Abstract
Hydrolysis in bioethanol production is one of the most limiting stages in the entire production process since it is the stage where the sugars to be converted to ethanol are obtained. Ulva fasciata, Hydropuntia dentata and Sargassum vulgare seaweeds were examined in this study to determine the most efficient pretreatment, optimal hydrolysis conditions and predictive models with dilute sulphuric acid and cellulase enzyme as catalysts. Dilute acid pretreatment was found to be the most efficient in maximizing the catalytic efficiency of enzymes applied on all the three selected seaweeds. Ulva fasciata, however, was found to be efficiently hydrolysable without any form of pretreatment. The study also found dilute sulphuric acid hydrolysis to be less effective since it released up to 52.4% of reducing sugars in the seaweeds as compared to the 90.9% from hydrolysis with cellulase enzyme. Also, the most efficient regression model between the seaweed species studied was obtained for the enzymatic hydrolysis of U. fasciata with a correlation coefficient of 99.4%. This indicates a high precision in predicting the reducing sugar yields from the species within boundary conditions. Overall, the optimal enzymatic hydrolysis process was influenced most by substrate concentration for all three seaweeds examined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bioethanol is the most widely used transport biofuel globally with a combined total production of 106 billion litres in the year 2017 [1]. It is being used commercially in blends with gasoline in some countries in order to reduce carbon footprints [2, 3]. Commercial bioethanol production has been primarily limited to edible feedstock such as sugarcane, corn, sugar beet and rapeseed [4]. In Ghana, commercial bioethanol is produced from cassava, which is also cultivated as a staple food in the country [5]. The continued use of edible feedstock for commercial bioethanol production could result in issues over food security, competition for arable land and fresh water use and the excessive use of pesticides and fertilizers [6]. Seaweeds have been studied extensively as potential alternative biomass for use in bioethanol production [7, 8]. This is primarily due to their high growth rate, high yield per hectare, no arable land requirement, no fertilizer requirement and low pretreatment costs [9].
The production of bioethanol from seaweeds also goes through the typical processes of pretreatment, hydrolysis (saccharification), fermentation and ethanol recovery through distillation and dehydration. The hydrolysis process in bioethanol production is one of the most limiting stages in the entire production process since it is the stage where the sugars to be converted to ethanol are obtained. Hydrolysis of seaweeds for bioethanol production involves the breakdown of polysaccharides (complex sugars) such as cellulose, laminarin, ulvan, alginate, carrageenan, mannitol and agar to simple sugars (monosaccharides) such as glucose, galactose, rhamnose, mannose, fucose, xylose and arabinose for fermentation to ethanol [10]. A poor hydrolysis of the seaweed biomass would therefore lead to a poor ethanol yield [8]. The two most commonly used hydrolysis methods are the dilute acid hydrolysis and enzymatic hydrolysis.
Dilute acid hydrolysis (or pretreatment) is considered the most economical and time-saving form of hydrolysis currently available for algal biomass [11]. Acid concentrations as low as 0.006 M along with a 15 min reaction time have been reported with appreciable reducing sugar yields [12, 13]. Nonetheless, enzymatic hydrolysis is considered the most efficient form of hydrolysis for algal biomass available, despite concerns in various studies over the high cost of enzymes and longer reaction times [14, 15].
In this study, the interactions between the critical factors affecting dilute acid and enzymatic hydrolysis were separately examined on three tropical seaweeds: Ulva fasciata, Hydropuntia dentata and Sargassum vulgare. This was done in order to improve the fermentable sugar yields while attempting to minimize catalyst dosages and reaction times, which in turn reduces catalysts costs for bioethanol production. The hydrolytic data obtained were used to develop novel predictive models which describes the total reducing sugar yields from cellulase enzymes and sulphuric acid in the hydrolysis of the selected tropical seaweeds representing the three groups of macroalgae.
2 Materials and methods
2.1 Harvesting and pre-processing of selected seaweeds
The seaweed species selected for this study were U. fasciata, H. dentata (an agarophyte) and S. vulgare. These species were selected because they are the most densely distributed across both the east and west coast of Ghana. They are also representative of the three groups of seaweeds, which are known to vary in composition and cellular structure [16].
The selected seaweeds were harvested in February 2016 from Prampram (5.5717°N, 0.1332°W) and Mumford (5.2660°N, 0.7542°E) on the east and west coast of Ghana (West Africa), respectively. The seaweeds were pre-processed through washing and sorting to remove sand, debris and any unwanted material. They were then sun-dried for 3–4 days to reduce the moisture content to < 15% from an initial moisture content of 80–90%. The dry seaweeds were milled to a particle size of < 1 mm, bagged in zip lock bags and stored in a dry cabinet before use.
2.2 Characterisation of seaweeds
The seaweeds were characterized for their total carbohydrates, total proteins, lipid content, moisture content, total solids, volatile solids and ash content. The total solids and moisture content were determined as described by Sluiter et al. [17] (adapted from ASTM E1756-01). The lipids content was obtained through Soxhlet extraction as described in Borines et al. [3]. It involved the heating of the sample in a solvent (petroleum ether) using a Soxhlet extraction apparatus for 16 h. The solvent was recovered through evaporation while the flask containing the lipids extract was cooled and weighed. Total proteins were determined as described in Hames et al. [18]. The volatile solids and ash content were determined as described in Sluiter et al. [19] (adapted from ASTM E1755-01). It involved the dry oxidation of the biomass at 575 °C until a constant residue weight is obtained. The total carbohydrate was obtained using a modified form of the method described in Van Wychen and Laurens [20] (adapted from ASTM E1758-01). It involved the sequential hydrolysis of the biomass with 72% sulphuric acid at 30 °C for 1 h and 4% sulphuric acid at 121 °C for 1 h. The liquid fraction of the hydrolysate obtained was analysed for total sugars using the PAHBAH (4-hydroxybenzoic acid hydrazide) assay [21].
Since seaweeds are known to be composed of a heterogeneous mixture of monomeric sugars in their structure, it was essential to determine the fractions of monomeric sugars present in the selected species. The monomeric sugars composition was determined using a modified form of the method described in Van Wychen and Laurens [20] (adapted from ASTM E1758-01). It also involved the sequential hydrolysis of the biomass with 72% sulphuric acid at 30 °C for 1 h and 4% sulphuric acid at 121 °C for 1 h. The liquid fraction of the hydrolysate obtained was analysed for monomeric sugars via high-performance liquid chromatography (HPLC) as specified in Sect. 2.6.
2.3 Seaweed pretreatment methods
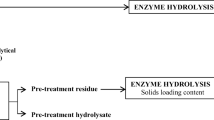
In this study, various pretreatments were examined to assess their relevance in algal ethanol production, their effect on sugar recovery and the best condition that supports enzymatic hydrolysis. Six unique pretreatment conditions and two controls were considered. Each condition was followed by enzymatic hydrolysis with a commercial enzyme, Cellic CTEC II (Novozymes, Denmark) at 5 FPU/g DM (dry matter) for 72 h at 50 °C with 5% substrate concentration while shaking at 150 rpm in an incubator shaker (Lab Companion SIF5000, Jeio Tech-Korea). All pretreatments considered were carried out at a substrate concentration of 10%. The pretreatments used were dilute acid, dilute alkaline, hot buffer, extremely low acid, dry heat and hot water wash with buffer-less and buffered enzymatic hydrolysis as controls. All three pre-processed seaweeds were screened with these pretreatments using the conditions summarized in Table 1.
2.4 Optimisation of dilute acid hydrolysis of selected seaweeds
The general full factorial experimental design was used in finding the ideal conditions for dilute acid hydrolysis of the selected seaweeds. The design was composed of three factors with three levels per factor. The factors examined were sulphuric acid concentration (0.1, 0.2, 0.3 M), reaction time (15, 30, 60 min) and reaction temperature (100, 120, 130 °C). The boundary conditions were kept narrow due to the extensive reports on the optimal conditions of dilute acid hydrolysis of seaweeds [11]. The experimental design matrix was generated with Minitab 17 statistical software. A total of 27 experimental runs were performed on each seaweed species in triplicates. The interactions between the factors (hydrolysis conditions) and the response variable (TRS: Total reducing sugars) were modelled with the aid of multiple regression analysis using Minitab 17 statistical software.
For each unit, pre-processed seaweed was added to 10 ml of acid solution (of known concentration) in 100 ml Duran bottles to form a substrate concentration of 10% w/v dry basis. The mixture was heated in a convection oven (VWR Dry-Line, Germany) at each specified temperature and time from the experimental design matrix. The mixture was cooled to room temperature and centrifuged at 6000 rpm for 5 min. An aliquot of the liquid fraction was analysed for total reducing sugars using the PAHBAH assay [21].
2.5 Optimisation of enzymatic hydrolysis of selected seaweeds
The central composite rotational factorial experimental design was used to examine the interactions between enzyme dosage, reaction times and substrate concentration to optimize the total reducing sugar yield. The design was composed of 3 factors with 3 levels per factor. The factors examined were enzyme dosage (2, 5, 8 FPU/g dry biomass), reaction time (24, 48, 72 h) and substrate concentration (5, 10, 15% w/v dry basis). The experimental design matrix was generated with Minitab 17. A total of 20 experimental runs were performed on each seaweed species in triplicates. The interactions between the factors (hydrolysis conditions) and the response variable (TRS) were modelled with the aid of multiple regression analysis using Minitab 17.
For each unit, pre-processed seaweed was first pretreated with 0.2 M H2SO4 at 130 °C in a convection oven for 15 min with a substrate concentration of 20% w/v dry basis (adapted from Meinita et al. [8]). The mixture was cooled to room temperature after heating. The pH was adjusted to the range of 5–6 with 4.5 M NaOH. The amount of enzyme as specified in the experimental design matrix was added to the mixture. The enzyme applied in this study was Cellic Ctec II (Novozymes, Denmark), a commercial enzyme whose cellulase activity, measured in filter paper units (FPU), was determined using a modified form of the method described by Adney and Baker [24]. The final substrate concentration of the mixture was also adjusted to 5% w/v dry basis with distilled water. The mixture was incubated in an incubator shaker (Lab Companion SIF5000, Jeio Tech-Korea) at 50 °C while shaking at 150 rpm for the length of time specified in the experimental design matrix. After the specified time, the mixture was cooled to room temperature and centrifuged at 6000 rpm for 5 min. A 100 μl aliquot of the liquid fraction (supernatant) was taken and analysed for total reducing sugars using the PAHBAH assay. The experimental data obtained were analysed using multiple regression analysis in Minitab 17 statistical software to obtain the optimal process conditions.
2.6 Analysis of sugar recovery
In the analysis of sugars, individual monomeric sugars were identified and quantified via HPLC while the total reducing sugars were quantified via PAHBAH assay. The analysis of monomeric sugars in the seaweeds was performed on a Shimadzu LC10/20 HPLC equipped with a refractive index detector. Monomeric sugars were analysed in the HPLC on a Rezex RPM column (Phenomenex, USA) operating at a column temperature of 80 °C and a detector temperature of 40 °C with ultrapure water as mobile phase at a flow rate of 0.6 ml/min. The HPLC was calibrated with high-purity standards of glucose, xylose, mannose, cellobiose, rhamnose, arabinose, fucose, galactose, galacturonic acid and mannitol. The sample injection volume for all the analytes was 10 μl, which were filtered (0.2 μm) before the analysis.
The concentration of total reducing sugars in the various hydrolysates obtained was quantitatively measured using the PAHBAH assay [21]. This assay exploits the reduction effect of the aldehyde group in the structure of reducing sugars with chromogenic agents. In this study, 0.5% w/v of the chromogenic reagent, 4-hydroxybenzoic acid hydrazide (PAHBAH) in 0.5 M NaOH was reacted with aliquots of the hydrolysates obtained to form a bright yellow colour when heated in a test tube at 100 °C for 5 min. The absorbance of the colour formed was measured at a wavelength of 410 nm with a spectrophotometer (Genesys 10S VIS, Thermoscientific-USA). The absorbance obtained was measured against a standard glucose calibration curve to obtain the concentration of total reducing sugars in the hydrolysate. The total reducing sugar yields obtained were used to evaluate the catalytic efficiency of the catalysts used during the hydrolysis of the seaweeds. Catalytic efficiency in this context refers to the ratio of total reducing sugars released to the total carbohydrates in the biomass expressed as a percentage. The total reducing sugar yield and catalytic efficiency were calculated using Eqs. 1 and 2.
For total reducing sugar yield,
For catalytic efficiency,
3 Results and discussion
3.1 Composition of the selected seaweeds
The composition of seaweeds or any other biomass considered for bioethanol production is critical in determining whether it may be a suitable raw material for this purpose. The three seaweeds sampled in this study were examined for various components, which are relevant to bioethanol production. After pre-processing through washing, sun drying and grinding, the total solids increased to 82–90% from 10 to 15% in the freshly harvested biomass (Table 2). Sun drying which is regarded as one of the oldest and simplest methods of biomass preservation and drying was effective in reducing the moisture content in the selected seaweeds. The high efficiency of the drying method is very favourable and cost-efficient for potential seaweed farming and processing in Ghana, where coastal fishing communities are mostly poor.
The selected seaweeds were composed of 31.2–32.6% total carbohydrates (Table 2) with no significant difference between them (p value < 0.05). The total carbohydrate content is of utmost importance in bioethanol production since it is the principal component that is converted by fermenting organisms such as yeast to ethanol. The carbohydrate values obtained for U. fasciata (31.3%), H. dentata (31.2%) and S. vulgare (32.6%) compare favourably with 45% from Marquez et al. [25], 39% from Rhein-Knudsen et al. [26] and 19.4% from Marinho-Soriano et al. [27], respectively, for the same species. The carbohydrate content in the selected seaweed species can be considered high enough for substantial ethanol recovery of up to 15% of the biomass assuming the theoretical maximum ethanol recovery of 51.2% of the reducing sugars can be achieved. The selected species were therefore considered as adequate potential substrates for bioethanol production.
3.2 Monomeric sugar composition of the selected seaweeds
The total carbohydrates fraction in seaweeds and most biomass are made up of various monomeric sugars (reducing sugars). Seaweeds are known to be composed of a broad diversity of monomeric sugars or monosaccharides as compared to other biomass such as cassava, sugarcane and elephant grass used in bioethanol production [28]. This study found the major monomeric sugars in U. fasciata to be glucose, xylose and rhamnose at 15.1, 7.6 and 5.4%, respectively; S. vulgare had glucose and fucose at 15.3 and 4.0%, respectively; and H. dentata had galactose and glucose at 13.1 and 12.0%, respectively, of dry biomass (Table 3). There was no significant difference between the glucose content, which formed the largest fraction of monomeric sugar in both U. fasciata and S. vulgare species at 15.1 and 15.3%, respectively. The glucose fraction for H. dentata was, however, significantly lower at 12.0%. In H. dentata, galactose formed the largest monomeric sugar fraction at 13.1%. These results indicate that the overall fermentable hexose (C-6 sugars) fraction of the monomeric sugars was higher than the pentose (C-5 sugars) fraction in all three seaweeds studied. This was cumulatively between 16 and 27%. This is particularly preferred since the hexose fraction of carbohydrates is much easier to ferment via the glycolytic metabolic pathway used by most yeast strains in the fermentation of sugars to ethanol [29]. It is reported that most common fermenting organisms such as the yeast, Saccharomyces cerevisiae, used in bioethanol production prefer hexose monomeric sugars such as glucose and mannose over pentose monomeric sugars such as xylose and arabinose [29]. The difference in hexose and pentose fraction could, however, impose a challenge through a possible reduction in ethanol yield unless the fermenting organism chosen demonstrates some significant pentose to ethanol conversion capability. The selection of the fermenting organism to process seaweeds to ethanol would, therefore, have a critical impact on ethanol yield.
3.3 Effect of pretreatments on enzymatic catalytic efficiency
In this study, various pretreatments were examined to assess their relevance in macroalgal ethanol production, their effect on reducing sugar recovery efficiency and the best condition that supports enzymatic hydrolysis. Six unique pretreatment conditions and two controls were considered in this study. Each condition was followed by the application of the commercial enzyme, Cellic CTEC II. The catalytic efficiency of the enzymes applied after each pretreatment was used as the metric of comparison.
Ulva fasciata responded best to dilute acid, hot buffer and interestingly, the buffered control (with no treatment) with catalytic efficiencies of 69.6, 67.2 and 55.1%, respectively (Fig. 1). There was no significant difference between them (p value < 0.05). The dilute acid and hot buffer done at 130 °C for 60 min with 0.2 M H2SO4 and 0.05 M citrate buffer as catalysts, respectively, were both considered the most extreme between selected treatment methods. This was due to the high heat energy required and toxic catalysts used. The buffered control, which was not subjected to any pretreatment but was hydrolysed directly with the enzyme in a citrate buffer medium, was, however, milder and less energy intensive. Trivedi et al. [22] adopted a similar approach in screening several pretreatments on U. fasciata. They identified pretreatment with hot acetate buffer at 120 °C for 60 min as most efficient for high sugar recovery.
The results for U. fasciata first imply that high temperatures favour structural breakdown. However, in the case of dilute acid treatment, higher concentrations of catalysts are also required since both the extremely low acid and dry heat treatments were done at the same temperature as dilute acid treatment. High catalyst loads have been reported to increase the number of active catalytic sites for the substitution reaction by acids [30]. The second implication of the results (Fig. 1) is that buffers used in the hot buffer pretreatment and buffered medium play a significant role especially for the enzymatic hydrolysis that follows pretreatment. Enzymes are known to be very pH sensitive; thus, stable pH provided by the buffers during hydrolysis improved the reducing sugar recovery.
Sargassum vulgare responded best to dilute acid and hot buffer treatments with catalytic efficiencies of 56.0 and 39.3%, respectively (Fig. 1). They were significantly different from each other and significantly higher than all the other treatments (p value < 0.05). Hot water wash also recorded a considerable catalytic efficiency of 24.2%. All the other treatments recorded similar catalytic efficiencies ranging between 2.0 and 6.5% after the enzyme was applied (Fig. 1). The response of S. vulgare to the pretreatments is quite similar to the case of U. fasciata particularly, their preference for heat application. The use of an acid catalyst such as sulphuric acid in the presence of heat as used in the dilute acid pretreatment and citric acid as used in the citrate buffer medium were both favourable for recovering reducing sugars. The high concentrations of the acids also greatly impact the cellular breakdown as seen in the change in difference in catalytic efficiencies between the dilute acid and extremely low acid treatments (Fig. 1).
The buffered medium control, which was effective in supporting the hydrolysis of U. fasciata without any pretreatment, was ineffective in S. vulgare. This could be attributed to the significant structural difference between the two seaweeds. The structure of S. vulgare is considered more stable than U. fasciata due to the presence of the hydrocolloid, alginate as compared to the weak hydrocolloid, ulvan in U. fasciata.
Dilute acid pretreatment gave the highest catalytic efficiency (67.9%) for H. dentata (Fig. 1). The H. dentata seaweed formed a gel when treated with dry heat, extremely low acid, hot buffer and dilute base. These gels were not analysed further since solid-state hydrolysis of biomass to recover reducing sugars would be ineffective. The gel formation which was also reported by Kim et al. [31] using 0.05–0.20 N Ca(OH)2 could be attributed to the interactions between the hydrocolloid, agar found in the H. dentata species and the OH- ions in water and bases at high temperatures. This phenomenon of gel formation in water is what gives hydrocolloids their gelling properties for use as thickeners in the food and pharmaceutical industries [25].
From the six pretreatments applied, only dilute acid and hot water wash were favourable for use on H. dentata. Dilute acid gave a significantly higher catalytic efficiency of 67.9% as against 5.2% from the hot water wash after enzyme application (p value < 0.05) (Fig. 1). The buffered control also gave a significantly higher catalytic efficiency (14.2%) than the hot water wash (p value < 0.05). Since only the acid catalyst interacted favourably with the sulphated-galactans predominant in agarophytes such as H. dentata, the selection of pretreatments for use on red seaweeds is very limited. This would in turn impact considerably on pretreatment and hydrolysis costs for commercial-scale bioethanol production.
All three seaweeds responded well to the dilute acid pretreatment with catalytic efficiencies between 55 and 70% (Fig. 1). This emphasizes its extensive use by numerous seaweed bioethanol production researchers [8]. Dilute acid pretreatment was selected as the most efficient pretreatment method from the six studied. However, U. fasciata maintains an important hydrolytic advantage over the other two seaweeds since it can be efficiently hydrolysed without any pretreatment.
3.4 Dilute acid hydrolysis of the selected seaweed
3.4.1 Optimal acid-catalysed hydrolysis of the selected seaweed
Dilute acid hydrolysis was optimized using response surface methodology, where the independent variables considered were time, temperature and acid concentration. Multiple regression analysis was used to analyse the data obtained to generate model equations that can describe and predict yields from each species within boundary conditions. Table 4 shows the three model equations for each seaweed hydrolysed. The equations were made up of the total reducing sugar yields expressed as a function of the independent variables, time (X1), temperature (X2) and acid concentration (X3). The model equation obtained for U. fasciata was expressed as a second-degree (quadratic) polynomial equation while the models for S. vulgare and H. dentata were first degree (linear) polynomials (Table 4). The model equation for H. dentata excludes the X2 term indicating that reaction temperature has the least effect on its TRS yield. This observation is further highlighted in the optimal conditions (Table 5) obtained, which shows that the ideal condition for hydrolysing H. dentata species is independent of temperature.
The correlation coefficient (R2) value, which represents the percentage variation explained by the model, was highest for U. fasciata at 79.9%. Sargassum vulgare and H. dentata were, however, marginally lower at 68.8 and 41.7%, respectively (Table 4). This implies that the models can predict the interactions between the variables under study for each species but with considerable limitation. The model for U. fasciata had the highest adequacy in predicting the TRS yield based on its correlation coefficient which is a measure of model strength. The lower R2 value for H. dentata from dilute acid hydrolysis could be attributed to the poor interactions between hydrolysis conditions. The relationship between the Y (TRS yield) and X values in all three models was statistically significant at p ≤ 0.005. The use of fitted models for the dilute acid hydrolysis optimization of all three types of seaweed is not only informative but also novel in comparison with literature on bioethanol from seaweed.
The optimal conditions for dilute acid hydrolysis of all three selected species were similar, with a time of 60 min and an acid concentration of 0.3 M with minimal variation in temperature (Table 5). The reaction temperature of U. fasciata must be reduced to 120 °C from 130 °C to obtain its optimal yield. This temperature reduction for only the green seaweed can be attributed to the weaker α-1,4-glycosidic linkages found in the starch fraction of U. fasciata. These are easier to breakdown than the β-1,4-glycosidic linkages (between mannuronic and guluronic acid) found in the alginate fraction of S. vulgare and the β-1,3-glycosidic linkages in agar found in H. dentata which are structurally more stable [32].
Meinita et al. [33] noted a reaction time, temperature and catalyst concentration of 15 min, 130 °C and 0.2 M, respectively, as the optimal dilute acid hydrolysis condition from their work on the red seaweed, Kappaphycus alvarezii. They obtained a TRS yield of 38.5% DM (dry matter). The difference between the optimal conditions in this study and theirs can be attributed primarily to the use of the carrageenophyte (a group of red seaweed), K. alvarezii. This is structurally different from the agarophyte, H. dentata used in this study due to the presence of the hydrocolloid, agar. Other factors that could account for the variation include geographical location, light intensity, water quality and harvest season.
Even though considered cost-effective, the dilute acid hydrolysis optimization released a maximum of 16.3 g TRS/100 g DM from H. dentata from a possible 31 g/100 g carbohydrate content. This represents a sugar recovery efficiency of 52.6%. This indicates that a more efficient hydrolysis method may be needed to complement or replace the dilute acid hydrolysis process to maximize sugar recovery. This study, therefore, examined enzymatic hydrolysis as an alternative.
3.4.2 Effect of acid hydrolysis conditions on TRS yields
The effects of the independent variables considered in the dilute acid hydrolysis process were examined to determine which variable had the most influence on TRS yields as shown in Fig. 2. The results are represented using contour plots which allow the examination of the relationship between three variables in a two-dimensional view. Two independent variables are represented on the x and y-axis of the plot while the response variable is represented by the contours (red gradient: low yields, green gradient: high yields).
From the contour plots of U. fasciata, acid concentration and time had the biggest combined effect on TRS yield as seen in Fig. 2a. Longer reaction times and higher catalyst concentrations favoured TRS yields of 15% DM or higher (Fig. 2a). Temperatures below 105 °C were less favourable with yields of less than 9% DM. This implies that longer reaction time, higher catalyst loading, and higher temperatures are required to maximize TRS yields for U. fasciata.
For S. vulgare, acid concentration had the highest influence on TRS yield even though marginal (Fig. 2b). The plot for S. vulgare is dominated by red regions, which indicates TRS yields below 9% DM. Low yields are found in all the interactions between the variables tested. This indicates the upper boundary conditions set for the S. vulgare optimization may not be favourable since TRS yields greater than 9% DM were reported after reaction temperatures of 125 °C and catalyst concentrations of greater than 0.26 M (Fig. 2b). This further implies that stronger conditions may be required to breakdown the cellular structure of S. vulgare to release the reducing sugars.
For H. dentata, reaction time was the most influential factor with temperature having no visible effect on TRS yield (Fig. 2c). TRS yields were greater than 12% DM for reaction times higher than 40 min. This was, however, limited to acid concentrations greater than 0.15 M. Trends for interactions between acid concentration and temperature with H. dentata were, however, difficult to predict.
Generally, the three seaweeds studied favoured high temperatures, high catalysts loading and longer retention times to maximize TRS yields. But further increase in these variables has been reported to result in fermentation inhibitor formation. Meinita et al. [8] noted that even though these conditions favour high TRS yields, thresholds exist which if exceeded decreased TRS yields. In their study, high temperatures and high acid catalyst loads which exceeded 120 °C and 0.2 M, respectively, caused the formation of sugar degradation products or inhibitors in the form of hydroxymethylfurfural and levulinic acid. These inhibitors were, however, not found in this study even though those thresholds were exceeded. The thresholds of acid hydrolysis must, therefore, be identified uniquely in each study for each species when dilute acid hydrolysis is used. The TRS yields from this study indicate that the boundary conditions for dilute acid hydrolysis with the selected species especially H. dentata must be redefined to maximize yield. This, nonetheless, must be done with the risk of inhibitor formation in mind.
3.5 Enzymatic hydrolysis of the selected seaweed
3.5.1 Optimal enzyme-catalysed hydrolysis of the selected seaweed
Enzymatic hydrolysis was also optimized in this study using response surface methodology. The independent variables considered were time, substrate concentration and enzyme concentration. Multiple regression analysis was also used to analyse the TRS yields obtained in order to generate model equations that can predict the possible yields from each species within boundary conditions. Table 6 shows the three model equations for each species with TRS yields expressed as a function of the independent variables considered. The models obtained for all three seaweeds were expressed as second-degree polynomial equations (Table 6). The relationship between the Y (TRS yield) and the X values in all three models was statistically significant with p < 0.001, a good initial indicator that the three models will be quite efficient in predicting the reducing sugar yields.
The correlation coefficient (R2) for U. fasciata was highest at 99.4%, a strong indicator of its precision in predicting the TRS yields from the species within the boundary conditions. This also indicates that the model can explain the bulk of the variation between variables and that the experimental values are almost fitted perfectly by the model. The models from S. vulgare and H. dentata were also acceptable with R2 values 61.3 and 75.2%, respectively, even though considerably lower than U. fasciata (Table 6). Pilavtepe et al. [34] also used response surface methodology along with regression modelling to describe the relationship between enzyme concentration, substrate loading and reaction time on TRS yields for the green seaweed, Posidonia oceanica. Their correlation coefficient of 93.2% was lower than that obtained for the green seaweed, U. fasciata in this study. This was, however, expected due primarily to variations in the species and their geographical harvest location. Generally, the models obtained in this study from the optimization of the enzymatic hydrolysis for all three seaweeds were adequate in describing the relationship between the variables under study.
Interestingly, the same optimal condition of 8 FPU/g DM, enzyme dosage; 5% w/v, substrate concentration and 24 h, hydrolysis time were obtained for all three seaweeds studied (Table 7). This implies that the hydrolysis of the seaweed was favoured by high enzyme concentration for a shorter time with a lower substrate concentration. Generally, a high enzyme load is reported to result in high TRS yield due to an increase in the ratio of substrate to enzyme [34]. Nonetheless, the enzyme dosage levels used in this study were lower than the range of 15–30 FPU/g DM often used [35]. This was done due to the positive corresponding effect it would have on overall hydrolysis costs.
3.5.2 Effect of enzymatic hydrolysis conditions on TRS yields
The effects of the various independent variables considered in the enzymatic hydrolysis process were examined to determine their influence on the TRS yields as shown in Fig. 3. It was observed that U. fasciata seaweed recorded TRS yields below 20% DM at substrate concentrations above 7.5% w/v irrespective of the enzyme concentration as shown in Fig. 3a. Similarly, TRS yields below 20% DM were obtained at substrate concentrations above 10% w/v irrespective of the reaction time. This indicates that both time and enzyme concentration had the least influence on TRS yields from U. fasciata. This variation could be attributed to efficient mobility and cleavage of the enzyme to the substrate particles to cause their break down due to an increased enzyme to substrate ratio. A system with a high substrate loading may, therefore, require more time for effective hydrolytic activity by the enzyme. It can be inferred from the TRS yields obtained for U. fasciata that efficient enzymatic hydrolysis can be achieved over a short period with minimal enzyme dosage.
A similar observation was made for H. dentata for interactions between hydrolysis time and enzyme dosage (Fig. 3c). Both were the least influential with substrate concentration being the predominant factor. TRS yields of 20% DM or greater were obtained for substrate concentrations below 7.5% w/v. Sargassum vulgare, however, showed a higher tolerance for high substrate concentration since it recorded TRS yields greater than 16.5% DM at substrate concentrations as high as 12% w/v regardless of the reaction time (Fig. 3b).
Generally, the TRS yields after the enzymatic hydrolysis of all the three seaweeds studied were very high (> 20% DM) and should be considered strongly as the hydrolysis method of choice for seaweeds. This was, however, preceded by dilute acid pretreatment which means additional cost would be incurred for species such as S. vulgare and H. dentata which require pretreatment before enzyme application. This study also shows that in the enzymatic hydrolysis of seaweeds, substrate concentration should receive the most consideration to maximize TRS yield.
3.6 Comparison of acid and enzyme-catalysed hydrolysis of seaweed
Dilute acids and enzymes are currently the most common catalysts used during the hydrolysis stage of seaweed bioethanol production. Sulphuric and hydrochloric acids are the most extensively used chemical catalysts in the acid form of hydrolysis while cellulases are the dominant enzymes used in hydrolysing seaweeds enzymatically [3, 8]. The fundamental factors apart from one being chemical (acids) and the other biological (enzymes) that differentiate the acids and enzymes are: reaction time, catalyst costs, by-products and environmental effects. The dilute acids are considered efficient, lower in cost and faster in reaction time than other methods but come with the risk of inhibitor formation [36]. Enzymes are considered more efficient and non-toxic but more expensive [14]. Neither catalysts are considered effectively recoverable after use.
Table 8 shows a comparison of the catalytic efficiencies of dilute acid and enzymes after their application to the three selected seaweeds. Catalytic efficiencies significantly increased by 39.4, 39.7, and 42.4% for U. fasciata, S. vulgare and H. dentata, respectively, when enzymes were used in place of acids. This shows the use of enzymes has a considerably higher efficiency when used to catalyse the hydrolysis of seaweeds. For each of the seaweeds studied the enzymatic hydrolysis process was the most efficient in recovering the reducing sugars. The results indeed emphasize that the enzymes are more efficient in sugar recovery. Concerns over costs are indeed valid since the seaweeds were pretreated before enzymes were applied. However, this additional cost can be avoided based on the seaweed species selected for use as evident in Fig. 1 for U. fasciata, which can be hydrolysed efficiently without pretreatment.
4 Conclusions
This study established that dilute acid pretreatment was the most efficient in treating all the three selected seaweeds before hydrolysis. Ulva fasciata, however, can be hydrolysed with cellulase in a buffered medium without any form of pretreatment. The study also found dilute sulphuric acid hydrolysis to be less effective since it released up to 52.4% of reducing sugars in the seaweeds as compared to the 90.9% from hydrolysis with cellulase enzymes. The optimal enzymatic hydrolysis process was influenced most by the substrate concentration used for all three seaweeds examined. Individual fitted regression models were also developed for the dilute acid and enzymatic hydrolysis of all three seaweeds studied. The TRS yields from the three seaweeds showed that seaweeds in Ghana have considerable prospects as efficient substrates for commercial bioethanol production. Nonetheless, a techno-economic analysis of the entire process chain including the cultivation stage must be appropriately evaluated before commercial consideration.
References
REN21 (2018) Advancing the global renewable energy transition. Renewables 2018 global status report 2018 in perspective. http://www.ren21.net/wp-content/uploads/2018/06/GSR_2018_Highlights_final.pdf. Accessed 9 Nov 2018
Adams JM, Gallagher JA, Donnison IS (2009) Fermentation study on Saccharina latissima for bioethanol production considering variable pre-treatments. J Appl Phycol 21(5):569
Borines MG, de Leon RL, Cuello JL (2013) Bioethanol production from the macroalgae Sargassum spp. Bioresour Technol 138:22–29
Chakraborty S, Das Mondal R, Mukherjee D, Bhattacharjee C (2013) Production of bio-based fuels: bioethanol and biodiesel. In: Piemonte V (ed) Sustainable development in chemical engineering innovative technologies, 1st edn. Wiley, Chichester, pp 153–180
Ghana trade portal (2016) http://www.ghanatrade.gov.gh/Food-Beverage/calteh-ventures-limited.html. Accessed 9 Nov 2018
Kraan S (2013) Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig Adapt Strateg Glob Change 18(1):27–46
Schultz-Jensen N, Thygesen A, Leipold F, Thomsen ST, Roslander C, Lilholt H, Bjerre AB (2013) Pretreatment of the macroalgae Chaetomorpha linum for the production of bioethanol—comparison of five pretreatment technologies. Bioresour Technol 140:36–42
Meinita MDN, Marhaeni B, Winanto T, Setyaningsih D, Hong Y (2015) Catalytic efficiency of sulfuric and hydrochloric acids for the hydrolysis of Gelidium latifolium (Gelidiales, Rhodophyta) in bioethanol production. J Ind Eng Chem 27:108–114
Jones CS, Mayfield SP (2012) Algae biofuels: versatility for the future of bioenergy. Curr Opin Biotechnol 23(3):346–351
Lee KT, Ofori-Boateng C (2013) Biofuels: production technologies, global profile, and market potentials. In: Singh A (ed) Sustainability of biofuel production from oil palm biomass. Springer, Singapore, pp 31–74
Mutripah S, Meinita MDN, Kang JY, Jeong GT, Susanto AB, Prabowo RE, Hong YK (2014) Bioethanol production from the hydrolysate of Palmaria palmata using sulfuric acid and fermentation with brewer’s yeast. J Appl Phycol 26(1):687–693
ye Lee J, Li P, Lee J, Ryu HJ, Oh KK (2013) Ethanol production from Saccharina japonica using an optimized extremely low acid pretreatment followed by simultaneous saccharification and fermentation. Bioresour Technol 127:119–125
Meinita MDN, Marhaeni B, Winanto T, Jeong GT, Khan MNA, Hong YK (2013) Comparison of agarophytes (Gelidium, Gracilaria, and Gracilariopsis) as potential resources for bioethanol production. J Appl Phycol 25(6):1957–1961
Lee SB, Kim SK, Hong YK, Jeong GT (2016) Optimization of the production of platform chemicals and sugars from the red macroalga, Kappaphycus alvarezii. Algal Res 13:303–310
Kim HM, Wi SG, Jung S, Song Y, Bae HJ (2015) Efficient approach for bioethanol production from red seaweed Gelidium amansii. Bioresour Technol 175:128–134
McHugh DJ (2003) A guide to the seaweed industry FAO fisheries technical paper 441. Food and Agriculture Organization of the United Nations, Rome
Sluiter A, Hames B, Hyman D, Payne C, Ruiz R, Scarlata C et al (2008) Determination of total solids in biomass and total dissolved solids in liquid process samples. National Renewable Energy Laboratory, Golden, CO, NREL Technical Report No. NREL/TP-510-42621, 1-6
Hames B, Scarlata C, Sluiter A (2008) Determination of protein content in biomass. National Renewable Energy Laboratory, 1–5
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2004) Determination of ash in biomass: LAP-005 NREL analytical procedure. National Renewable Energy Laboratory, Golden
Van Wychen S, Laurens L (2013) Determination of total carbohydrates in algal biomass. National Renewable Energy Laboratory, Golden, pp 275–3000
Du F, Wolger E, Wallace L, Liu A, Kaper T, Kelemen B (2010) Determination of product inhibition of CBH1, CBH2, and EG1 using a novel cellulase activity assay. Appl Biochem Biotechnol 161(8):313–317
Trivedi N, Gupta V, Reddy CRK, Jha B (2013) Enzymatic hydrolysis and production of bioethanol from common macrophytic green alga Ulva fasciata Delile. Bioresour Technol 150:106–112
Gao F, Gao L, Zhang D, Ye N, Chen S, Li D (2015) Enhanced hydrolysis of Macrocystis pyrifera by integrated hydroxyl radicals and hot water pretreatment. Bioresour Technol 179:490–496
Adney B, Baker J (1996) Measurement of cellulase activities. Lab Anal Proced 6:1996
Marquez GPB, Santiañez WJE, Trono GC Jr, Montaño MNE, Araki H, Takeuchi H, Hasegawa T (2014) Seaweed biomass of the Philippines: sustainable feedstock for biogas production. Renew Sust Energy Rev 38:1056–1068
Rhein-Knudsen N, Ale MT, Ajalloueian F, Yu L, Meyer AS (2017) Rheological properties of agar and carrageenan from Ghanaian red seaweeds. Food Hydrocoll 63:50–58
Marinho-Soriano E, Fonseca PC, Carneiro MAA, Moreira WSC (2006) Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour Technol 97(18):2402–2406
Kostas ET, White DA, Du C, Cook DJ (2016) Selection of yeast strains for bioethanol production from UK seaweeds. J Appl Phycol 28(2):1427–1441
van Maris AJ, Abbott DA, Bellissimi E, van den Brink J, Kuyper M, Luttik MA et al (2006) Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Antonie Van Leeuwenhoek 90(4):391–418
Gensch T, James MJ, Dalton T, Glorius F (2018) Increasing catalyst efficiency in C–H activation catalysis. Angew Chem Int Ed 57(9):2296–2306
Kim NJ, Li H, Jung K, Chang HN, Lee PC (2011) Ethanol production from marine algal hydrolysates using Escherichia coli KO11. Bioresour Technol 102(16):7466–7469
Rhein-Knudsen N, Ale MT, Ajalloueian F, Meyer AS (2017) Characterization of alginates from Ghanaian brown seaweeds: Sargassum spp. and Padina spp. Food Hydrocoll 71:236–244
Meinita MDN, Hong YK, Jeong GT (2012) Comparison of sulfuric and hydrochloric acids as catalysts in hydrolysis of Kappaphycus alvarezii (cottonii). Bioprocess Biosyst Eng 35(1–2):123–128
Pilavtepe M, Celiktas MS, Sargin S, Yesil-Celiktas O (2013) Transformation of Posidonia oceanica residues to bioethanol. Ind Crops Prod 51:348–354
Leitner V, Lindorfer J (2016) Evaluation of technology structure based on energy yield from wheat straw for combined bioethanol and biomethane facility. Renew Energy 87:193–202
Ho SH, Huang SW, Chen CY, Hasunuma T, Kondo A, Chang JS (2013) Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour Technol 135:191–198
Acknowledgements
The authors are grateful to the Danida Fellowship Centre (DFC) for supporting through the research project, Seaweed Biorefinery in Ghana (DFC No. 14–01DTU). The authors are also grateful to Gertrude Opoku and Robert Aryeetey of the KNUST and; Gloria Addicco, K.A. Degraft Johnson and Anthony Adu-Gyamfi of the CSIR-WRI, Ghana, for their support with field work.
Funding
This research was funded by DANIDA Fellowship Centre (Grant Number 14-01DTU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Offei, F., Mensah, M. & Kemausuor, F. Cellulase and acid-catalysed hydrolysis of Ulva fasciata, Hydropuntia dentata and Sargassum vulgare for bioethanol production. SN Appl. Sci. 1, 1469 (2019). https://doi.org/10.1007/s42452-019-1501-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1501-5
Keywords
- Seaweed pretreatment
- Enzymatic hydrolysis
- Acid hydrolysis
- Ulva fasciata
- Hydropuntia dentata
- Sargassum vulgare