Abstract
Fabrication of anti-bacterial nanomaterials made considerable progress in the catalytic method of green synthesis. In the current research work, a simple and environmentally convenient method was used for the synthesis of zinc oxide nanomaterials (ZnO-NMs) using the root extract of the Moringa oleifera. The fabricated MOR-ZnONMs were analysed by various analytical techniques such as UV–Vis absorption spectroscopy, Fourier transforms infrared spectroscopy (FT-IR), X-ray diffraction (XRD), Field emission scanning electron microscopy (FE-SEM), energy dispersive X-ray analysis (EDX), and photoluminescence spectroscopy. XRD analysis revealed that the synthesized MOR-ZnONMs have the hexagonal wurtzite structure. FT-IR confirmed the presence of various functional groups were responsible for the reduction of the metal ion into MOR-ZnONMs. The particles size, morphology and topography of the synthesized MOR-ZnONMs were determined by Dynamic Light Scattering and Transmission Electron Microscopy with significant measures. The intense and narrow widths of the zinc and oxygen present in the MOR-ZnONMs have been identified by utilizing EDX of high purity and crystalline nature. The prepared MOR-ZnONMs were tested for anti-bacterial activities and revealed that the maximum zones of inhibition were observed against Gram-positive and Gram-negative pathogenic bacteria.
Similar content being viewed by others
1 Introduction
Nowadays nanotechnology has an increase of vital role in most dynamic areas of research modern materials science. Nanotechnology is the technology that performed on the nanoscale that has applications in the real world. Nanotechnology can give the production and application of chemical, physical and biological materials at a scale ranging from individual atoms and molecules to submicron dimensions, as well as the incorporation of the resulting nanostructures into bigger structures. Recently, the nanotechnology has extended the scope of elevating the research in various scientific disciplines due to the unique properties of the nanomaterials compared with bulk materials of the individual atoms are molecules. Nanomaterials of the noble metals have attracted interest and applications in various field including biomedical applications because of their quantum detention effects, anti-bacterial activity and their large reactive surface area [1, 2]. The sizes of the nanomaterials are similar to the most biological molecules and their molecular structures. Therefore these nanomaterials can be used in the biomedical research field and applications. In recent years nanomedicine has generated great enthusiasm because of important discoveries, especially in cancer therapy [3, 4].
Nanotechnology mainly includes synthesis, characterization and biological applications of synthesized nanoparticles. There are many chemicals and physical methods for the preparation of nanoparticles. A physicochemical method has some disadvantages due to high cost, high temperature, high pressure and usage of chemicals. To avoid all the disadvantages of a physicochemical method there is a need an alternative method for the preparation of nanoparticles. Therefore current researchers focused on the fabrication of alternative and non-toxic nanomaterials by using of the cost-effective biological materials and environmentally safety components [5]. Green fabrication method gives the progression over chemical and physical methods as eco and environment friendly and easily scaled up for large scale production of the nanomaterials. The chemical and physical methods there need to use high pressure, energy, temperature and toxic chemicals as reducing agents [6, 7]. The bio-fabrication of various nanoparticles has been completed by using various plants and assessing the antimicrobial action, for example, a leaf extract from Rangoon Creeper leaves [8], Syzygium Cumini Stem Bark Aqueous Extract [9], and Tridax Procumbens and Prosopis Juliflora leaves [10, 11], Limonia acidissima and Terminalia Chebula leaves Aqueous Extract [12, 13].
Zinc oxide nanomaterials are used as a foliar fungicide on fruits and vegetables, in the manufacturing of rayons, good precursors for the synthesis of ZnO, in kill mould in paints and ceramic as the colourant. There are different methods available for the synthesis of Zinc oxide nanomaterials such as aqueous organic interface method [14], chemical co-precipitation method [15], a two-step template approach [16], wet chemical route [17], anion exchange method [18], controlling the PH of an aqueous Zn ion solution with weak bases [19], and the solvothermal method with surfactants [20] and an electrochemical method [21].
The present study focuses on the preparation of new, rapid, clean, non-toxic and environmentally acceptable green route for the production of zinc oxide nanomaterials by green fabrication process using hexahydrate Zinc nitrate using the Moringa oleifera root (MOR) extract at room temperature. To the best of the author’s knowledge till date, there have been no reports on the bio-synthesis of MOR-ZnONMs using the aqueous root extract of the MOR and also their spectro-chemical and anti-bacterial studies.
2 Experimental
2.1 Materials and methods
Moringa Oleifera fresh roots were collected from the Panyam village, nearby Nandyal, Kurnool District, and Andhra Pradesh, India. Hexahydrate Zinc nitrate (Zn(NO3)2∙6H2O) purchased from the Sigma-Aldrich Chemicals Pvt. Ltd., Hyderabad, India. Muller Hinton Agar nutrient broth was obtained from Himedia Laboratories, Mumbai, India. The pathogenic bacterial strains such as gram-negative cultures (Escherichia coli) and gram-positive cultures (Bacillus Substillis) were obtained from Microbial Type Culture Collection, Bangalore, India.
2.2 Preparation of Moringa Oleifera root extract
Moringa oleifera roots were collected and 20 g of roots were weighed by using an electronic balance and washed thoroughly with tap water initially followed by twice in double distilled (DD) water. The air-dried roots were coped keen on fine pieces and transferred into a clean conical flask with 100 mL DD water and allowed to boil at 60 °C for 1 h, then the residues were filtered with Whatman No.1 and preserved at 4 °C for further utilization. The filtered extract of root was used for the fabrication of ZnONMs and it plays a dual role as reducing and capping agent in the formation of nanomaterials.
2.3 Synthesis of MOR-ZnONMs
An aqueous solution of 0.1 M of Zn metal solution was prepared by the measured amount of hydrated Zn(NO3)2 salt was taken in a 100 mL beaker and dissolved in 50 mL of DD water under significant stirring for 15 min at laboratory conditions. After continuous stirring, the raw solution of 5 mL of root extract was added and the solution mixture was allowed to heat at around 60–90 °C for 2 h. The MOR extract acts as both reducing and capping agent in the reduction mechanism and the visual observations take plays by a colour change from clear white to brownish colour paste, the colour change is the primary confirmation of the formation of MOR-ZnONMs. The paste was transferred into a clean ceramic crucible and allowed it into muffle furnace maintaining 400 °C for 4 h and gave fine powder. The powder was preserved and used for further analytical characterization and biological application. The schematic graphical representation of green fabrication of MOR-ZnONMs is shown in Fig. 1.
2.4 Characterization of synthesized zinc oxide nanomaterials
Shimadzu 2400 UV–Visible double beam model UV–Visible spectrophotometer was used for the analysis of maximum absorption band in the range from 200 to 800 nm at room temperature. The dried MOR-ZnONMs are ground with KBr pellets and subjected to ALPHA interferometer (ECO-ATR), Bruker, Ettlingen, Karlsruhe, Germany instrument in the range of 400-4000 cm−1 for finding the functional groups present in the synthesized samples. Oxford Inca Penta FET × 3EDAX instrument was used for the finding of mean diameter and size circulation using Dynamic Light Scattering (DLS) method. XRD analysis of synthesized MOR-ZnONMs cast on glass slides are recorded using a Bruker-Binary V2 (Raw) (Cu radiation, λ = 0.1546 nm) instrument running at 40 kV and 40 mA and are recorded with 2θ angle from the range of 10-90°. TEM (Carl Zeiss EVO ma 15) experiments are performed to find out the size and shape of bio reduced MOR-ZnONMs.
2.5 Anti-bacterial activity
For the finding of antibacterial activity of synthesized MOR-ZnONMs, a Regular disc diffusion method [22] was used. The biological test organisms were grown in nutrient agar broth for 24 h. Sterilized petri plates were poured with agar media followed by standard protocols and then sterilized and solidified. For prepare bacterial lawns, after solidification, 100 µL overnight culture of each organism was poured on the petri plates using a sterile glass rod. Sterilized discs were placed on the solidified agar media with 5 mm diameter and MOR-ZnONMs were loaded at required volumes on discs and incubated at 37 °C for 24 h. For antibacterial activity, distilled water is used as a positive control. After the incubation period, a significant zone of inhibition was observed around the discs. Using meter ruler the diameter of all zones were measured and mean values for each organism were also recorded and represented in millimetres.
3 Results and discussion
3.1 UV–visible analysis
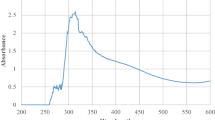
The powder of the bio-fabricated MOR-ZnONMs was used for the primary confirmation of reaction mixture of aqueous roots extract of Moringa oleifera and hydrated zin nitrate in solution produced an interesting colour change after 24 h of incubation time from clear white to yellow colour powder. The colour change was confirmed the formation of MOR-ZnONMs, and it was due to the excitation of Surface Plasmon Resonance (SPR) of the various phytochemicals present in the root extract which were involved in the reduction mechanism. Optimized diluted MOR-ZnONMs solution was used for the primary confirmation of formation of nanomaterials by using UV–Vis spectrophotometric analysis and noticed the absorption peak maxima at 375 nm [23], and it is due to the electron transitions from the valence band to the conduction band in the reduction of Zinc oxide by the phytochemicals (Fig. 2). The initial plain root extract doesn’t show any significant absorption peak in the recorded spectra. Based on these observations the intensity of absorption band growths with increasing of reaction time and subsequent colour fluctuations were observed without shifting of wavelength during the reaction. The shape of the bio-fabricated MOR-ZnONMs is hypothetical to be sphere-shaped in line with Mei’s theory [24].
3.2 FT-IR analysis
For initial find of the possible functional groups present in the root extract of Moringa oleifera which leads to the formation of ZnONMs by reduction mechanism FTIR spectrum was recorded for both aqueous root extract and the fabricated fine oven-dry powder of MOR-ZnONMs. Figure 3.A. corresponds to the aqueous root extract of Moringa oleifera and Fig. 3.B. related to the bio-fabricated MOR-ZnONMs. The recorded spectrum of the broad stretch at 331.2 and 3414.07 cm−1 indicates the phenolic hydroxyl stretch vibrations that indicate the H-bonding groups in the phenolic compounds as well as the bio-synthesized nanomaterials [25]. The peaks rise at 2928.45 cm−1 and 2851 cm−1 and their combination peaks were associated with –CH stretching vibrations of aldehydes [26]. The strong absorption peaks at 1731.96 cm-1 correspond to the –C = O of the stretching vibrations of the aldehydes present in the phenolic mixture. The strong intense peaks at 1621.11 cm−1 are the –NH bending vibrational peak of amine functional group and 1450.44 cm−1 refers to the –C–N of amide groups in the prepared material. The frequency around 1355.43 and 1180 cm−1 combination indicates the –C=O of phenolic compounds and the sharp absorption at 1036.95 cm−1 indicates the symmetric stretching vibration of ethers (R–O–R). The frequency at 820 cm−1 indicates the bending vibrations of the alkene –C–H groups of the aliphatic compounds in the reaction mixture. The vibrational frequency in between 459 to 600 cm−1 region denoted the metal oxides [27]. The stabilization and capping agents of fabricated MOR-ZnONMs may be due to the coordination of ZnONMs with hydroxyl and C=O groups present in the root extract. Fluctuations or shifting of vibrations in the spectra indicates the involvement of functional groups present in the root extract in the reduction mechanism and the formation of MOR-ZnONMs. The above results may conclude the involvement of the phytochemicals in the reduction mechanism and the stabilization of MOR-ZnONMs.
3.3 X-ray diffraction analysis
Structure and nature of the fabricated MOR-ZnONMs were analyzed by powder XRD spectrometer and Fig. 4 show the XRD pattern of green bio-synthesized MOR-ZnONMs. The diffraction measurement peaks of the MOR-ZnONMs are indexed which corresponds to the hexagonal wurtzite structure of zinc oxide with lattice constants of a = 0.320 nm and c = 0.526 nm. Figure 4 represents the XRD pattern and the noticeable reflection planes are identified at 110, 002, 101, 102, 110, 103, 201, 202 and which corresponds to diffraction angles are 31.66°, 34.43°, 36.24°, 47.46°, 56.60°, 62.76°, 67.89°, and 76.96°. These resultant peaks were matches with the previous reports and confirm the wurtzite structure of bio-fabricated MOR-ZnONMs [28]. The sharp peaks and the fine peaks were designate the pure crystalline nature of the MOR-ZnONMs. The increased intense peak of (002) for zinc-oxide nanomaterials exhibits the anisotropic behaviour could be observed in spectra which confirmed the obtained appropriate orientation of the material was in the respective direction [29]. The maximum diffraction peaks of MOR-ZnONMs were observed at the crystalline planes 101 and 110 and the average crystalline size of the nanomaterials 22.89 nm was calculated by using Scherer’s formula, the various parameters for the measuring of crystalline size were placed in Table 1 and finally these reports associates with dynamic light scattering analysis present in Fig. 6.
where, 0.89 = Scherer’s constant, D = Average crystal size, λ = Wavelength of the X-ray radiation (Cu Kα), β = Full width at half-maximum (FWHM) of the MOR-ZnONMs of (1 0 1) line. θ = Bragg’s angle of diffraction.
3.4 Energy dispersive X-ray (EDX) analysis
EDX spectral data was supported to the detection of the metal–ligand along with the elements present in the composition and provides the average % of each element present in the fabricated MOR-ZnONMs. Figure 5 exhibited the EDX spectrum of MOR-ZnONMs prepared by the aqueous solution of MOR root extract with 0.1 M of hydrated zinc nitrate solution. In Fig. 5 Strong intense signals detected which were related to zinc and oxygen and the results were confirmed that the fabricated nanomaterials were in pure and state of chemical nature [30]. The high single intense peaks of Zn and O are found between 0 and 8, and three peaks of Zn atom were found at 1, 8.5 and 9.5. These results associated with the previous reports in which similar peaks have been observed in ZnONMs synthesis using Corriandrum leaf extract [31]. Insert in Fig. 5 were done to find the % of weight (65.42) and atomic % (41.99) of zinc and oxygen elements present in the bio-fabrication MOR-ZnONMs.
3.5 Particle size distribution by DLS and TEM analysis of bio-synthesized MOR-ZnONMs
One of the most important properties of the nanomaterials is size and surface volume ratio of each particle and plays a very important role in the biomedical field. The size distribution of the nanomaterials was carried out by DLS analysis and the obtained graph shown the sharp intensity peaks (Fig. 6). The size distribution was dispersed widely from 15 to 40 in nano range (nm); the average size of the MOR-ZnONMs was to be at around ~ 25 nm with 2.5 of the peak width. The size distribution of MOR-ZnONMs gives a sphere-shaped cluster which confirmed by the TEM pictures in Fig. 7. TEM pictures are captured with a dehydrated compound of synthesized MOR-ZnONMs and recorded at different magnifications. Size distribution, morphology and surface volume ratio of the synthesized Zn oxide nanomaterials were playing the most important role in evaluating the toxicity of various types of pathogenic bacteria’s [32]. TEM pictures expose that the MOR-ZnONMs were in distributed in nano size, monodispersed and were spherical in shape with an average size of ~ 25 nm.
3.6 Photoluminescence spectroscopy (PLS) analysis
PLS is a contactless and non-destructive method of probing the electronic structure of synthesized nanomaterials. UV–Visible light is subjected directly onto a material, where these are absorbed and imparts excess energy into the material in a process called photo-excitation. Figure 8 was the PLS of the bio-fabricated nanomaterials measured at room temperature and confirms the formation of MOR-ZnONMs. The emission spectra of the prepared MOR-ZnONMs was plotted between 400 and 750 nm of UV–Visible region and noticed that most of the sharp intense peaks in the visible region with respect to the various colours. The intensity and width of the peaks in the respective band of emission, one can conclude the structure of the ZnO nanomaterial [33]. PLS of the bio-fabricated MOR-ZnONMs showed several emission bands of blue in between 435 and 480 nm, the bands for green are from 490 to 550 nm. The yellow and orange is in between 560 to 595 nm and above 600 nm indicates the red colour emission bands are concern with the defect structure of the MOR-ZnONMs. The green emission band is recognized to the occurrence of independent ionized oxygen vacancies. This representative emission is due to the radiative recombination of the photo-generated gap with an electron involving in the oxygen opening [34]. The emission of the fabricated MOR-ZnONMs was captured under UV visible light radiation and clearly emits the radiation with clear visibility (Fig. 9). These results and the emission spectra of the MOR-ZnONMs clearly showed that these are spherical in shape having nano-dimensions which is also confirmed by the TEM images of the nanomaterials.
3.7 Anti-bacterial activity
Recently, ZnO nanoparticles showed good anti-bacterial activity on the pathogenic microorganism like Streptococcus mutans, Streptococcus pyogenes, Vibrio cholerae, Shigella flexneri and Salmonella typhi [35]. The anti-bacterial activity of MOR-ZnONMs was studied by measuring the zone inhibition diameter. The pathogenic bacterial strains used in the present study (Escherichia coli and Bacillus Subtilis) causes transmissible diseases in human beings. To evaluate the ability of the nanomaterials, the anti-bacterial studies were conducted by agar well diffusion method. For the experimental purpose initially different concentrations with the dilution of fine powder of MOR-ZnONMs in double-distilled water. The dilution parameters were followed by 0.10 gm of nanomaterial powder was dissolved in 2 mL of DD water (1. DD water, 2. 0.15 gm in 2 mL, 3. 0.20 gm in 2 mL, 4. 0.25 gm in 2 mL and 5. 0.30 gm in 2 mL) and after complete dispersion of the nanomaterial in the water 200 microliters of the solution of each dilution was used in the anti-bacterial evolution study. Figure 10 gives the detailed zone of inhibition diameter (ZID) of the synthesized MOR-ZnONMs and the measurements of the ZID placed in Table 2. By analyzing the above both Fig. 10 and Table 2, the ZID was increasing with the increasing of the addition of the nanomaterials and by the action of MOR-ZnONMs on each strain of organisms.
4 Conclusion
In the present study on “Green bio-fabrication of MOR-ZnONMs using root extract of Moringa oleifera and their anti-bacterial activity”, MOR-ZnONMs were prepared using aqueous root extract of Moringa oleifera, a plant commonly grown for ornamental purposes. These fabricated nanomaterials were optimized and resultant nanopowder was analyzed using UV- Visible spectroscopy, FTIR, EDX, XRD, DLS, PLS and TEM. Initial confirmation of formation of MOR-ZnONMs was characterized by UV–Visible spectrophotometer and the sharp intense peak of absorption was obtained at 375 nm. FTIR analysis significant changes in functional groups related to the various polyphenols present in the root extract. Identification of the Zn and ZnO present in the fabricated nanomaterial was confirmed by the EDX reports. DLS and TEM photographs revealed that the separable particle size range of 15 nm to 40 nm which were associates with the XRD reports and also this reports of the nanomaterials are present in the form of aggregates. The morphological and crystalline nature of the MOR-ZnONMs were analyzed by XRD and confirms the hexagonal wurtzite structure and also establishes the role of a lesser intense capping layer on the surface of the nanomaterials. Although in the anti-bacterial study the effect of nanomaterials for their anti-bacterial potential, these forms the good ZID against both gram-positive and gram-negative tested pathogenic bacteria’s. The reports of the present study conclusively an eco-friendly approach for fabrication of zinc oxide nanomaterials and these studies have the potential for developing good anti-bacterial formulations having nano-sized particles in the material.
References
Abou El-Nour KMM, Eftaiha A, Al-Warthan A, Ammar RAA (2010) Synthesis and applications of silver nanoparticles. Arab J Chem 3:135–140. https://doi.org/10.1016/j.arabjc.2010.04.008
Laura MS, Vera AA (2019) Advances in magnetic noble metal/iron-based oxide hybrid. Nanoparticles Biomed Dev 6:75. https://doi.org/10.3390/bioengineering6030075
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MP, Acosta-Torres LS, Diaz-Torres LA, Grillo R, Swamy MK, Sharma S, Habtemariam S, Shin HS (2018) Nano based drug delivery systems: recent developments and future prospects. Nanobiotechnol 16:71. https://doi.org/10.1186/s12951-018-0392-8
Bjornmalm M, Kristofer JT, Michael M, Scott AM, Caruso F (2017) Bridging bio-nano science and cancer nanomedicine. ACS Nano 11:9594–9613. https://doi.org/10.1021/acsnano.7b04855
Das RK, Pachapur VL, Lonappan L, Naghdi M, Pulicharla R, Maiti S, Cledon M, Dalila LMA, Sarma SJ, Brar SK (2017) Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects Nanotechnol. Environ Eng 2:18. https://doi.org/10.1007/s41204-017-0029-4
Rafique M, Sadaf I, Rafique MS, Tahir MB (2017) A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol 45:1272–1291. https://doi.org/10.1080/21691401.2016.1241792
Mohammadinejad R, Karimi S, Iravani S, Varma RS (2015) Plant-derived nanostructures: types and applications. Green Chem. https://doi.org/10.1039/C5GC01403D
Birusanti AB, Umamahesh M, Devanna N, Espenti CS, Sreenivasulu M (2019) Sustainable green synthesis of silver nanoparticles by using rangoon creeper leaves extract and their spectral analysis and anti-bacterial studies. IET Nanobiotechnol 13:71–76. https://doi.org/10.1049/iet-nbt.2018.5117
Espenti CS, Rao KM, Krishna Rao KSV (2018) A simple biosynthesis of silver nanoparticles from syzygium cumini stem bark aqueous extract and their specrtochemical and antimicrobial studies. J Appl Pharm Sci 8:073–079. https://doi.org/10.7324/JAPS.2018.8111
Rao KM, Espenti CS, Krishna Rao KSV (2017) Biosynthesis of microbial resistance Au-nanoparticles from Aqueous Extract of Tridax Procumbens Leaves. Ind J Adv Chem Sci 5:24–29. https://doi.org/10.22607/IJACS.2017.501004
Krishna Rao KSV, Reddy PRS, Espenti CS, Rao KM (2017) Green synthesis of gold nanoparticles using Prosopis Juliflora leaves extract and evaluation of their anti-bacterial. Act Ind J Adv Chem Sci 5:102–107. https://doi.org/10.22607/IJACS.2017.502005
Espenti CS, Rao KM, Krishna Rao KSV (2016) A green approach to synthesize controllable silver nanostructures from Limonia acidissima for inactivation of pathogenic bacteria. Cognet Chem 2:1144296. https://doi.org/10.1080/23312009.2016.1144296
Espenti CS, Rao KM, Krishna Rao KSV (2016) Bio-synthesis and characterization of silver nanoparticles using Terminalia Chebula leaf extract and evaluation of its antimicrobial potential. Mater. Let. 174:129–133. https://doi.org/10.1016/j.matlet.2016.03.106
Ul Haq AN, Nadhman A, Ullah I, Mustafa G, Yasinzai M, Khan I (2017) Synthesis approaches of zinc oxide nanoparticles: the dilemma of ecotoxicity. J Nanomaterm 2017, Article ID 8510342, p 14. https://doi.org/10.1155/2017/8510342
Adam RE, Pozina G, Willander M, Nur O (2018) Synthesis of ZnO nanoparticles by co-precipitation method for solar driven photodegradation of Congo red dye at different pH. Photonics Nanostruct-Fundam Appl. https://doi.org/10.1016/j.photonics.2018.08.005
Rajesh D, Vara Lakshmi B, Sunandana CS (2012) Two-step synthesis and characterization of ZnO nanoparticles. Phys B 407:4537–4539. https://doi.org/10.1016/j.physb.2012.07.050
Nikam AV, Prasad BLV, Kulkarni AA (2013) Wet chemical synthesis of metal oxide nanoparticles: a review. Cryst Eng Commun. https://doi.org/10.1039/C8CE00487K
Cho G, Park Y, Hong Y-K, Ha D-H (2019) Ion exchange: an advanced synthetic method for complex nanoparticles. Nano Converg 6:17. https://doi.org/10.1186/s40580-019-0187-0
Zhang Y, Ram MK, Stefanakos EK, Goswami DY (2012) Synthesis, characterization, and applications of ZnO nanowires. J Nanomater, volume 2012, Article ID 624520, 22. https://doi.org/10.1155/2012/624520
Radzimska AK, Jesionowski T (2014) Zinc oxide—from synthesis to application: a review. Materials 7:2833–2881. https://doi.org/10.3390/ma7042833
Singh J, Dutta T, Kim KH, Rawat M, Samddar P, Kumar P (2018) ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. Nanobiotechnol 16:84. https://doi.org/10.1186/s12951-018-0408-4
Jorgensen JH, Turnidge JD, Washington JA (1999) Antibacterial susceptibility tests: dilution and disk diffusion methods. In: Murray PR, Pfaller MA, Tenover FC, Baron EJ, Yolker RH (eds) Mannual of clinical microbiology, 7th edition, 1526–1543, ASM Press, Washington, DC. NII Article ID (NAID):10019777527
Al-Shabib NA, Husain FM, Ahmed F, Khan RA, Ahmad I, Alsharaeh E, Khan MS, Hussain A, Rehman MT, Yusuf M, Hassan I, Khan JM, Ashraf GM, Alsalme A, Al-Ajmi MF, Tarasov VV, Aliev G (2016) Biogenic synthesis of zinc oxide nanostructures from Nigella sativa seed: prospective role as food packaging material inhibiting broad-spectrum quorum sensing and biofilm. Sci Rep 6:36761. https://doi.org/10.1038/srep36761
Preeti D, Mausumi M (2016) Noble metal nanoparticles: plant mediated synthesis, mechanistic aspects of synthesis and applications. Ind Eng Chem Res. https://doi.org/10.1021/acs.iecr.6b00861
Santhoshkumar J, Rajeshkumar S, Venkat Kumar S (2017) Phyto-assisted synthesis, characterization and applications of gold nanoparticles: a review. Biochem Biophys Rep 11:46–57. https://doi.org/10.1016/j.bbrep.2017.06.004
Dostert KH, O’Brien CP, Mirabella F, Ivars-Barcelo F, Schauermann S (2016) Adsorption of acrolein, propanal, and allyl alcohol on Pd(111): a combined infrared reflection–absorption spectroscopy and temperature programmed desorption study Phys. Chem Chem Phys 18:13960. https://doi.org/10.1039/C6CP00877A
Balan V, Mihai CT, Cojocaru FD, Uritu CM, Dodi G, Botezat D, Gardikiotis I (2019) Vibrational spectroscopy fingerprinting in medicine: from molecular to clinical practice. Materials 12:2884. https://doi.org/10.3390/ma12182884
Saif S, Tahir A, Asim T, Chen Y, Khan M, Adil SF (2019) Green synthesis of ZnO hierarchical microstructures by Cordia myxa and their antibacterial activity. Saudi J Biol Sci 26:1364–1371. https://doi.org/10.1016/j.sjbs.2019.01.004
Fakhar-e-Alam M, Akram MW, Iqbal S, Alimgeer KS, Atif M, Sultana K, Willander M, Wang ZM (2017) Empirical modeling of physiochemical immune response of multilayer zinc oxide nanomaterials under UV exposure to melanoma and Foreskin Fibroblast. Sci Rep 7:46603. https://doi.org/10.1038/srep46603
Siddiqi KS, Rahman A, Tajuddin SM, Husen A (2018) Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett 13: 141. https://doi.org/10.1186/s11671-018-2532-3
Ansari MA, Murali M, Prasad D, Alzohairy MA, Almatroudi A, Alomary MN, Udayashankar AC, Singh SB, Asiri SMM, Ashwini BS, Gowtham HG, Kalegowda N, Amruthesh KN, Lakshmeesha TR, Niranjana SR (2020) Cinnamomum verum bark extract mediated green synthesis of ZnO nanoparticles and their antibacterial potentiality. Biomolecules 10(2):336. https://doi.org/10.3390/biom10020336
Yusof HM, Mohamad R, Zaidan UH, Rahman NAA (2019) Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J Anim Sci Biotechnol 10:57. https://doi.org/10.1186/s40104-019-0368-z
Lokesh KJ, Yogita K, Anil K, Manoj K, Kamlendra A (2017) Investigation of luminescence and structural properties of ZnO nanoparticles, synthesized with different precursors. Mater Chem Front 1:1413. https://doi.org/10.1039/C7QM00058H
Milot RL, Eperon GE, Green T, Snaith HJ, Johnston MB, Herz LM (2016) Radiative monomolecular recombination boosts amplified spontaneous emission in HC(NH2)2SnI3 Perovskite films. J Phys Chem Lett 7:4178–4184. https://doi.org/10.1021/acs.jpclett.6b02030
Aditya A, Chattopadhyay S, Jha D, Gautam HK, Maiti S, Ganguli M (2018) Zinc oxide nanoparticles dispersed in ionic liquids show high antimicrobial efficacy to skin-specific bacteria. ACS Appl Mater Interfaces 10:15401–15411. https://doi.org/10.1021/acsami.8b01463
Acknowledgements
We sincerely express our thanks to Chemistry department and authorities, RGMCET, Nandyal, for providing needful requirements and Sri Venkateswara University Tirupati for the providing the essential characterization.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Human and animal rights
The research work of this paper has not involved any human participants and animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Espenti, C.S., Rama Krishna, A.G. & Rami Reddy, Y.V. Green biosynthesis of ZnO nanomaterials and their anti-bacterial activity by using Moringa Oleifera root aqueous extract. SN Appl. Sci. 2, 1424 (2020). https://doi.org/10.1007/s42452-020-2945-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2945-3