g-C3N4 for the photocatalytic activation of PMS to effectively degrade emerging contaminants in wastewater.
Graphical abstract

Highlights
-
Wood pulp cellulose biochar (WPBC) improved the catalytic performance of g-C3N4 via both radical and nonradical mechanisms.
-
The photoelectrical properties and PMS activation capability were improved by WPBC.
-
WPBC/g-C3N4 presented high stability for multiple runs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pharmaceuticals and personal care products (PPCPs) have caused increasing public concern for their adverse effects (Guo et al. 2022; Wang and Wang 2016; Wang et al. 2021). Diclofenac (DCF), as one of the PPCPs, is produced extensively and widely used in human medical care. The damage of DCF to different organs even at low concentrations has been reported (Horl 2010). DCF is one of the most frequently detected PPCPs in sewage treatment plant effluents, estuaries, rivers, surface water, ground water, and even in drinking water (Bu et al. 2013). Therefore, effective methods are needed to minimize DCF discharge to natural water body.
The SO4·−-based advanced oxidation technologies (SR-AOTs) have been intensively investigated to degrade organic contaminants due to the high oxidation potential (2.60–3.10 V), wide pH adaptation range and long half-life period (30–40 μs) of SO4·− (Oh et al. 2016). Sulfate radicals are mainly produced by peroxymonosulfate (PMS) or persulfate (PS) activation, and PMS is easier to be activated due to its asymmetry structure. Compared with the mostly investigated activating methods like transition metals and those needing large energy input (e.g., ultrasound, UV, heat), carbon materials have shown distinct advantages mainly due to the environmental friendly property. However, the active species produced in PMS activation by a single carbon material is always dominated by singlet oxygen (1O2) (Lee et al. 2020), which shows limited oxidation capacity.
Among various carbon catalysts, graphitic carbon nitride (g-C3N4) has received increasing attention as a photocatalyst due to the visible light responsive property, facile synthesis processes, and easily accessible precursors (Zhang et al. 2019). The photoelectron excited from g-C3N4 has been reported to be able to activate PMS (Ghanbari and Moradi 2017), and the dominant radicals could change from ·O2– in direct photocatalysis to SO4·–/OH in the presence of PMS (Xu et al. 2020). However, being limited by the relatively narrow absorption range of visible light (< 460 nm) (Masih et al. 2017), small specific surface area, and rapid recombination of electron–hole pairs, the photocatalytic capability of g-C3N4 is moderate (Cao et al. 2015). A series of studies have shown that carbon materials with g-C3N4 could improve its photocatalytic activity. Graphene could facilitate the separation of photogenerated carriers to enhance the photocatalytic capability of g-C3N4 (Yu et al. 2014). Activated carbon could enhance the photocatalytic activity of g-C3N4 mainly by intensifying adsorption (Chen et al. 2016). Compared to the above carbon materials, biochar has the advantages of facile preparation and high cost-effectiveness. However, limited studies have been carried out investigating the combination of g-C3N4 with biochar to activate PMS under visible light.
Some studies reported that biochar alone could activate PMS to degrade pollutants. Utilizing wet sludge to prepare biochar could activate PMS to degrade sulfamethoxazole, and 1O2 was the dominant contributor (Xu et al. 2020). Pine needle biochar could also activate PMS to degrade 1,4-dioxane, and the dominant reactive oxygen species (ROS) were detected as ·OH and 1O2 (Ouyang et al. 2019). In addition, biochar was also found to improve the activity of photocatalysts. The introduction of reed straw biochar could enhance the photocatalytic performance of Fe3O4/BiOBr by 65% (Li et al. 2019). Biochar was also reported to enhance the photocatalytic performance of g-C3N4. The separation efficiency of electron–hole pairs of g-C3N4 was enhanced by introducing fade magnolia biochar (Zhu et al. 2018a, b). The presence of rice straw biochar narrowed the bandgap and broadened light absorption range of g-C3N4 (Meng et al. 2020). However, there are hardly any studies reporting the performance of PMS activation by biochar/g-C3N4 composite, which is the main interest of this study.
Wood pulp cellulose (WP), as an important biomass waste during the pulp and paper mills production, is the dominant component of lignocellulosic biomass and an excellent raw material for producing fibrous biochar (Mandeep et al. 2020). Effective conversion of these wastes to useful catalysts for wastewater treatment may provide a sustainable alternative to their disposal. Besides, WP is mainly composed of cellulose, and high graphitized carbon materials can be prepared by carbonization. Herein, wood pulp cellulose biochar (WPBC)/g-C3N4 composites were prepared and the capability for activating PMS under visible light to degrade DCF was evaluated. The role of WPBC in improving the catalytic performance of g-C3N4 was investigated emphatically from the aspects of catalyst properties, DCF degradation kinetics, and the generation of ROS. The effects of WPBC doping amount, reagents dosage, solution pH and common anions were also investigated. Finally, the underlying mechanisms of DCF degradation were proposed.
2 Experimental
2.1 Materials and chemical reagents
The WP was obtained from Nippon Paper Chemicals (Japan). The diclofenac sodium, PMS (Oxone®), tert butyl alcohol (TBA, ≥ 99.0%) and p-benzoquinone (p-BQ, ≥ 98.0%) were purchased from Sigma-Aldrich, USA. The phenol (≥ 99.0%), ethanol (EtOH, ≥ 99.7%), l-histidine, Na2S2O3 (≥ 99.0%), Na2SO4 (≥ 99.0%), NaCl (≥ 99.5%), NaNO3 (≥ 99.0%), NaHCO3 (≥ 99.7%) and NaH2PO4 (≥ 99.0%) were all purchased from Sinopharm Chemical, China. The ammonium oxalate monohydrate (AO, ≥ 99.8%), 5,5-Dimethyl-1-pyrroline N-oxide (DMPO, ≥ 97.0%) and 2,2,6,6-tetramethylpiperidine (TMP, ≥ 98.0%) were obtained from Shanghai Aladdin Biochemical, China. The deuterium oxide (D2O, ≥ 95%) was purchased from J&K, China. The melamine was obtained from Shanghai Lingfeng Chemical, China. Solution pH was adjusted by 0.5 M H2SO4 and 0.5 M NaOH. Ultrapure water prepared by the HHitech water purification machine (HHitech Smart-DUV) was used exclusively.
2.2 Synthesis of samples
2.2.1 Preparation of WPBC
The WPBC was prepared by pyrolysis. The WP was put into a tubular furnace and heated at 5 °C/min to 200 °C for 1 h, and then heated at 5 °C/min to 800 °C for 3 h in N2 atmosphere. After that, the samples were naturally cooled down to ambient temperature, ground into powder and denoted as WPBC.
2.2.2 Synthesis of WPBC/g-C3N4 composites
The composites of WPBC/g-C3N4 were also prepared by pyrolysis. Briefly, a certain amount of WPBC powder was added to 25 mL ultrapure water and sonicated for 24 h to achieve a well-dispersed mixture. The weight of WPBC was set as 5, 10, 20, 50, 100, 200, 400 and 500 mg, respectively. Then, 2 g melamine was added into the mixture and stirred until uniformly dispersed, which was further put into an oven and dried at 80 °C for 24 h to paste. The obtained products were put into a tubular furnace and heated at 5 °C/min to 200 °C for 1 h, then heated at 5 °C/min to 550 °C for 3 h in N2 atmosphere. The obtained samples were cooled down to room temperature and ground into powder, resulting in the WPBC/g-C3N4 with different WPBC doping amounts.
2.3 Characterization
The morphologies of the obtained samples were investigated by scanning electron microscopy (SEM, FEI Quanta 200) and transmission electron microscopy (TEM, FEI F20). Raman spectroscopy (Raman, Thermo DXR532) was employed to characterize the surface properties of the samples with a 780 nm Ar laser. The crystal structures of the samples were measured by X-ray diffractometer (XRD, Rigaku Smartlab) with a Cu-Kα radiation source (1.54 Å). X-ray photoelectron spectroscopy (XPS, Kratos Axis Ultra DLD) was used to analyze surface chemical compositions and element valence states. The optical properties were investigated by diffuse reflectance spectra (DRS, Perkin Elmer Lambda 950). Brunauer–Emmett–Teller (BET) specific surface area and pore size distribution were investigated by N2 adsorption–desorption method on a Micromeritics ASAP 2020 HD88 instrument. The point of zero charge (pHzpc) of catalyst was determined using the pH drift method. The thermal stability of the samples was analyzed through thermogravimetric analysis (TGA, Netzsch STA 2500 Regulus) with the temperature rising from 30 to 1000 °C at a constant heating rate of 10 °C/min in N2 atmosphere.
2.4 Experimental conditions
Reactions were conducted in a quartz beaker with continuous stirring, which was soaked in a tank filled with cooling water to maintain the solution temperature at 22 ± 1 °C. The photocatalytic experiments were performed under visible light irradiation using a 200 W light-emitting diode (LED) lamp (λ > 420 nm). The solution volume was 100 mL for all experiments. Except for experiments examining different parameters, the initial DCF concentration, catalyst dosage and PMS concentration were fixed at 0.01 mM, 0.5 g/L, and 2.0 mM, respectively.
In a typical experiment, the as-prepared catalyst was added in 50 mL of ultrapure water and a 10 min ultrasonic period was allowed to ensure well dispersion. Then, 50 mL of DCF water solution was added to obtain the required DCF concentration. The mixture was stirred in the dark for 30 min to reach an adsorption–desorption equilibrium. Afterwards, PMS stock solution (regardless of the volume change) was added and light was turned on simultaneously to start the reaction. The solution pH during the reaction was not adjusted nor controlled. At specified time intervals, 1.0 mL samples were withdrawn and quenched with 0.5 mL concentrated sodium thiosulfate, which were further filtered through a membrane (0.22 μm) for analysis after good shake.
For experiments under anoxic conditions, N2 purging was conducted beforehand during the dark adsorption period to remove O2 and still kept during the whole catalytic reaction, which maintained the DO at 0.3–1.0 mg/L.
All experiments were repeated 2–3 times until the error was below 5% and the average values were used to plot.
2.5 Analytical methods
The DCF concentration was determined by high performance liquid chromatography (HPLC; Dionex Ultimate 3000) equipped with a reverse-phase C18 column (250 mm × 4.6 mm × 3 μm) and a UV detector. The mobile phase was a mixture of 80/20% (v/v) methanol and 0.1% phosphoric acid water solution at a flow rate of 1.0 mL/min. The detection wavelength for DCF was set as 276 nm. The degradation intermediates of DCF were analyzed by HPLC/MS (Thermo LTQ Orbitrap XL) equipped with an electrospray ionization source and an electrostatic orbitrap mass analyzer. The gradient programme of the mobile phase is given in Fig. S1. Negative mode was adopted to identity the intermediates. The reactive species produced in the catalytic oxidation system were determined by the electron paramagnetic resonance (EPR) spectroscopy (Bruker E500-9.5/12). The total organic carbon (TOC) concentration was quantified by Analytic Jena multi N/C 3100 TOC. The photocurrent response, electrochemical impedance spectra (EIS) and linear sweep voltammetry (LSV) analysis were all determined by an electrochemical workstation (CHI660E) with a standard three-electrode method.
3 Results and discussion
3.1 Characterization of the catalysts
Figure 1a–c shows the SEM images of WP, WPBC and WPBC50/g-C3N4. Fibrous structures can be observed for both WP and WPBC, while WPBC has smaller fiber diameter due to the decomposition of functional groups on WP during pyrolysis. On the surface of WPBC50/g-C3N4 composite, the fragmented g-C3N4 are attached to fibrous WPBC and no obvious interface is observed between two phases, which proves the successful hybridization of WPBC50/g-C3N4. Figure S2a displays the SEM image of g-C3N4. It can be seen that g-C3N4 exhibited tightly stacked layer morphology, which was significantly exfoliated by WPBC. The TEM image of WPBC50/g-C3N4 further demonstrates the successful synthesis of WPBC50/g-C3N4 (Fig. 1d), in which WPBC fibers are wrapped by sheet-structured g-C3N4.
The XRD analysis was conducted to examine the structures of prepared samples. As shown in Fig. 2a, WP presents a strong single peak at 22.52° and two overlapped weaker peaks at 15.38° and 16.08°, ascribing to the (002), (101) and \(\left( {10\overline{1}} \right)\) lattice planes of typical cellulose structure (Zhao et al. 2017). After pyrolyzation, the obtained WPBC shows two new peaks at 24.00° and 42.04°, corresponding well to the (002) and (100) layers of the graphitized carbonaceous materials (Hu et al. 2020). As presented in Fig. 2b, two typical peaks of g-C3N4 can be seen. One intensive peak at 27.42° representing the (002) plane is ascribed to the aromatic interlayer stacking in g-C3N4, and the small peak near 13.10° is a typical (100) plane peak due to the in-plane structure of aromatic system (Liu et al. 2011). After compositing WPBC, the XRD patterns of WPBC50/g-C3N4 are similar to that of g-C3N4, proving that the doping of WPBC does not influence the crystal structure of g-C3N4.
Figure S2b shows the Raman spectra of WP and WPBC. After pyrolysis, two significant peaks can be found at around 1340 cm−1 and 1597 cm−1 for WPBC, which represents the disordered carbon or defective graphitic structures (D band) and graphitized sp2 carbon (G band) in a graphitized carbon (Hu et al. 2017). The intensity ratio between these two bands (ID/IG) can be used as an indicator of disorientated degree caused by structure defect and heteroatom doping (Pachfule et al. 2016). WPBC shows a ID/IG value of 0.69, suggesting that WPBC contains highly ordered carbons with relatively limited structural defects (El-Naggar et al. 2018).
TGA was performed to quantify WPBC ratio in different composites according to the residual weight of WPBC after heating to 1000 °C (Fig. S2c). The mass ratio of WPBC in composites was determined to be 0.6–33.2% corresponding to 5–500 mg of WPBC dopant.
UV–Vis diffuse reflectance spectra of g-C3N4 and WPBC50/g-C3N4 are shown in Fig. 2c. WPBC50/g-C3N4 exhibited stronger absorption in visible range than g-C3N4. The energy band gaps of g-C3N4 and WPBC50/g-C3N4 based on Kubelka–Munk function are shown in Fig. 2d, where WPBC50/g-C3N4 showed lower value (2.49 eV) than g-C3N4 (2.56 eV), indicating a stronger capability of WPBC50/g-C3N4 for visible light absorption.
The adsorption capability and pore distribution property were further examined by N2 adsorption/desorption measurement (Fig. 3). The detailed values of specific surface area (SSA), pore volume (VP) and pore diameter (Dp) of different catalysts are shown in Table S1. As illustrated in Fig. 3a, WPBC showed a typical IV isotherm with a H2-type hysteresis and relatively high SSA (444.39 m2/g) and VP (0.24 cm3/g), which reflects the intrinsic nature of biochar material. On the contrary, pristine g-C3N4 demonstrated relatively poorer porosity (SSA ~ 5.45 m2/g, VP ~ 0.05 cm3/g). When WPBC was incorporated, the obtained WPBC50/g-C3N4 exhibited a similar typical IV isotherm with a H3-type hysteresis as g-C3N4, which is mainly caused by the existence of non-rigid aggregates of plate-like g-C3N4 (Thommes 2010). Owing to the rich porosity of WPBC, the WPBC50/g-C3N4 showed higher SSA (10.44 m2/g) and Vp (0.09 cm3/g) than g-C3N4.
3.2 Degradation kinetics of DCF
3.2.1 Degradation of DCF during WPBC/g-C3N4 photocatalysis
The photocatalytic activities of WPBC/g-C3N4 with different WPBC doping amounts were investigated by examining the kinetics of DCF degradation under visible light irradiation. It can be seen in Fig. 4 that both g-C3N4 and WPBC/g-C3N4 demonstrated weak adsorption capability toward DCF (< 8%), although WPBC alone could show moderate adsorptive capability (18%) (Fig. S3). It was probably due to the occupation of WPBC surface by g-C3N4 particles embedded in thus preventing WPBC from exerting the adsorption capacity, which is consistent with SEM observations.
When light was turned on (from 0 min), obvious degradation of DCF could be observed. About 56.2% DCF was degraded within 120 min photocatalysis by pure g-C3N4. The photocatalytic property of WPBC was precluded (Fig. S3). After introducing a small amount of WPBC (5 mg) to g-C3N4, the resulting WPBC5/g-C3N4 showed improved DCF degradation performance, indicating that the dopant WPBC could improve the photocatalytic activity of g-C3N4. Obviously, WPBC50/g-C3N4 exhibited the best photocatalytic performance. The DCF removal efficiency increased as the WPBC load increased from 5 to 50 mg and then decreased when the WPBC loading was excessive (> 50 mg), which was probably due to the shading effect by excessive WPBC. Moreover, the DCF degradation during g-C3N4 and WPBC/g-C3N4 photocatalysis were found well following the pseudo-first-order kinetics. The rate constants (kobs) and corresponding R2 were displayed in Table S2. It was observed that the kobs of DCF degradation by applying WPBC50/g-C3N4 was higher (kobs = 11.9 × 10–3 min−1) than the others. Therefore, it was concluded that the introduction of appropriate amount of WPBC could enhance the photocatalytic performance of g-C3N4. This result was also consistent with the DRS analysis that stronger light absorption was obtained by WPBC50/g-C3N4 than that by pure g-C3N4.
3.2.2 Degradation of DCF in the presence of PMS
To further investigate the PMS activation capabilities of WPBC/g-C3N4, the degradation kinetics of DCF was evaluated. Interactions between PMS and DCF with and without visible light were precluded by control tests (Fig. 5b). As can be seen in Fig. 5a, the removal of DCF proceeded much faster in the presence of PMS than that isshown in Fig. 4, which implied that both g-C3N4 and WPBC/g-C3N4 composites could react with PMS under visible light. Beneficial effects could also be obtained toward PMS activation by combining WPBC with g-C3N4. Generally, the WPBC/g-C3N4 composites exhibited higher DCF degradation efficiencies than pure g-C3N4, and the composite WPBC50/g-C3N4 showed the best performance.
It was also clearly seen in Fig. 5a that the degradation kinetics of DCF in the presence of PMS demonstrated obvious two stages, a fast decay period followed by a much slower degradation phase, which likely resulted from the involved multiple mechanisms for the activation of PMS. Dark experiments were conducted to examine the PMS activation capabilities of g-C3N4, WPBC, and WPBC/g-C3N4 (Fig. 5b). It was found that all three carbon materials could activate PMS in the dark showing typical two stage DCF degradation kinetics, and WPBC showed the highest PMS activation efficiency. This arose the assumption that the very rapid removal of DCF in the initial period shown in Fig. 5a was most likely attributed to the fast PMS activation by carbon materials. It was also noted that, since WPBC did not show photoactivity (Fig. S3), the DCF removal efficiencies by WPBC-PMS system were the same with and without light. However, visible light could significantly enhance the PMS activation capability of both g-C3N4 and WPBC/g-C3N4 by comparing the DCF removal rates shown in Fig. 5a and b, which suggested that the photoelectrons of g-C3N4 and WPBC/g-C3N4 were beneficial and contributed dominantly to PMS activation. The better performance of WPBC/g-C3N4 composites than that of g-C3N4 in PMS activation was probably due to two reasons: (i) the improved photocatalytic capability of the composites induced by WPBC as discussed in Fig. 4; (ii) stronger PMS activation capability of the dopant WPBC than that of g-C3N4 in the dark. The degradation kinetics of DCF could be expressed well using Behnajady-Modirshahla-Ghanbery model (Behnajady et al. 2007) (Text S1, Table S3), and the strongest maximum oxidation capacity (1/b = 1.159) was obtained by WPBC50/g-C3N4. Therefore, WPBC50/g-C3N4 was selected for further study because of its best performance under visible light.
3.3 Identification of ROS
3.3.1 Quenching experiments
To identify the principal ROS for the degradation of DCF in WPBC50/g-C3N4/Vis-PMS process and determine the role of WPBC in the evolution of ROS during PMS activation, competitive tests were performed in different systems using various quenchers. As shown in Fig. 6a, the addition of either ethanol (EtOH) or tert butyl alcohol (TBA) made similar moderate inhibition. Since TBA was effective scavenger for ·OH and inert toward SO4·– (kOH = 3.8–7.6 × 108 M−1 s−1, kSO4·– = 4.0–9.1 × 105 M−1 s−1), but EtOH could effectively quench both ·OH and SO4·– (k·OH = 1.6–7.7 × 107 M−1 s−1, kSO4·– = 1.2–2.8 × 108 M−1 s−1) (Fang et al. 2013; Pan et al. 2020), the results indicated the minor role of ·OH and neglectable impact of SO4·– on DCF removal in WPBC50/g-C3N4/Vis-PMS process. As seen in Fig. 6b, the inhibition by EtOH or TBA was weaker in g-C3N4/Vis-PMS than that in WPBC50/g-C3N4/Vis-PMS, which suggested that the incorporation of WPBC may make some improvement for the production of ·OH. In addition, ammonium oxalate (AO) was used as a hole quencher (Liu et al. 2019). It was found that DCF removal decreased to 62% by adding AO, which indicated that h+ might be partly responsible for the degradation of DCF. However, when p-benzoquinone (p-BQ) was applied as the quencher for ·O2– (k·O2– = 9 × 108 M−1 s−1) and L-histidine was used to quench 1O2 (k1O2 = 3.2 × 107 M−1 s−1) (Ma et al. 2018), only 13% and 40% of DCF were degraded in WPBC50/g-C3N4/Vis-PMS process, respectively (Fig. 6a), which could imply the possible important contributions of ·O2– and 1O2 to DCF degradation.
Competitive experiments were also conducted to clarify the contributing ROS involved in direct photocatalytic processes of g-C3N4 and WPBC50/g-C3N4 (Fig. 6c, d). It could be observed that TBA, l-histidine and AO all had relatively weak inhibition on DCF degradation, but p-BQ showed significant inhibition. It implied that ·OH, 1O2 and h+ contributed weakly but ·O2– may make major contribution to the photocatalytic degradation of DCF in both g-C3N4/Vis and WPBC50/g-C3N4/Vis processes. Similar findings were also reported in previous studies (Wang et al. 2017).
As can be seen from Fig. 5b, the carbon materials of WPBC, g-C3N4, and WPBC50/g-C3N4 all could activate PMS in the dark, so different scavengers were also applied to examine the contributing ROS. Among three carbon materials, WPBC demonstrated the strongest PMS activation capability. The great inhibition of DCF degradation in WPBC-PMS process by adding l-histidine suggested the crucial role of 1O2, and the weak inhibition by EtOH could support a minor role of ·OH and SO4·– (Fig. 6e). The observed stronger inhibition by TBA than EtOH was probably due to the high viscosity of TBA that could mask the active sites on WPBC surface (Huang et al. 2017). Similar inhibition effects were also obtained in WPBC50/g-C3N4-PMS (Fig. 6f) and g-C3N4-PMS processes (Fig. S4), which implied that similar contributing ROS might be involved. It should be noted that, since p-BQ could also react with PMS (Oh et al. 2017), the observed inhibition of DCF degradation by adding p-BQ in PMS-involved processes may result from the competition for PMS between p-BQ and DCF, and therefore the generation of ·O2– needs to be further verified by EPR.
3.3.2 EPR analysis
EPR was conducted to further investigate the possible ROS in different systems. Figure 7a shows the obtained signals in all relevant processes by adding 80 mM DMPO in water solvent.
EPR spectra of different reactive oxygen species in different systems, a DMPO-OH and DMPOX spectra using 80 mM DMPO for 10 min reaction, b DMPO-OOH spectra using 80 mM DMPO for 10 min reaction, c, d TMP-1O2 spectra using 5.0 mM TMP for 10 min reaction (condition: [DCF]0 = 0.01 mM, [catalyst]0 = 0.5 g/L, [PMS]0 = 2.0 mM)
The signal of DMPO-OH (αN = αHβ = 14.9 G (Timmins et al. 1999)) was detected in the dark g-C3N4-PMS system, which might be due to the direct delivery of electrons from active sites to PMS resulting in PMS reduction to generate ·OH (Gao et al. 2020). Interestingly, the signal of DMPO-OH was not detected in the dark WPBC50/g-C3N4-PMS system, which contradicted with the quenching results (Fig. 6f). The above phenomenon might originate from the fact that the produced ·OH radicals were bonded on the surface of WPBC during the activation process due to the existence of numerous micro and mesoporous. Phenol, as a frequently used surface-bound-radical scavenger (Liang and Su 2009), was adopted to examine the influence (Fig. S5). When phenol was added, the degradation of DCF was partially inhibited, which suggested that the surface-bound ·OH may exist and contribute to DCF removal in the dark WPBC50/g-C3N4-PMS system. The DMPOX (αN = 7.3 ± 0.1 G, αHβ = 3.9 ± 0.1 G) with characteristic seven-line signal was detected in dark WPBC-PMS, which should be mainly attributed to the direct oxidation of DMPO by nonradical species like 1O2 (Verstraeten et al. 2009).
For direct photocatalytic processes, the load of WPBC increased the intensity of DMPO-OH a bit. The ·OH should be generated by the reductive pathway (Eqs. 1–3) because the valence band (VB) potential is not positive enough to generate ·OH directly (VB = 1.62 VNHE, E0 (·OH/H2O) = 2.68 VNHE) (Hao et al. 2016). Therefore, the benefit of incorporating WPBC into g-C3N4 for the production of ·OH was likely due to the effective separation of e−/h+ pairs, which was supported by the enhanced photocurrent in WPBC/g-C3N4 photocatalytic process compared with the photocatalysis of g-C3N4 (Fig. 8). This was consistent with some previous studies that carbon materials could be used as efficient interface transfer accelerators for photogenerated electrons (Dikdim et al. 2019; Kumar et al. 2018).
In the processes of PMS activation under visible light, no obvious peak of DMPO-SO4 could be observed. It might be because of the fast transformation from DMPO-SO4 to DMPO-OH via nucleophilic substitution (Timmins et al. 1999). Besides, the intensity of DMPO-OH decreased remarkably either with g-C3N4 or WPBC/g-C3N4 as the catalyst, which, however, was inconsistent with the quenching results (Fig. 6a, b). As seen in Fig. 7b, strong signals of DMPO-OOH were observed in both g-C3N4/Vis-PMS and WPBC50/g-C3N4/Vis-PMS systems, which proved the generation of ·O2−. It was inferred that the failure of ·OH detection by EPR may be caused by the fast consumption of ·OH by ·O2− (Eq. 7). It should be noted that although evident DMPO-OOH signal was also detected in WPBC50/g-C3N4/Vis process, it was weaker than that in PMS existing condition. In dark processes, almost no DMPO-OOH signal could be observed, which confirmed that the ·O2– should be produced from the reaction between photoelectrons and O2 (Eq. 6). Thus it was concluded that the presence of PMS could also promote the generation of photoelectrons via effectively capturing the hole (Eq. 8) (Dikdim et al. 2019).
As can be seen in Fig. 7c, d, the apparent signal representing 1O2 (aN = 16.9 G) arose in all investigated systems, which was consistent with the results of quenching experiment (Song et al. 2006). In the absence of PMS (Fig. 7c), the ·OH could react with ·O2– to generate 1O2 during photocatalytic processes (Eq. 7) (Yu et al. 2020), leading to the formation of relatively weak 1O2. Since the involvement of WPBC improved the photocatalytic performance of WPBC50/g-C3N4, the intensity of 1O2 was higher during WPBC50/g-C3N4 photocatalysis compared with g-C3N4 photocatalysis. In the presence of PMS (Fig. 7d), 1O2 could be generated in the dark, mainly attributed to the activation of PMS by active sites of WPBC and g-C3N4 (Gao et al. 2018), which agreed well with the quenching results. It was found that during the photoactivation of PMS process, the signal of 1O2 became significantly stronger than that in darkness. To further understand the source of 1O2 in WPBC50/g-C3N4/Vis-PMS system, nitrogen was purged to blow out the dissolved oxygen. However, the removal efficiency of DCF was only reduced by 10% (Fig. S6), which indicated that the generation of 1O2 weakly depended on dissolved oxygen. The 1O2 should be mainly produced by two sources: (i) the active sites of catalyst; (ii) photogenerated e−/h+ pairs to react with PMS (Eqs. 4–9).
3.3.3 Solvent exchange experiment
To further confirm the contribution of 1O2 to DCF degradation in different systems, solvent exchange tests were conducted using D2O to replace H2O for reactions. The 1O2 has a longer lifetime in D2O (55 μs) compared with that in H2O (4.2 μs) (Luo et al. 2019). As shown in Fig. S7, DCF was degraded faster in D2O-mediated condition compared with H2O, which further attested the contribution of 1O2 to DCF degradation in all investigated systems.
3.3.4 Electrochemical analysis
The electrochemical analysis was further conducted to analyze other possible non-radical pathways like electron-transfer for DCF degradation. Electrochemical impedance spectroscopy (EIS) analysis in Fig. 9a demonstrated that WPBC50/g-C3N4 showing a smaller arc may have lower charge migration resistance than g-C3N4, implying that WPBC50/g-C3N4 could possess better electron transfer capability under visible light than g-C3N4, and similar results were also found by compositing actvated carbon with g-C3N4 (Dikdim et al. 2019; Wang et al. 2022). Additionally, the linear sweep voltammograms (LSV) analysis results were given in Fig. 9b. It can be clearly seen thatthe current density of WPBC50/g-C3N4 increased a little upon the addition of PMS, which indicated the formation of a metastable reactive complex between PMS and WPBC50/g-C3N4 (Lee et al. 2016). Moreover, a significant increase of current density could be observed with the coexist of PMS and DCF, which could well imply the effective electron transfer from DCF to PMS via WPBC50/g-C3N4 (Zhu et al. 2018a; b).
3.3.5 Active sites analysis of WPBC50/g-C3N4
The nature and coordination of C, N, O elements in WPBC50/g-C3N4 catalyst were investigated by XPS to further examine the contribution of different functional groups during the catalytic process. Pure g-C3N4 (before use), fresh WPBC50/g-C3N4 and the used WPBC50/g-C3N4 (after 5 rounds) were all examined for comparison. As shown in the XPS survey spectra (Fig. 10a), strong signals denoting C, N and O elements could be observed. The spectrum of C 1s could be deconvoluted into four peaks corresponding to C=C (284.4 eV), C–C/C–N (285.0 eV), C–O (286.0 eV) and C=N/C=O (287.9 eV) functional groups (Fig. 10b) (Zhao et al. 2014). Similarly, four peaks were observed after the deconvolution in the N 1s region at 398.2, 399.1, 400.5 and 403.9 eV, corresponding to two-coordinated N atoms (C=N–C), three-coordinated N atoms (N−(C)3), terminal amino functional groups (C–N–H) and the π-excitation (Fig. 10c) (Tao et al. 2015). The spectrum of O 1s could be deconvoluted into three single peaks with binding energies of 531.7, 532.8 and 533.6 eV representing C=O, N–C–O groups and the adsorbed water, respectively (Fig. 10d) (Ren et al. 2022; Wei et al. 2016).
The relative abundance of different functional groups calculated based on the integral area for catalysts are listed in Table S4. It can be seen that after compositing WPBC, the content of C=N–C group increased, suggesting that the structure of g-C3N4 was not destroyed after the combination, in consistent with XRD results. In addition, the content of C=O group also increased due to the load of WPBC. It was also seen in Fig. 10b, c that both the C 1s and N 1s peaks of WPBC50/g-C3N4 composite showed a slight negative shift with respect to that of g-C3N4, implying the interactions between WPBC and g-C3N4 (Li et al. 2014).
By comparing the XPS spectra between fresh WPBC50/g-C3N4 and used WPBC50/g-C3N4, it was observed that the relative abundance of C=O and N–(C)3 decreased after reaction (Table S4). These groups participated in the reaction and might be the potential active sites, which was consistent with some previous studies (Gao et al. 2020; Guo et al. 2018). The C=O groups possess substantial electron density at the oxygen atom, which could activate PMS to produce 1O2 (Eqs. 10–13) (Huang et al. 2018; Zhao et al. 2014). The N–(C)3 group has a high electronegativity and small covalent radius to facilitate the electron transfer from the neighboring C, forming electron-rich N atoms and electron-deficient C3 atoms to activate PMS (Eqs. 9, 14–15) (Duan et al. 2015; Gao et al. 2018; Sun et al. 2020). These should be the main possible ways for active sites of WPBC50/g-C3N4 to produce 1O2 and ·OH.






3.4 Effects of various reaction parameters
3.4.1 Effect of reagent dosage and pH
The influence of catalyst dosage, PMS and DCF concentrations on DCF removal was investigated. As shown in Fig. 11a, generally higher catalyst dosages could accelerate the degradation of DCF. The improvement could be divided into two stages, a faster acceleration and a weaker enhancement before and after 0.5 g/L, respectively. It was because more active sites could be provided by increasing catalyst dosage, but the “inner filter” effect of incident light could also happen with excessive catalyst. As seen in Fig. 11b, the degradation of DCF increased rapidly with the increase of PMS concentration from 0.1 to 2.0 mM. However, when further increasing PMS to 5.0 mM, DCF degradation did not obviously change and even decreased, which may be caused by the limited amount of electrons and active sites produced from photocatalyst to activate excess PMS. Figure 11c shows the influence of DCF concentration. As expected, faster degradation was observed at lower [DCF]0. This occurred mainly because the quantity of the reactive species in WPBC50/g-C3N4/Vis-PMS system was sufficient when [DCF]0 was low, and each DCF molecule was more likely to be oxidized. Whereas, with the gradual increase of DCF concentration, the reactive species were relatively insufficient, leading to the decline of DCF degradation efficiency.
Due to the wide pH range of real sewage, it is meaningful to study the degradation of DCF at different pH conditions. The DCF degradation efficiency generally decreased as the initial solution pH increased from 3 to 11 (Fig. 11d). This should be attributed to the combined effects related to the different charged states of WPBC50/g-C3N4, PMS and DCF molecules at different pH values. The point of zero charge (pHzpc) of WPBC50/g-C3N4 was measured as 6.07 (Fig. S8), and therefore when pH was lower than 6.07, the positively charged WPBC50/g-C3N4 surface easily attracted the negatively charged HSO5−. On the contrary, when the pH was higher than 6.07, the catalyst surface was negatively charged and DCF molecules also existed in the anionic form (pKa = 4.15) (Fig. S9), so that the repulsion interactions between WPBC50/g-C3N4 and PMS, and WPBC50/g-C3N4 and DCF both resulted in the unsatisfactory degradation of DCF. In addition, the HSO5− also shows minimum stability to be dissociated to SO52− at pH > 9 (pKa2 = 9.4) (Ghanbari and Moradi, 2017), leading to poor DCF removal performance at strong alkaline condition.
3.4.2 Effect of common anions
In practical operations, many anions may exist in natural water that can affect the removal efficiency of organic pollutants. Herein, the common anions including Cl−, HCO3−, H2PO4−, NO3− and SO42− were added individually to examine the influence on DCF removal. As shown in Fig. S10, HCO3−, H2PO4−, NO3− and SO42− exhibited weak inhibiting effect on DCF degradation, and the inhibition follows the order of HCO3− > SO42− > H2PO4− > NO3−. The inhibition caused by HCO3−, H2PO4− and NO3− was likely due to the radical scavenging effect (Eqs. 16–20) (Lau et al. 2007; Wang and Wang 2020). The inhibition by high concentration of SO42− was probably caused by the competition for catalyst surface between PMS and SO42−. However, Cl− had remarkable accelerating effect on DCF degradation, which was due to the generation of reactive chlorine species from the reaction between Cl− and PMS (Eqs. 21–25) (Lutze et al. 2015), which may further react with DCF.
3.4.3 Principal component analysis
Furthermore, principal component analysis (PCA) was used to investigate the correlation of DCF removal rates and typical environmental factors such as catalyst dosage, PMS concentration, DCF concentration and initial pH. As shown in Fig. S11, it was clear that the increase of catalyst dosage and PMS concentration separately substantially promoted the degradation of DCF. In contrast, the elevation of DCF concentration and initial pH significantly suppressed the DCF removal. Obviously, the DCF concentration is the most influential factor to inhibit the degradation of DCF in WPBC50/g-C3N4/Vis-PMS system.
3.5 Catalyst stability
Cycling tests were conducted to evaluate the reusability of WPBC50/g-C3N4. The catalyst was easily separated through a standing and layering process after the reaction, washed with ultrapure water several times, dried in a vacuum oven, and finally kept at ambient temperature for next round use. Repeated experiments were run by adding a fresh solution of DCF and PMS. According to the results shown in Fig. S12, the DCF degradation still maintained comparable efficiency after 5 consecutive cycles, indicating the high stability of WPBC50/g-C3N4.
3.6 Degradation pathways of DCF and mineralization performance
The degradation intermediates of DCF in WPBC50/g-C3N4/Vis-PMS system were analyzed by HPLC/MS. The mineralization kinetic was shown in Fig. S13. The TOC decreased by 19% within 1 h when the complete removal of DCF was achieved. Fig. S14 shows the HPLC spectra of samples at different sampling time. It can be clearly seen that as the degradation of DCF (27.6 min), primary intermediates (e.g., 24.5 min) were produced, which could be effectively destructed after accumulating to maximum concentration (ca. 30 min). Four intermediates (m/z = 310, 264, 228, 197) could be identified (Fig. S15), and the possible degradation pathways are shown in Fig. 12. Combined with the above analysis of ROS, 1O2 attack on diclofenac molecules played a dominant role in diclofenac oxidation. However, there were no oxidative products observed after the reaction because they were quickly transformed to hydroxylated derivatives through substitution or hydrolysis (Chong et al. 2017). The intermediate with a m/z of 310 was the mono-hydroxylated derivatives of diclofenac. The intermediate with a m/z of 264 corresponded to quinine imine structures, which could be easily obtained by the oxidation of the intermediate with a m/z of 310 (Perez-Estrada et al. 2005). The DCF would be dechlorinated, and intermediates with the m/z of 228 or 197 could be detected. Under further reaction in WPBC50/g-C3N4/Vis-PMS process, these organic substances could be further oxidized into other ring opening products, which may escape the effective detection by HPLC/MS, and eventually be transformed into inorganic materials such as CO2, HCl and H2O.
4 Conclusions
In this study, WPBC/g-C3N4 catalysts were successfully prepared, which could effectively degrade DCF via the activation of PMS under visible light. The visible light absorption ability, quantum efficiency and electrical conductivity were all improved by the combination of WPBC, which led to higher efficiency for both photocatalysis and PMS activation of WPBC/g-C3N4 than that of pure g-C3N4. The composite WPBC50/g-C3N4 showed the optimum catalytic performance. In WPBC50/g-C3N4/Vis-PMS system, the active species including ·OH, h+, ·O2–, 1O2 and the electron-transfer all made contributions to DCF degradation, and 1O2 was the most abundant ROS. The 1O2 should be mainly produced by the active sites (C=O and N–(C)3) of WPBC/g-C3N4 and photogenerated e−/h+ pairs to react with PMS. Based on PCA results, the DCF concentration should be the most influential factor for DCF degradation. Moreover, the composites could maintain good stability in cyclic runs. The hydroxylation and dechlorination were found to be responsible for the decomposition and mineralization of DCF.
Data availability
The data and material used in this study will be made available by the authors upon request.
References
Behnajady MA, Modirshahla N, Ghanbary F (2007) A kinetic model for the decolorization of CI Acid Yellow 23 by Fenton process. J Hazard Mater 148(1–2):98–102. https://doi.org/10.1016/j.jhazmat.2007.02.003
Bu Q, Wang B, Huang J, Deng S, Yu G (2013) Pharmaceuticals and personal care products in the aquatic environment in China: a review. J Hazard Mater 262:189–211. https://doi.org/10.1016/j.jhazmat.2013.08.040
Cao S, Low J, Yu J, Jaroniec M (2015) Polymeric photocatalysts based on graphitic carbon nitride. Adv Mater 27(13):2150–2176. https://doi.org/10.1002/adma.201500033
Chen X, Kuo D-H, Lu D (2016) Nanonization of g-C3N4 with the assistance of activated carbon for improved visible light photocatalysis. RSC Adv 6(71):66814–66821. https://doi.org/10.1039/c6ra10357j
Chong S, Zhang G, Zhang N, Liu Y, Huang T, Chang H (2017) Diclofenac degradation in water by FeCeOx catalyzed H2O2: Influencing factors, mechanism and pathways. J Hazard Mater 334:150–159. https://doi.org/10.1016/j.jhazmat.2017.04.008
Dikdim JMD, Gong Y, Noumi GB, Sieliechi JM, Zhao X, Ma N, Yang M, Tchatchueng JB (2019) Peroxymonosulfate improved photocatalytic degradation of atrazine by activated carbon/graphitic carbon nitride composite under visible light irradiation. Chemosphere 217:833–842. https://doi.org/10.1016/j.chemosphere.2018.10.177
Duan X, Sun H, Wang Y, Kang J, Wang S (2015) N-doping-induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation. ACS Catal 5(2):553–559. https://doi.org/10.1021/cs5017613
El-Naggar A, Lee SS, Awad YM, Yang X, Ryu C, Rizwan M, Rinklebe J, Tsang DCW, Ok YS (2018) Influence of soil properties and feedstocks on biochar potential for carbon mineralization and improvement of infertile soils. Geoderma 332:100–108. https://doi.org/10.1016/j.geoderma.2018.06.017
Fang G-D, Dionysiou DD, Al-Abed SR, Zhou D-M (2013) Superoxide radical driving the activation of persulfate by magnetite nanoparticles: implications for the degradation of PCBs. Appl Catal B 129:325–332. https://doi.org/10.1016/j.apcatb.2012.09.042
Gao Y, Zhu Y, Lyu L, Zeng Q, Xing X, Hu C (2018) Electronic structure modulation of graphitic carbon nitride by oxygen doping for enhanced catalytic degradation of organic pollutants through peroxymonosulfate activation. Environ Sci Technol 52(24):14371–14380. https://doi.org/10.1021/acs.est.8b05246
Gao Y, Li T, Zhu Y, Chen Z, Liang J, Zeng Q, Lyu L, Hu C (2020) Highly nitrogen-doped porous carbon transformed from graphitic carbon nitride for efficient metal-free catalysis. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121280
Ghanbari F, Moradi M (2017) Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review. Chem Eng J 310:41–62. https://doi.org/10.1016/j.cej.2016.10.064
Guo F, Lu J, Liu Q, Zhang P, Zhang A, Cai Y, Wang Q (2018) Degradation of acid orange 7 by peroxymonosulfate activated with the recyclable nanocomposites of g-C3N4 modified magnetic carbon. Chemosphere 205:297–307. https://doi.org/10.1016/j.chemosphere.2018.04.139
Guo H, Wang Y, Liao L, Li Z, Pan S, Puyang C, Su Y, Zhang Y, Wang T, Ren J, Li J (2022) Review on remediation of organic-contaminated soil by discharge plasma: plasma types, impact factors, plasma-assisted catalysis, and indexes for remediation. Chem Eng J 436:135239. https://doi.org/10.1016/j.cej.2022.135239
Hao R, Wang G, Tang H, Sun L, Xu C, Han D (2016) Template-free preparation of macro/mesoporous g-C3N4/TiO2 heterojunction photocatalysts with enhanced visible light photocatalytic activity. Appl Catal B 187:47–58. https://doi.org/10.1016/j.apcatb.2016.01.026
Horl WH (2010) Nonsteroidal anti-inflammatory drugs and the kidney. Pharmaceuticals (basel, Switzerland) 3(7):2291–2321. https://doi.org/10.3390/ph3072291
Hu P, Su H, Chen Z, Yu C, Li Q, Zhou B, Alvarez PJJ, Long M (2017) Selective degradation of organic pollutants using an efficient metal-free catalyst derived from carbonized polypyrrole via peroxymonosulfate activation. Environ Sci Technol 51(19):11288–11296. https://doi.org/10.1021/acs.est.7b03014
Hu H, Sun L, Gao Y, Wang T, Huang Y, Lv C, Zhang Y-F, Huang Q, Chen X, Wu H (2020) Synthesis of ZnO nanoparticle-anchored biochar composites for the selective removal of perrhenate, a surrogate for pertechnetate, from radioactive effluents. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2019.121670
Huang G-X, Wang C-Y, Yang C-W, Guo P-C, Yu H-Q (2017) Degradation of bisphenol A by peroxymonosulfate catalytically activated with Mn1.8Fe1.2O4 nanospheres: synergism between Mn and Fe. Environ Sci Technol 51(21):12611–12618. https://doi.org/10.1021/acs.est.7b03007
Huang B-C, Jiang J, Huang G-X, Yu H-Q (2018) Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate. J Mater Chem A 6(19):8978–8985. https://doi.org/10.1039/c8ta02282h
Kumar P, Boukherroub R, Shankar K (2018) Sunlight-driven water-splitting using two-dimensional carbon based semiconductors. J Mater Chem A 6(27):12876–12931. https://doi.org/10.1039/c8ta02061b
Lau TK, Chu W, Graham NJD (2007) The aqueous degradation of butylated hydroxyanisole by UV/S2O82−: study of reaction mechanisms via dimerization and mineralization. Environ Sci Technol 41(2):613–619. https://doi.org/10.1021/es061395a
Lee H, Kim H-I, Weon S, Choi W, Hwang YS, Seo J, Lee C, Kim J-H (2016) Activation of persulfates by graphitized nanodiamonds for removal of organic compounds. Environ Sci Technol 50(18):10134–10142. https://doi.org/10.1021/acs.est.6b02079
Lee J, von Gunten U, Kim J-H (2020) Persulfate-based advanced oxidation: critical assessment of opportunities and roadblocks. Environ Sci Technol 54(6):3064–3081. https://doi.org/10.1021/acs.est.9b07082
Li Y, Wu S, Huang L, Wang J, Xu H, Li H (2014) Synthesis of carbon-doped g-C3N4 composites with enhanced visible-light photocatalytic activity. Mater Lett 137:281–284. https://doi.org/10.1016/j.matlet.2014.08.142
Li S, Wang Z, Zhao X, Yang X, Liang G, Xie X (2019) Insight into enhanced carbamazepine photodegradation over biochar-based magnetic photocatalyst Fe3O4/BiOBr/BC under visible LED light irradiation. Chem Eng J 360:600–611. https://doi.org/10.1016/j.cej.2018.12.002
Liang C, Su H-W (2009) Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind Eng Chem Res 48(11):5558–5562. https://doi.org/10.1021/ie9002848
Liu J, Zhang T, Wang Z, Dawson G, Chen W (2011) Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J Mater Chem 21(38):14398–14401. https://doi.org/10.1039/c1jm12620b
Liu W, Li Y, Liu F, Jiang W, Zhang D, Liang J (2019) Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: mechanisms, degradation pathway and DFT calculation. Water Res 151:8–19. https://doi.org/10.1016/j.watres.2018.11.084
Luo R, Li M, Wang C, Zhang M, Khan MAN, Sun X, Shen J, Han W, Wang L, Li J (2019) Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition. Water Res 148:416–424. https://doi.org/10.1016/j.watres.2018.10.087
Lutze HV, Kerlin N, Schmidt TC (2015) Sulfate radical-based water treatment in presence of chloride: formation of chlorate, inter-conversion of sulfate radicals into hydroxyl radicals and influence of bicarbonate. Water Res 72:349–360. https://doi.org/10.1016/j.watres.2014.10.006
Ma W, Wang N, Fan Y, Tong T, Han X, Du Y (2018) Non-radical-dominated catalytic degradation of bisphenol A by ZIF-67 derived nitrogen-doped carbon nanotubes frameworks in the presence of peroxymonosulfate. Chem Eng J 336:721–731. https://doi.org/10.1016/j.cej.2017.11.164
Mandeep K, Gupta G, Shukla P (2020) Insights into the resources generation from pulp and paper industry wastes: challenges, perspectives and innovations. Bioresour Technol 297:122496. https://doi.org/10.1016/j.biortech.2019.122496
Masih D, Ma Y, Rohani S (2017) Graphitic C3N4 based noble-metal-free photocatalyst systems: a review. Appl Catal B 206:556–588. https://doi.org/10.1016/j.apcatb.2017.01.061
Meng L, Yin W, Wang S, Wu X, Hou J, Yin W, Feng K, Ok YS, Wang X (2020) Photocatalytic behavior of biochar-modified carbon nitride with enriched visible-light reactivity. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.124713
Oh W-D, Dong Z, Lim T-T (2016) Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: current development, challenges and prospects. Appl Catal B 194:169–201. https://doi.org/10.1016/j.apcatb.2016.04.003
Oh W-D, Dong Z, Ronn G, Lim T-T (2017) Surface-active bismuth ferrite as superior peroxymonosulfate activator for aqueous sulfamethoxazole removal: performance, mechanism and quantification of sulfate radical. J Hazard Mater 325:71–81. https://doi.org/10.1016/j.jhazmat.2016.11.056
Ouyang D, Chen Y, Yan J, Qian L, Han L, Chen M (2019) Activation mechanism of peroxymonosulfate by biochar for catalytic degradation of 1,4-dioxane: important role of biochar defect structures. Chem Eng J 370:614–624. https://doi.org/10.1016/j.cej.2019.03.235
Pachfule P, Shinde D, Majumder M, Xu Q (2016) Fabrication of carbon nanorods and graphene nanoribbons from a metal-organic framework. Nat Chem 8(7):718–724. https://doi.org/10.1038/nchem.2515
Pan Y, Zhou M, Wang Q, Cai J, Tian Y, Zhang Y (2020) EDTA, oxalate, and phosphate ions enhanced reactive oxygen species generation and sulfamethazine removal by zero-valent iron. J Hazard Mater 391:122210. https://doi.org/10.1016/j.jhazmat.2020.122210
Perez-Estrada LA, Malato S, Gernjak W, Aguera A, Thurman EM, Ferrer I, Fernandez-Alba AR (2005) Photo-fenton degradation of diclofenac: identification of main intermediates and degradation pathway. Environ Sci Technol 39(21):8300–8306. https://doi.org/10.1021/es050794n
Ren J, Yao Z, Wei Q, Wang R, Wang L, Liu Y, Ren Z, Guo H, Niu Z, Wang J, Zhen Y (2022) Catalytic degradation of chloramphenicol by water falling film dielectric barrier discharge and FeO catalyst. Sep Purif Technol 290:120826. https://doi.org/10.1016/j.seppur.2022.120826
Song B, Wang G, Tan M, Yuan J (2006) A europium(III) complex as an efficient singlet oxygen luminescence probe. J Am Chem Soc 128(41):13442–13450. https://doi.org/10.1021/ja062990f
Sun P, Liu H, Feng M, Zhai Z, Fang Y, Zhang X, Sharma VK (2020) Strategic combination of N-doped graphene and g-C3N4: efficient catalytic peroxymonosulfate-based oxidation of organic pollutants by non-radical-dominated processes. Appl Catal B. https://doi.org/10.1016/j.apcatb.2020.119005
Tao Y, Ni Q, Wei M, Xia D, Li X, Xu A (2015) Metal-free activation of peroxymonosulfate by g-C3N4 under visible light irradiation for the degradation of organic dyes. RSC Adv 5(55):44128–44136. https://doi.org/10.1039/c5ra06223c
Thommes M (2010) Physical adsorption characterization of nanoporous materials. Chem Ing Tech 82(7):1059–1073. https://doi.org/10.1002/cite.201000064
Timmins GS, Liu KJ, Bechara EJ, Kotake Y, Swartz HM (1999) Trapping of free radicals with direct in vivo EPR detection: a comparison of 5,5-dimethyl-1-pyrroline-N-oxide and 5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide as spin traps for HO* and SO4*. Free Radic Biol Med 27(3–4):329–333. https://doi.org/10.1016/s0891-5849(99)00049-0
Verstraeten SV, Lucangioli S, Galleano M (2009) ESR characterization of thallium(III)-mediated nitrones oxidation. Inorg Chim Acta 362(7):2305–2310. https://doi.org/10.1016/j.ica.2008.10.013
Wang J, Wang S (2016) Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: a review. J Environ Manage 182:620–640. https://doi.org/10.1016/j.jenvman.2016.07.049
Wang J, Wang S (2020) Reactive species in advanced oxidation processes: formation, identification and reaction mechanism. Chem Eng J. https://doi.org/10.1016/j.cej.2020.126158
Wang F, Chen P, Feng Y, Xie Z, Liu Y, Su Y, Zhang Q, Wang Y, Yao K, Lv W, Liu G (2017) Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl Catal B 207:103–113. https://doi.org/10.1016/j.apcatb.2017.02.024
Wang L, Ye J, Zhang J, Meng Q, Li X, Chen Z, Yu H, Zhang A, Bu Z, Jiao Y, Pan Y (2021) Removal of sulfamethazine using peracetic acid activated by Fe0 and UV: Efficiency and mechanism study. J Environ Chem Eng 9(6):106358. https://doi.org/10.1016/j.jece.2021.106358
Wang Q, Pan Y, Fu W, Wu H, Zhou M, Zhang Y (2022) Aminopolycarboxylic acids modified oxygen reduction by zero valent iron: proton-coupled electron transfer, role of iron ion and reactive oxidant generation. J Hazard Mater 430:128402. https://doi.org/10.1016/j.jhazmat.2022.128402
Wei M, Gao L, Li J, Fang J, Cai W, Li X, Xu A (2016) Activation of peroxymonosulfate by graphitic carbon nitride loaded on activated carbon for organic pollutants degradation. J Hazard Mater 316:60–68. https://doi.org/10.1016/j.jhazmat.2016.05.031
Xu L, Qi L, Sun Y, Gong H, Chen Y, Pei C, Gan L (2020) Mechanistic studies on peroxymonosulfate activation by g-C3N4 under visible light for enhanced oxidation of light-inert dimethyl phthalate. Chin J Catal 41(2):322–332. https://doi.org/10.1016/s1872-2067(19)63447-9
Yu Q, Guo S, Li X, Zhang M (2014) Template free fabrication of porous g-C3N4/graphene hybrid with enhanced photocatalytic capability under visible light. Mater Technol 29(3):172–178. https://doi.org/10.1179/1753555714y.0000000126
Yu G, Wang Y, Cao H, Zhao H, Xie Y (2020) Reactive oxygen species and catalytic active sites in heterogeneous catalytic ozonation for water purification. Environ Sci Technol 54(10):5931–5946. https://doi.org/10.1021/acs.est.0c00575
Zhang H, Wu W, Li Y, Wang Y, Zhang C, Zhang W, Wang L, Niu L (2019) Enhanced photocatalytic degradation of ciprofloxacin using novel C-dot@ Nitrogen deficient g-C3N4: synergistic effect of nitrogen defects and C-dots. Appl Surf Sci 465:450–458. https://doi.org/10.1016/j.apsusc.2018.09.183
Zhao Z, Dai Y, Lin J, Wang G (2014) Highly-ordered mesoporous carbon nitride with ultrahigh surface area and pore volume as a superior dehydrogenation catalyst. Chem Mater 26(10):3151–3161. https://doi.org/10.1021/cm5005664
Zhao D, Yang F, Dai Y, Tao F, Shen Y, Duan W, Zhou X, Ma H, Tang L, Li J (2017) Exploring crystalline structural variations of cellulose during pulp beating of tobacco stems. Carbohydr Polym 174:146–153. https://doi.org/10.1016/j.carbpol.2017.06.060
Zhu S, Huang X, Ma F, Wang L, Duan X, Wang S (2018a) Catalytic removal of aqueous contaminants on N-doped graphitic biochars: inherent roles of adsorption and nonradical mechanisms. Environ Sci Technol 52(15):8649–8658. https://doi.org/10.1021/acs.est.8b01817
Zhu Z, Fan W, Liu Z, Yu Y, Dong H, Huo P, Yan Y (2018b) Fabrication of the metal-free biochar-based graphitic carbon nitride for improved 2-mercaptobenzothiazole degradation activity. J Photochem Photobiol A 358:284–293. https://doi.org/10.1016/j.jphotochem.2018.03.027
Acknowledgements
The Advanced Analysis and Testing Center of Nanjing Forestry University is acknowledged.
Funding
This study was financially supported by China Postdoctoral Science Foundation (Grant No. 2019 M661856), Qing Lan Project of Jiangsu Province (Grant No. 2020), Natural Science Foundation of Jiangsu Province, China (Grant No. BK20201385), National Key Research and Development Project (Grant No. 2017YFC0505803), National Natural Science Foundation of China (NSFC) (Grant No. 41977354).
Author information
Authors and Affiliations
Contributions
YH: writing the manuscript, data analysis, literature research. LG: supervision of experiment, discussion. HG: revision of text. JH: funding acquisition and visualization. WQ: project administration. LX: conceptualization, funding acquisition, and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, Y., Gan, L., Gong, H. et al. Photoactivation of peroxymonosulfate by wood pulp cellulose biochar/g-C3N4 composite for diclofenac degradation: the radical and nonradical pathways. Biochar 4, 35 (2022). https://doi.org/10.1007/s42773-022-00155-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-022-00155-0