Abstract
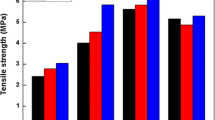
Investigation of Maillard reaction in natural rubber (NR) latex was carried out using two reducing sugars, glucose and ribose. Both reducing sugars together with a 1:1 mixture were added as 5 wt% solution to concentrated NR latex preserved with ammonia in a 1:1 ratio and preheated at 27, 70 and 90 °C for 2 h. The changes in the latex due to the reaction were characterised using pH and L values for 216 h (9 days). Cast latex films were then prepared and investigated for their swelling index (SI), gel content and tensile properties. Results obtained showed reduction in both pH and L values with time for the latexes with reducing sugars indicating progress of the Maillard reaction. For the Maillard reacted films, SI reduced whereas gel content, tensile stress and tensile strength increased. However, no significant changes were observed for the elongation at break of the reacted samples compared to the unreacted samples. Increasing the preheating temperature of the latex and reducing sugar mixtures to 70 and 90 °C from 27 °C increased the Maillard reaction which further improved the film properties. Although both reducing sugars improved the film properties, the improvements brought by reacting NR latex with ribose were more significant compared to reacting NR latex with glucose. From the current findings, it is evident that reducing sugars could be used to enhance the physical properties of natural rubber latex films. This technique will benefit the latex industry as it is environmentally friendly, easily applicable and cheaper compared to currently available techniques.









Similar content being viewed by others
References
D'Auzac J, Jacob JL, Chrestin H (1989) Physiology of rubber tree latex. CRC Press, London, Boca Raton
Swanson CL, Buchanan RA, Otey FH (1979) Molecular weights of natural rubbers from selected temperate zone plants. J Appl Polym 23:743–748
Cornish K (2001) Similarities and differences in rubber biochemistry among plant species. Phytochemistry 57:1123–1134
Bateman L (1963) Chemistry and physics of rubber-like substance. Maclaren and Sons, London
Blackley DC (1997) Polymer latices science and technology:types of lattices, vol 2. Chapman & Hall, London
Archer BL, Sekhar BC (1955) The proteins of Hevea brasiliensis latex: I. Protein constituents of fresh latex serum. Biochem J 61(3):503–508
Na-Ranong N, de Livonniere H, Jacob JL (1995) Natural rubber: doubts about the PRI. Plant Rech Dév 2(2):44–45
Nawamawat K, Sakdapipanich JT, Ho CC, Ma Y, Song J, Vancso JG (2011) Surface nanostructure of Hevea brasiliensis natural rubber latex particles. Colloids Surf A Physicochem Eng Asp 390(1–3):157–166
Sansatsadeekul J, Sakdapipanich J, Rojruthai P (2011) Characterization of associated proteins and phospholipids in natural rubber latex. J Biosci Bioeng 111(6):628–634
Ho CC, Kondo T, Muramatsu N, Ohshima H (1996) Surface structure of natural rubber latex particles from electrophoretic mobility data. J Colloid Interface Sci 178(2):442–445
Yu H, Wang Q, Li J, Liu Y, He D, Gao X, Yu H (2017) Effect of lipids on the stability of natural rubber latex and tensile properties of its films. J Rubber Res 20(4):213–222
Chong KL (1977) The chemistry of sulphur vulcanisation of natural rubber latex. PhD Thesis, CNAA, NCRT, Polytechnic of North London
Loh ACP (1982) Further investigations of prevulcanisation of natural rubber latex. PhD Thesis, CNAA, NCRT, Polytechnic of North London
Gorton ADT (1977) The effect of aqueous detergent solutions on dipped natural rubber latex vulcanizates. NR Tech 8(4):79–88
Bloomfield GF (1951) Studies in Hevea rubber. J Rubb Res Inst Malaya Commun 13:271–273
Sekhar BC (1962) Abnormal groups in rubber and microgel. In: Proc of the 4th Rubb Tech Conf, London, 22–25 May 1962, pp 460–469
Maillard LC (1912) Action of amino acids on sugars: formation of melanoidins in a methodical way. Compt Rend 154:66–68
Hodge JE (1953) Chemistry of browning reactions in model systems. J Agric Food Chem 1:928–941
Mauron J (1981) The Maillard reaction in foods: a critical review from the nutritional standpoint. Prog Food Nutr Sci 5:5–35
Lee MS, Nagy S (1983) Quality changes and non enzymatic browning intermediates in grapefruit juice during storage. J Food Sci 53:168–176
Ledl F, Schleicher E (1990) New aspects of the Maillard reaction in food and the human body. Angew Chem Int Ed Engl 29:565–594
Reynolds TM (1963) Chemistry of nonenzymic browning. I. The reaction between aldoses and amines. Adv Food Res 12:1–52
Montha S, Suwandittakul P, Poonsrisawat A, Oungeun P, Kongkaew C (2016) Maillard reaction in natural rubber latex: characterization and physical properties of solid natural rubber. Adv Mater Sci Eng 2016:1–6
Francis FJ, Clydesdale FM (1975) Food colorimetry: theory and applications. AVI Publishing Company Inc, Wesport
Pathare PB, Opara UL, Al-Said FAJ (2013) Colour measurement and analysis in fresh and processed foods: a review. Food Bioprocess Technol 6:36–60
Treloar LRG (1975) The physics of rubber elasticity, 3rd edn. Clarendon Press, Oxford
Singh M (2007) Effect of Maillard reaction on the properties and extractable protein content of cast natural rubber latex films. M.Sc. Thesis, Universiti Sains Malaysia
ASTM D3616-95 Standard test method for rubber—determination of gel, swelling index, and dilute solution viscosity
ASTM D 412-92 Standard test methods for vulcanized rubber and thermoplastic rubbers and thermoplastic elastomers—tension
Hashiba H (1981) The browning reaction of Amadori compounds derived from various sugars. Agric Biol Chem 46(2):547–548
Gee G (1945) The recent advances in the physics and chemistry of rubber III. The solubility and swelling of rubbers. Rubber Chem Technol 18(4):716–724
Johns J, Nakason C, Thitithammawong A, Klinpituksa P (2012) Method to vulcanize natural rubber from medium ammonia latex by using glutaraldehyde. Rubber Chem Technol 85(4):565–575
Johns A, Sham Aan MP, Johns J, Bhagyasekhar MS, Nakason C, Kalkornsurapranee E (2015) Optimization study of ammonia and glutardahyde contents on vulcanization of natural rubber latex. Iran Polym J 24:901–909
Nimpaiboon A, Sriring M, Kumarn S, Sakdapipanich J (2020) Reducing and stabilizing the viscosity of natural rubber by using sugars: interference of the maillard reaction between proteins and sugars. J Appl polym Sci. https://doi.org/10.1002/app.49389
Mark JE, Erman B, Roland M (2013) The science and technology of rubber, 4th edn. Elsevier B.V, Amsterdam
Huneau B (2011) Strain-induced crystallization of natural rubber: a review of X-ray diffraction investigations. Rubber Chem Technol 84(3):425–452
Acknowledgements
The authors would like to thank the Director General of Malaysian Rubber Board for permission to publish this paper. This work was part of a MSc program conducted in the Universiti Sains of Malaysia (USM) and it was made possible through funding via its internal grant. The technical assistances rendered by Mr. Zandar Md Saman, Mr. Mohamad Hassan and Mr. Segaran are highly appreciated. The authors would also like to acknowledge Veronica Charlotte for language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we do not have any commercial or associate interest that represents a conflict of interest in connection with the work submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, M., Azhar Mat Easa & Azahari, B. Effect of Maillard reaction in ammonia preserved natural rubber latex using reducing sugars. J Rubber Res 23, 365–374 (2020). https://doi.org/10.1007/s42464-020-00064-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42464-020-00064-6