Abstract
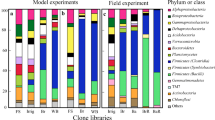
Biological Soil Disinfestation (BSD) is used to control soil-borne phytopathogens like Ralstonia solanacearum by introducing fresh organic matter and covering the soil with thick, opaque plastic sheets to induce soil anaerobiosis. In this study, BSD was implemented for the first time in Egypt to manage R. solanacearum in five naturally-infested fields. To improve BSD’s efficiency, biocontrol agents, including Pseudomonas putida (PD3142), P. fluorescens (PD3339), Acinetobacter baumannii (PD3138), and Stenotrophomonas maltophilia (PD4560), or plant-animal compost were applied after removing the plastic. The pathogen was eliminated when the soil oxygen concentration was less than or equal to 1%. However, this method resulted in a decrease in potato production. Application of P. putida and P. fluorescens after BSD led to a significant increase in potato production. Furthermore, BSD led to a significant decrease in fungal biodiversity, measured by the Shannon index (H’). PCR-DGGE analysis showed that P. putida and S. maltophilia reduced bacterial biodiversity as indicated by species richness (S) and H’. Next generation sequencing assays showed Moraxellaceae and Pseudomonadaceae decrease in their abundance after BSD treatment, while Bacillaceae were less affected . Moreover, applying of compost following BSD was associated with an increase in beneficial bacterial genera such as Luteolibacter, Dyadobacter, Sphingobium, Flavobacterium, and Pseudomonas.






Similar content being viewed by others
Data Availability
The data and material used during the current study are available from the author on reasonable request.
Change history
23 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s42360-023-00679-w
Abbreviations
- BSD:
-
Biological soil disinfestation
- Poor BSD:
-
Soil Oxygen concentration > 1
- PD:
-
Plantenziektenkundige Dienst (Plant protection Service, Wageningen, the NL)
- S :
-
Species richness
- H’:
-
Shannon index
- DGGE:
-
Denaturing gradient gel electrophoresis
- NGS:
-
Next generation sequencing
- CMC:
-
Carboxymethyl cellulose
- ARC:
-
Agricultural Research Center
- FITC:
-
Fluorescein Isothiocyanate
- OM:
-
Organic matter
- SFOM:
-
Soil mixed with the fresh OM
References
Angelopoulou DJ, Naska EJ, Paplomatas EJ, Tjamos SE (2014) Biological control agents (BCAs) of verticillium wilt: influence of application rates and delivery method on plant protection, triggering of host defence mechanisms and rhizosphere populations of BCAs. Plant Pathol 63:1062–1069. https://doi.org/10.1111/ppa.12198
Anonymous (1998) Council Directive 98/57/EC of 20 July 1998 on the control of Ralstonia solanacearum (Smith) Yabuuchi et al. Publication 97/647/EC Official Journal European Communities L235:8–39
AOAC (1990) Official methods of analysis. Association of Analytical Chemists, Virginia, USA. APHA, 1998. Standard methods for the examination of waters and wastewaters. APHAAWWA-WEF, Washington, DC
Athira S, Anith KN (2020) Plant growth promotion and suppression of bacterial wilt incidence in tomato by rhizobacteria, bacterial endophytes and the root endophytic fungus Piriformospora indica. Indian Phytopathol 73:629–642. https://doi.org/10.1007/s42360-020-00283-2
Biswal G, Singh D, Dhal NK (2018) Synergistic effect of Bacillus subtilis and boric acid on management of bacterial wilt disease of potato caused by Ralstonia solanacearum in coastal plains of Odisha under field conditions. Indian Phytopathol 71:431–434. https://doi.org/10.1007/s42360-018-0053-8
Blok WJ, Lamers JG, Termorshuizen AJ, Bollen GJ (2000) Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathol 90:253–259.https://doi.org/10.1094/PHYTO.2000.90.3.253
Conrad R (2020) Methane Production in Soil Environments-Anaerobic Biogeochemistry and Microbial Life between Flooding and Desiccation. Microorganisms. 2020;8(6):881. https://doi.org/10.3390/microorganisms8060881
Eichner CA, Erb RW, Timmis KN, Wagner-Döbler I (1999) Thermal gradient gel electrophoresis analysis of bioprotection from pollutant shocks in the activated sludge microbial community. Appl Environ Microbiol 65:102–109. https://doi.org/10.1128/aem.65.1.102-109.1999
Elphinstone JG, Stanford HM, Stead DE (1998) Detection of Ralstonia solanacearum in potato tubers, Solanum dulcamara, and associated irrigation water. In: Prior P, Allen C, Elphinstone JG (eds) Bacterial Wilt Disease: molecular and ecological aspects. Springer, Berlin, Germany, pp 133–139. https://doi.org/10.1007/978-3-662-03592-4_19
Farag NS, Eweda WE, Mostafa MI, Balabel NM (2004) Preliminary observations on the bacteriology and pathology of Ralstonia solanacearum. Egypt J Agric Res 82:1519–1523
Fu L, Penton CR, Ruan Y, Shen Z, Xue C, Li R (2017) Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol Biochem 104:39–48. https://doi.org/10.1016/j.soilbio.2016.10.008
Gamliel A, Austerweil M, Kritzman G (2000) Non Chemical Approach to soilborne pest management - Organic amendments. Crop Prot 19:847–853
García R, García A, Delgado L (1999) Distribution, incidence and variability of Ralstonia solanacearum, causal agent of bacterial wilt of potato, in Mérida state. Venezuela Bioagro 11(1):12–23
Glaeser S, Kämpfer P (2013) The Family Sphingomonadaceae. The Prokaryotes: Alphaproteobacteria and Betaproteobacteria 641–707. https://doi.org/10.1007/978-3-642-30197-1_302
Goud JC, Termorshuizen AJ, Blok WJ, van Bruggen AHC (2004) Long-term effect of biological soil disinfestation on Verticillium wilt. Plant Dis 88:688–694. https://doi.org/10.1094/PDIS.2004.88.7.688
Graham J, Jones DA, Lloyd AB (1979) Survival of Pseudomonas solanacearum race 3 in plant debris and latently infected potato tubers. Phytopathol 69:1100–1103
Heuer H, Smalla K (1997) Application of denaturing gradient gel electrophoresis for studying soil microbial communities. In: van Elsas JD, Trevors JT, Wellington EMH (eds) Modern soil Microbiology. Marcel Dekker Inc, New York, pp 353–373
Hewavitharana SS, Klarer E, Reed AJ, Leisso R, Poirier B, Honaas L, Rudell DR, Mazzola M (2019) Temporal Dynamics of the Soil Metabolome and Microbiome during simulated anaerobic soil Disinfestation. Front Microbiol 10:2365. https://doi.org/10.3389/fmicb.2019.0236
Hewavitharana SS, Klarer E, Muramoto J, Shennan C, Mazzola M (2021) Analysis of environmental variables and Carbon Input on Soil Microbiome, Metabolome and Disease Control Efficacy in Strawberry Attributable to Anaerobic Soil Disinfestation. Microorganisms 9:1638. https://doi.org/10.3390/microorganisms9081638
Huang XQ, Liu LL, Zhao J, Zhang JB, Cai ZC (2019) The families Ruminococcaceae, Lachnospiraceae, and Clostridiaceae are the dominant bacterial groups during reductive soil disinfestation with incorporated plant residues. Appl Soil Ecol 135:65–72
ICARDA (2013) Methods of Soil, Plant, and Water Analysis: A manual for the West Asia and North Africa region. Eds. Estefan G, Sommer R, and Ryan J (Third Edition) 243pp
Janse JD (1988) A detection method for Pseudomonas solanacearum in symptomless potato tubers and some data on its sensitivity and specificity. Bull OEPP/EPPO Bull 18:343–351
Kabir Z, Fennimore SA, Duniway JM, Martin FN, Browne GT, Winterbottom CQ, Ajwa HA, Westerdahl BB, Goodhue RE, Haar MJ (2005) Alternatives to methyl bromide for strawberry runner plant production. HortScience 40:1709–1715. https://doi.org/10.21273/HORTSCI.40.6.1709
Katan J (2000) Physical and culture methods for the management of soil borne pathogens. Crop Prot 19:725–731
Kawicha P, Laopha A, Chamnansing W et al (2020) Biocontrol and plant growth-promoting properties of Streptomyces isolated from vermicompost soil. Indian Phytopathol 73:655–666. https://doi.org/10.1007/s42360-020-00267-2
Kissel DE, Sander DH, Ellis R (1985) Fertilizer-plant interaction in alkaline soils. In: Engelstad OP (ed) Fertilizer Technology and Use. Soil Science Society of America, Madison, WI, pp 153–196
Kolton M, Erlacher A, Berg G, Cytryn E (2016) The flavobacterium genus in the plant holobiont: ecological, physiological, and applicative insights. Microb Model Environ Ind Sustain 189–207. https://doi.org/10.1007/978-981-10-2555-6_9
Kumar R, Tiwari RK, Jeevalatha A et al (2021) Potato apical leaf curl disease: current status and perspectives on a disease caused by tomato leaf curl New Delhi virus. J Plant Dis Prot 128:897–911. https://doi.org/10.1007/s41348-021-00463-w
Kumar S, Biswas SK, Kumar A et al (2023) Effect of Integrated Disease Management (IDM) Practices on Disease Severity and Incidence of Common Scab of Potato. Potato Res (2023). https://doi.org/10.1007/s11540-023-09629-5
Lee J-C, Whang K-S (2020) Agriterribacter humi gen. nov., sp. nov., a novel bacterium of the family Chitinophagaceae isolated from soil. Int J Syst Evol Microbiol 70(9):5123–5130. https://doi.org/10.1099/ijsem.0.004397
Madigan MT, Cox SS, Stegeman RA (1984) Nitrogen fixation and nitrogenase activities in members of the family Rhodospirillaceae. J Bacteriol 157:73–78. https://doi.org/10.1128/JB.157.1.73-78.1984
Meliani A, Bensoltane A, Benidire L, Oufdou K (2017) Plant growth-promotion and IAA secretion with Pseudomonas fluorescens and Pseudomonas putida. Res Reviews: J Bot Sci 6(2):16–24
Messiha NAS, van Diepeningen AD, Farag NS, Abdallah SA, Janse JD, van Bruggen AHC (2007a) Stenotrophomonas maltophilia: a new potential biocontrol agent of Ralstonia solanacearum, the causal agent of potato brown rot. Eur J Plant Pathol 118:211–225. https://doi.org/10.1007/s10658-007-9136-6
Messiha NAS, van Diepeningen AD, Wenneker M, van Beuningen AAR, Janse JD, Coenen TGC, Termorshuizen AJ, van Bruggen AHC, Blok WJ (2007b) Biological Soil Disinfestation, a new control method for potato brown rot, caused by Ralstonia solanacearum race 3 biovar 2. Eur J Plant Pathol 117:403–415.https://doi.org/10.1007/s10658-007-9109-9
Messiha NAS, Elhalag KMA, Balabel NM, Farag SMA, Matar HA, Hagag MH, Khairy AM, Abd El-Aliem MM, Ali EE, Saleh OE et al (2019) Microbial Biodiversity as related to Crop Succession and Potato Intercropping for Management of Brown Rot Disease. Egypt J Biol Pest Co 29:84.https://doi.org/10.1186/s41938-019-0185-x
Messiha NAS, Elhalag KMA, Balabel NM, Matar HA, Farag SMA, Hagag MH, Khairy AM, Abd El-Aliem MM, Hanafy MS, Farag NS (2021) Efficiency of organic manuring and mineral fertilization regimes in potato brown rot suppression and soil microbial biodiversity under field conditions. Arch Phytopathol Plant Prot 54(9–10):534–556. https://doi.org/10.1080/03235408.2020.1844523
Molinuevo-Salces B, Gómez X, Morán A, García-González MC (2013) Anaerobic co-digestion of livestock and vegetable processing wastes: fibre degradation and digestate stability. Waste Manag 33:1332–1338. https://doi.org/10.1016/j.wasman.2013.02.021
Momma N (2008) Biological soil disinfestation (BSD) of soilborne pathogens and its possible mechanisms. Jarq-Jpn Agric Res 42:7–12. https://doi.org/10.6090/jarq.42.7
Momma N, Yamamoto K, Simandi P, Shishido M (2006) Role of organic acids in the mechanisms of biological soil disinfestation (BSD). J Gen Plant Pathol 72: 247-252. https://doi.org/10.1007/s10327-006-0274-z
Nakayama T, Homma Y, Hashidoko Y, Mizutani J, Tahara S (1999) Possible ole of xanthobaccins produced by Stenotrophomonas sp. Strain SB-K88 in suppression of sugar beet damping-off disease. Appl Environ Microbiol 65:4334–4339. https://doi.org/10.1128/AEM.65.10.4334-4339.1999
PM 7/21 (3) (2022) Ralstoniasolanacearum, R. pseudosolanacearum and R. syzygii (Ralstonia solanacearum species complex). EPPO Bull 52: 225– 261.https://doi.org/10.1111/epp.12837
Rahman M, Borah SM, Borah PK, Bora P, Sarmah BK, Lal MK, Tiwari RK, Kumar R (2023) Deciphering the antimicrobial activity of multifaceted rhizospheric biocontrol agents of solanaceous crops viz., Trichoderma harzianum MC2, and Trichoderma harzianum NBG. Front Plant Sci 14:2023. https://doi.org/10.3389/fpls.2023.1141506
Schlatter D, Kinkel L, Thomashow L, Weller D, Paulitz T (2017) Disease suppressive soils: new insights from the soil microbiome. Phytopath 107:1284–1297.https://doi.org/10.1094/PHYTO-03-17-0111-RVW
Sharma P (2023) Biocontrol strategies – retrospect and prospects. Indian Phytopathol 76:47–59. https://doi.org/10.1007/s42360-023-00601-4
Shennan C, Muramoto J, Baird G, Daugovish O, Koike S, Fenimore S, Bolda M, Rosskoph E, Butler D, Kokalis Burrelle N et al (2017) Anaerobic soil disinfestation is a potential alternative to fumigation for control of some soil-borne pathogens in strawberry production. Plant Pathol 67:51–66
Shinmura A (2004) Principle and effect of soil sterilization method by reducing redox potential of soil (in japanese). PSJ Soilborne Dis Workshop Rep 22:2–12
Stremińska MA, Runia WT, Termorshuizen AJ, Feil H, Van Der Wurff AWG (2014) Anaerobic soil disinfestation in microcosms of two sandy soils. Commun Agric Appl Biol Sci 79(2):15–19 PMID: 26084078
Takehara T (2004) Principles and effects of soil disinfestation with hot water. PSJ Soilborne Disease Workshop Report 22:22–37
Takeuchi S (2004) Control of soil-borne diseases by steam sterilization in Kochi Prefecture. PSJ Soilborne Disease Workshop Report 22:49–60
van Diepeningen AD, de Vos OJ, Korthals GW, van Bruggen AHC (2006) Effects of organic versus conventional management on chemical and biological parameters in agricultural soils. Appl Soil Ecol 31(1–2):120–135. https://doi.org/10.1016/j.apsoil.2005.03.003
van Keulen G, Alderson J, White J, Sawers RG (2007) The obligate aerobic actinomycete Streptomyces coelicolor A3(2) survives extended periods of anaerobic stress. Environ Microbiol 9:3143–3149
Walkley A, Black IA (1934) An examination of Degtjareff method for determining soil organic matter, and proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Weller SA, Elphinstone JG, Smith NC, Boonham N, Stead DE (2000) Detection of Ralstonia solanacearum strains with a quantitative, multiplex, real-time, fluorogenic PCR (TaqMan) assay. Appl Environ Microbiol 66(7):2853–2858. https://doi.org/10.1128/AEM.66.7.2853-2858.2000
Wu T, Qin Y, Li M (2021) Intercropping of tea (Camellia sinensis L.) and chinese Chestnut: variation in the structure of Rhizosphere Bacterial Communities. J Soil Sci Plant Nutr 21:2178–2190. https://doi.org/10.1007/s42729-021-00513-0
Yang T, Lupwayi N, Marc S-A, Siddique KHM, Bainard LD (2021) Anthropogenic drivers of soil microbial communities and impacts on soil biological functions in agroecosystems. Glob Ecol Conserv 27: e01521.https://doi.org/10.1016/j.gecco.2021.e01521
Acknowledgements
This study was conducted as part of the “Rehabilitation of Nile Valley and Delta to produce brown rot-free potato suitable for exportation” project, funded by The Science, Technology & Innovation Funding Authority (STIFA27859) of the Egyptian Ministry of Scientific Research. The author is deeply appreciative of the financial support and collaborative efforts.
Professor Nabil S. Farag, Emeritus Professor at the Plant Pathology Research Institute (PPATHRI), ARC, Egypt, is acknowledged for his invaluable review of the work and insightful feedback. Special thanks are extended to Professor Ahmed A. Gomah, Emeritus Professor at PPATHRI, ARC, for his valuable contributions to field trials and his expert advice on experiments in the field.
Funding
The study was carried out as part of the “Rehabilitation of Nile Valley and Delta to produce brown rot-free potato suitable for exportation” project, which was funded by The Science, Technology & Innovation Funding Authority (STIFA27859) of the Egyptian Ministry of Scientific Research.
Author information
Authors and Affiliations
Contributions
Single author.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval and consent to participate are not required for this manuscript.
Consent for publication
Not applicable.
Competing interests
The author declares that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised:
The Funding section was missing from this article and should have read:
Acknowledgements
This study was conducted as part of the “Rehabilitation of Nile Valley and Delta to produce brown rot-free potato suitable for exportation” project, funded by The Science, Technology & Innovation Funding Authority (STIFA27859) of the Egyptian Ministry of Scientific Research. The author is deeply appreciative of the financial support and collaborative efforts.
Professor Nabil S. Farag, Emeritus Professor at the Plant Pathology Research Institute (PPATHRI), ARC, Egypt, is acknowledged for his invaluable review of the work and insightful feedback. Special thanks are extended to Professor Ahmed A. Gomah, Emeritus Professor at PPATHRI, ARC, for his valuable contributions to field trials and his expert advice on experiments in the field.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Messiha, N.A.S. The impact of biological soil disinfestation in conjunction with antagonists and organic materials on potato brown rot control. Indian Phytopathology 76, 1001–1014 (2023). https://doi.org/10.1007/s42360-023-00668-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-023-00668-z