Abstract
Water pollution by heavy metals and nitrite ions is a public health concern around the world because they can degrade the quality of drinking water and cause serious diseases. Lead and cadmium are probably the most dangerous heavy metals. Indeed, lead contamination can affect fertility and pregnancy, cause infantile diseases and other mutagenic and carcinogenic effects. Exposure to cadmium can cause death in mammals, and nitrite ions are also very harmful. For all these reasons, it is necessary to find effective techniques to quantify the levels of these pollutants. Recently, there is great hope in the use of organic conducting polymers in the field of heavy metals detection. In this review, we presented studies of the toxicity of several heavy metals and nitrite ions and on their impact on the environment and human health. Also, the recent developments in the use of OCPs and their application in the detection of heavy metals and nitrite ions have been examined.

Reprinted from Wei et al. [82], Copyright 2013, with permission from Elsevier

Reprinted from Huang et al. [87], Copyright 2016, with permission from Elsevier

(Reproduced from Ref. [117] with permission from The Royal Society of Chemistry)

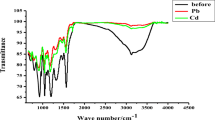
Reprinted from Guo et al. [120], Copyright 2005, with permission from Elsevier
Similar content being viewed by others
Abbreviations
- ABS:
-
Benzenesulfonic acid
- AES:
-
Atomic emission spectroscopy
- AP:
-
Aminophenyl
- ATT:
-
5-Amino-1,3,4-thiadiazole-2-thiol
- AuNP:
-
Gold nanoparticles
- AuNP:
-
Gold nanoparticles
- AuNPs:
-
Gold nanoparticles
- BIA:
-
Batch injection analysis
- Fbg:
-
Fibrinogen
- BiFE:
-
Bismuth films electrodes
- BMP:
-
1-Butyl-1-methylpyrrolidinium
- ChA:
-
Chronoamperometry
- CG:
-
Carboxyl graphene
- CNFs:
-
Carbon nanofibers
- CNTs:
-
Carbon nanotubes
- Co-NS:
-
Cobalt nanostructures
- CPE:
-
Carbon pencil electrode
- CQDs:
-
Carbon quantum dots
- CS:
-
Chitosan
- CV:
-
Cyclic voltammetry
- DAN:
-
Diaminonaphthalene
- DPASV:
-
Differential pulse-anodic stripping voltammetry
- DPV:
-
Differential pulse voltammetry
- EMIM:
-
1-Ethyl-3-methylimidazolium
- f-MWCNT:
-
Functionalized MWCNT
- FMWCNTs:
-
Acid functionalized MWCNTs
- FAAS:
-
Flame-atomic absorption spectrometry
- GO:
-
Graphene oxide
- GCE:
-
Glassy carbon electrodes
- IARC:
-
International Agency for Research on Cancer
- ICP:
-
Inductively induced plasma
- ITO:
-
Indium-doped tin oxide
- LOD(s):
-
Limit(s) of detection
- LSV:
-
Linear sweep voltammetry
- MAS:
-
Molecular absorption spectrometry
- MES:
-
2-Mercaptoethanesulfonate
- MS:
-
Mass spectrometry
- MWNT(s):
-
Multi-walled nanotube(s)
- NA:
-
Nafion
- nHAp:
-
Nano-sized hydroxyapatite
- OCPs:
-
Organic conducting polymers
- OD:
-
1,8-Octanediamine
- OES:
-
Optical emission spectroscopy
- OMt:
-
Organophilic montmorillonite clay
- PA:
-
Phytic acid
- PANI:
-
Polyaniline
- PANI-ES:
-
Emeraldine salt polyaniline
- PANI-GO:
-
Polyaniline/graphene oxide
- PANI-NTS:
-
Size-tunable polyaniline nanotubes
- PAOA:
-
Poly(aniline-co-o-aminophenol)
- P(DPA):
-
Polydiphenylamine
- P(DPA-co-2ABN):
-
Poly(diphenylamine-co-2-aminobenzonitrile)
- PEDOT:
-
Poly(ethylenedioxythiophene)
- PGE:
-
Pencil graphite electrode
- PHA:
-
1H-Pyrrole-1-hexanoic acid
- PLG:
-
Pencil lead graphite
- PProDOT:
-
Polypropylenedioxythiophene
- PPy:
-
Polypyrrole
- PPy/CNSs:
-
Polypyrrole/carbonaceous nanospheres
- PPy-GO:
-
Polypyrrole/graphene oxide
- PS:
-
Polystyrene
- PTB:
-
Poly(toluidine blue)
- PVF+:
-
Poly(vinylferrocenium)
- rGO:
-
Reduced graphene oxide
- SDS:
-
Sodium dodecyl sulfate
- SPE:
-
Screen printed electrode
- SWASV:
-
Square wave anodic stripping voltammetry
- SWV:
-
Square wave voltammetry
- TFSA:
-
Bis(trifluoromethylsulfonyl)amide
- UiO-66-NH2:
-
Zr/2-Amino-terephthalate metal–organic framework
- WHO:
-
World Health Organization
- ZIF:
-
Zeolitic imidazolate framework
References
Verla EN, Verla AW, Enyoh CE (2020) Bioavailability, average daily dose and risk of heavy metals in soils from children playgrounds within Owerri, Imo State, Nigeria. Chem Afr. https://doi.org/10.1007/s42250-020-00124-9
Kim J-J, Kim Y-S, Kumar V (2019) Heavy metal toxicity: an update of chelating therapeutic strategies. J Trace Elem Med Biol 54:226–231
Lentini P, Zanoli L, de Cal M, Granata A, Dell’Aquila R (2019) Chapter 222—Lead and heavy metals and the kidney. In: Ronco C, Bellomo R, Kellum JA, Ricci Z (eds) Critical care nephrology, 3rd edn, pp 1324–1330.e1. https://doi.org/10.1016/B978-0-323-44942-7.00222-3
Edogbo B, Okolocha E, Maikai B, Aluwong T, Zakari F, Uchendu C (2020) Assessment of potentially toxic elements in soils, water and vegetables around river Salanta area of Kano State, Nigeria: health risk analysis. Chem Afr. https://doi.org/10.1007/s42250-020-00141-8
U.S. Environmental Protection Agency (2015) Drinking water contaminants|environments and contaminants-America’s children and the environment|third edition. https://www.epa.gov/sites/production/files/2015-10/documents/ace3_drinking_water.pdf. Accessed 13 Apr 2020
World Health Organization (2011) Guidelines for drinking-water quality. http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/. Accessed 13 Apr 2020
Chauhan M, Bhardwaj SK, Bhanjana G, Kumar R, Dilbaghi N, Kumar S, Chaudhary GR (2019) Chapter 3—Conducting polymers and metal-organic frameworks as advanced materials for development of nanosensors. In: Deep A, Kumar S (eds) Advances in nanosensors for biological and environmental analysis, Elsevier, pp 43–62
Ballav N, Maity A, Mishra SB (2012) High efficient removal of chromium(VI) using glycine doped polypyrrole adsorbent from aqueous solution. Chem Eng J 198–199:536–546
Sall ML, Diaw AKD, Gningue-Sall D, Chevillot-Biraud A, Oturan N, Oturan MA, Aaron J-J (2017) Removal of Cr(VI) from aqueous solution using electrosynthesized 4-amino-3-hydroxynaphthalene-1-sulfonic acid doped polypyrrole as adsorbent. Environ Sci Pollut Res 24:21111–21127
Sall ML, Diaw AKD, Gningue-Sall D, Chevillot-Biraud A, Oturan N, Oturan MA, Fourdrin C, Huguenot D, Aaron J-J (2018) Removal of lead and cadmium from aqueous solutions by using 4-amino-3-hydroxynaphthalene sulfonic acid-doped polypyrrole films. Environ Sci Pollut Res 25:8581–8591
Deshmukh MA, Shirsat MD, Ramanaviciene A, Ramanavicius A (2018) Composites based on conducting polymers and carbon nanomaterials for heavy metal ion sensing (review). Crit Rev Anal Chem 48:293–304
Lange U, Roznyatovskaya NV, Mirsky VM (2008) Conducting polymers in chemical sensors and arrays. Anal Chim Acta 614:1–26
Lin M, Hu X, Ma Z, Chen L (2012) Functionalized polypyrrole nanotube arrays as electrochemical biosensor for the determination of copper ions. Anal Chim Acta 746:63–69
Dai H, Wang N, Wang D, Ma H, Lin M (2016) An electrochemical sensor based on phytic acid functionalized polypyrrole/graphene oxide nanocomposites for simultaneous determination of Cd(II) and Pb(II). Chem Eng J 299:150–155
Msaadi R, Ammar S, Chehimi MM, Yagci Y (2017) Diazonium-based ion-imprinted polymer/clay nanocomposite for the selective extraction of lead(II) ions in aqueous media. Eur Polym J 89:367–380
Elgrishi N, Rountree KJ, McCarthy BD, Rountree ES, Eisenhart TT, Dempsey JL (2018) A practical beginner’s guide to cyclic voltammetry. J Chem Educ 95:197–206
Bedioui F, Griveau S (2009) Voltampérométrie sur électrode solide; Perfectionnement des performances. Techniques de l'ingénieur Méthodes électrochimiques P2128 V2. https://www.techniques-ingenieur.fr
Zhang JXJ, Hoshino H (2014) Chapter 4—Electrical transducers: electrochemical sensors and semiconductor molecular sensors. In: Zhang JXJ, Hoshino K (eds) Molecular sensors and nanodevices, William Andrew Publishing, pp 169–232
Amine A, Mohammadi, H (2019) Amperometry. In: Worsfold P, Poole C, Townshend A, Miró M (eds) Encyclopedia of analytical science, 3rd edn. Academic Press, pp 85–98
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L (2016) A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res 23:8244–8259
Xie H, Wise SS, Holmes AL, Xu B, Wakeman TP, Pelsue SE, Singh NP, Wise JP Sr (2005) Carcinogenic lead chromate induces DNA double-strand breaks in human lung cells. Mutat Res 586:160–172
Xu J, Lian L, Wang XF (2008) Lead induces oxidative stress, DNA damage and alteration of p 53, Bax and Bcl-2 expressions in mice. Food Chem Toxicol 46:1488–1494
Zhang J, Caob H, Zhang Y, Zhang Y, Ma J, Wang J, Gao Y, Zhang X, Zhang F, Chu L (2013) Nephroprotective effect of calcium channel blockers against toxicity of lead exposure in mice. Toxicol Lett 218:273–280
Naik MM, Dubey SK (2013) Lead resistant bacteria: lead resistance mechanisms, their applications in lead bioremediation and biomonitoring. Ecotoxicol Environ Saf 98:1–7
Becker T, Dierchke H (2008) Vegetation response to high concentrations of heavy metals in the Harz Mountains, Germany. Phytocoenologia 38:255–265
Offem BO, Ayotunde EO (2008) Toxicity of lead to freshwater invertebrates (water fleas; Daphnia magna and Cyclop sp) in fish ponds in a tropical floodplain. Water Air Soil Pollut 192:39–46
Chen J, Chen Y, Liu W, Bai C, Liu X, Liu K, Li R, Zhu JH, Huang C (2012) Developmental lead acetate exposure induces embryonic toxicity and memory deficit in adult zebrafish. Neurotoxicol Teratol 34:581–586
Francisco ND, Troya RJD, Aguera EI (2003) Lead and lead toxicity in domestic and free-living birds. Avian Pathol 32:3–13
Ishii C, Nakayama SMM, Kataba A, Ikenaka Y, Saito K (2018) Characterization and imaging of lead distribution in bones of lead-exposed birds by ICP-MS and LA-ICP-MS. Chemosphere 212:994–1001
Patrick L (2006) Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern Med Rev 11:114–127
Cancer Environment (2018) Cadmium et ses composés. https://www.cancer-environnement.fr/411-Cadmium-et-ses-composes.ce.aspx. Accessed 13 Apr 2020
Rafati Rahimzadeh M, Rafati Rahimzadeh M, Kazemi S, Moghadamnia AA (2017) Cadmium toxicity and treatment: an update. Casp J Intern Med 8:135–145
Goullé J-P, Saussereau E, Mahieu L, Bouige D, Guerbet M, Lacroix C (2010) Une nouvelle approche biologique: le profil métallique. Ann Biol Clin 68:29–40
Pretto A, Loro VL, Morsch VM, Moraes BS, Menezes C, Santi A, Toni C (2014) Alterations in carbohydrate and protein metabolism in silver catfish (Rhamdia quelen) exposed to cadmium. Ecotoxicol Environ Saf 100:188–192
Othumpamgat S, Kashon M, Joseph P (2005) Eukaryotic translation initiation factor 4E Is a cellular target for toxicity and death due to exposure to cadmium chloride. J Biol Chem 280:162–169
Satarug S, Vesey DA, Gobe GC (2017) Kidney cadmium toxicity, diabetes and high blood pressure: the perfect storm. Tohoku J Exp Med 241:65–87
Poey J, Philibert C (2000) Toxicité des métaux. Rev Fr Lab 323:35–43
Hutchinson D, Müller J, McCarthy JE, Gun’ko YK, Verma NK, Bi X, Di Cristo L, Kickham L, Movia D, Prina-Mello A, Volkov Y (2018) Cadmium nanoparticles citrullinate cytokeratins within lung epithelial cells: cadmium as a potential cause of citrullination in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulm Dis 13:441–449
Liu J, Yu L, Castro L, Yan Y, Sifre MI, Bortner CD, Dixon D (2019) A nongenomic mechanism “metalloestrogenic” effects of cadmium in human uterine leiomyoma cells through G protein-coupled estrogen receptor. Arch Toxicol 93:2773–2785
Zhang Q, Zhang C, Ge J, Lv MW, Talukder M, Guo K, Li YH, Li JL (2020) Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy. Food Funct 11:1856–1868
Phillip CJC, Prankel SH (2011) Cadmium and the welfare of animals. In: Nriagu J (ed) Encyclopedia of environmental health, 2nd edn. Elsevier, Amsterdam, pp 470–474
Khafaga AF, Abd El-Hack ME, Taha AE, Elnesr SS, Alagawany M (2019) The potential modulatory role of herbal additives against Cd toxicity in human, animal, and poultry: a review. Environ Sci Pollut Res 26:4588–4604
Ogunkunle CO, Odulaja DA, Akande FO, Varun M, Vishwakarma V, Fatoba PO (2020) Cadmium toxicity in cowpea plant: effect of foliar intervention of nano-TiO2 on tissue Cd bioaccumulation, stress enzymes and potential dietary health risk. J Biotechnol 310:54–61
Fan SK, Ye JY, Zhang LL, Chen HS, Zhang HH, Zhu YX, Liu XX, Jin CW (2020) Inhibition of DNA demethylation enhances plant tolerance to cadmium toxicity by improving iron nutrition. Plant Cell Environ 43:275–291
Xiong X, Li H, Qiu N, Su L, Huang Z, Song L, Wang J (2020) Bioconcentration and depuration of cadmium in the selected tissues of rare minnow (Gobiocypris rarus) and the effect of dietary mulberry leaf supplementation on depuration. Environ Toxicol Pharm 73:103278
Rasn R, Rauj M (2018) Nitrate and nitrite content of vegetables: a review. J Pharmacogn Phytochem 7:322–328
Bahadoran Z, Ghasemi A, Mirmiran P, Azizi F, Hadaegh F (2016) Nitrate-nitrite-nitrosamines exposure and the risk of type 1 diabetes: a review of current data. World J Diabetes 7:433–440
Cantwell M, Elliott C (2017) Nitrates, nitrites and nitrosamines from processed meat intake and colorectal cancer risk. J Clin Nutr Diet 3:1–4
Commission Directive (EU) 2015/1787 of 6 October 2015. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01998L0083-20151027&from=EN,%202015. Accessed 13 Apr 2020
Miao LH, Lin Y, Pan WJ, Huang X, Ge XP, Zhou QL, Liu B, Ren MC, Zhang XX, Liang HL, Yu H, Ji K (2018) Comparative transcriptome analysis reveals the gene expression profiling in bighead carp (Aristichthys nobilis) in response to acute nitrite toxicity. Fish Shellfish Immunol 79:244–255
Medeiros R, Lopez B, Sampaio L, Romano L, Rodrigues R (2015) Ammonia and nitrite toxicity to false clownfish Amphiprion ocellaris. Aquac Int 24:985–993
Xing C, Shi Z, Yu M, Wang S (2008) Cationic conjugated polyelectrolyte-based fluorometric detection of copper(II) ions in aqueous solution. Polymer 49:2698–2703
Ogunfowokan AO, Adekunle AS, Oyebode BA, Oyekunle JAO, Komolafe AO, Omoniyi-Esan GO (2019) Determination of heavy metals in urine of patients and tissue of corpses by atomic absorption spectroscopy. Chem Afr 2:699–712
Bakırdere S, Yaroğlu T, Tırık N, Demiröz M, Fidan AK, Maruldalı O, Karaca A (2013) Determination of As, Cd, and Pb in tap water and bottled water samples by using optimized GFAAS system with Pd-Mg and Ni as matrix modifiers. J Spectrosc D 1(1). https://doi.org/10.1155/2013/824817
Tareen KA, Sultan NI, Parakulsuksatid Shafi M, Khan A, Khan WM, Hussain S (2014) Detection of heavy metals (Pb, Sb, Al, As) through atomic absorption spectroscopy from drinking water of District Pishin, Baluchistan, Pakistan. Int J Curr Micro Appl Sci 3:299–308
Abdul B, Attiq-ur-Rehman Samiullah, Naqeebullah K, Hayatullah Abdul B (2020) Physico-chemical and heavy metals analysis of drinking water and their effect on human health: a review. Pure Appl Biol 9(1):587–594
Dimpe KM, Ngila JC, Mabuba N, Nomngongo PN (2014) Evaluation of sample preparation methods for the detection of total metal content using inductively coupled plasma optical emission spectrometry (ICP-OES) in wastewater and sludge. Phys Chem Earth 76:42–48
Paredes E, Maestre SE, Prats S, Todolí JL (2006) Simultaneous determination of carbohydrates, carboxylic acids, alcohols, and metals in foods by high-performance liquid chromatography inductively coupled plasma atomic emission spectrometry. Anal Chem 19:6774–6782
Cengiz MF, Kilic S, Yalcin F, Kilic M, Gurhan Yalcin M (2017) Evaluation of heavy metal risk potential in Bogacayi River water (Antalya, Turkey). Environ Monit Assess 189:248
Bajenaru I, Josceanu AM, Guran C, Minca I (2015) Ion chromatographic method for determination of heavy metals in water. Rev Chim Buchar 66:1960–1964
Gao PF, Zhang XW, Kuang HZ, Li QQ, Li Y (2018) Study on simultaneous determination of Ni, Pb and Cd by ion chromatography. IOP Conf Ser Earth Environ Sci 146:012068
Bosch AC, O’Neill B, Sigge GO, Kerwath SE, Hoffman LC (2016) Heavy metal accumulation and toxicity in smoothhound (Mustelus mustelus) shark from Langebaan Lagoon, South Africa. Food Chem 190:871–878
Werner J (2018) Ionic liquid ultrasound-assisted dispersive liquid-liquid microextraction based on solidification of the aqueous phase for preconcentration of heavy metals ions prior to determination by LC-UV. Talanta 182:69–73
McGaw EA, Swain GM (2006) A comparison of boron-doped diamond thin-film and Hg-coated glassy carbon electrodes for anodic stripping voltammetric determination of heavy metal ions in aqueous media. Anal Chim Acta 575:180–189
Bedin KC, Mitsuyasu EY, Ronix A, Cazetta AL, Pezoti O, Almeida VC (2018) Inexpensive bismuth-film electrode supported on pencil-lead graphite for determination of Pb(II) and Cd(II) ions by anodic stripping voltammetry. Int J Anal Chem 2018. https://doi.org/10.1155/2018/1473706
Adraoui I, Rhazi ME, Amine A (2007) Fibrinogen-coated bismuth film electrodes for voltammetric analysis of lead and cadmium using the batch injection analysis. Anal Lett 40:349–368
Li J, Li Q, Lu C, Zhao L (2011) Determination of nitrite in tap waters based on fluorosurfactant-capped gold nanoparticles-enhanced chemiluminescence from carbonate and peroxynitrous acid. Analyst 136:2379–2384
Wang X, Adams E, Van Schepdael A (2012) A fast and sensitive method for the determination of nitrite in human plasma by capillary electrophoresis with fluorescence detection. Talanta 97:142–144
Lu L, Chen C, Zhao D, Yang F, Yang X (2015) A simple and sensitive assay for the determination of nitrite using folic acid as the fluorescent probe. Anal Methods 7:1543–1548
Kozub BR, Rees NV, Compton RG (2010) Electrochemical determination of nitrite at a bare glassy carbon electrode; why chemically modify electrodes? Sens Actuat B 143:539–546
Hutton EA, Ogorevc B, Hocevar SB, Weldon F, Smyth MR, Wang J (2001) An introduction to bismuth film electrode for use in cathodic electrochemical detection. Electrochem Commun 3:707–711
Gluck O, Schoning MJ, Luth H, Otto A, Emons H (1999) Trace metal determination by dc resistance changes of microstructured thin gold film electrodes. Electrochim Acta 44:3761–3768
Xing S, Xu H, Chen J, Shi G, Jin L (2011) Nafion stabilized silver nanoparticles modified electrode and its application to Cr(VI) detection. J Electroanal Chem 652:60–65
Dong Y, Ding Y, Zho Y, Chen J, Wang C (2014) Differential pulse anodic stripping voltammetric determination of Pb ion at a montmorillonites/polyaniline nanocomposite modified glassy carbon electrode. J Electroanal Chem 717–718:206–212
Bolado PF, Santos DH, Ardisana PJL, Pernia AM, Garcia AC (2008) Electrochemical characterization of screen-printed and conventional carbon paste electrodes. Electrochim Acta 53:3635–4364
Shams E, Torabi R (2006) Determination of nanomolar concentrations of cadmium by anodic-stripping voltammetry at a carbon paste electrode modified with zirconium phosphated amorphous silica. Sens Actuators B 117:86–92
Tang L, Chen J, Zeng G, Zhu Y, Zhang Y, Zhou Y, Xie X, Yang G, Zhang S (2014) Ordered mesoporous carbon and thiolated polyaniline modified electrode for simultaneous determination of cadmium(II) and lead(II) by anodic stripping voltammetry. Electroanalysis 26:2283–2291
March G, Nguyen TD, Piro B (2015) Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 5:241–275
Naveen MH, Gurudatt NG, Shim YB (2017) Application of conducting polymer composites to electrochemical sensors: a review. Appl Mater Today 9:419–433
Lu Y, Liang X, Niyungeko C, Zhou J, Xu J, Tian G (2018) A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 178:324–338
Bansod B, Kumar T, Thakur R, Rana S, Singh I (2017) A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens Bioelectron 94:443–455
Wei Y, Yang R, Liu JH, Huang XJ (2013) Selective detection toward Hg(II) and Pb(II) using polypyrrole/carbonaceous nanospheres modified screen-printed electrode. Electrochim Acta 105:218–223
Seenivasan R, Chang W-J, Gunasekaran S (2015) Highly sensitive detection and removal of lead ions in water using cysteine-functionalized graphene oxide/polypyrrole nanocomposite film electrode. ACS Appl Mater Interfaces 7:15935–15943
Muralikrishna S, Nagaraju DH, Balakrishna RG, Surareungchai W, Ramakrishnappa T, Shivanandareddy AB (2017) Hydrogels of polyaniline with graphene oxide for highly sensitive electrochemical determination of lead ions. Anal Chim Acta 990:67–77
Rahman MA, Won MS, Shim YB (2003) Characterization of an EDTA bonded conducting polymer modified electrode: its application for the simultaneous determination of heavy metal ions. Anal Chem 75:1123–1129
Philips MF, Gopalan AI, Lee K-P (2012) Development of a novel cyano group containing electrochemically deposited polymer film for ultrasensitive simultaneous detection of trace level cadmium and lead. J Hazard Mater 237–238:46–54
Huang H, Zhu W, Gao X, Liu X, Ma H (2016) Synthesis of a novel electrode material containing phytic acid-polyaniline nanofibers for simultaneous determination of cadmium and lead ions. Anal Chim Acta 947:32–41
Locatelli C (2010) Mutual interference problems in the simultaneous voltammetric determination of trace total mercury(II) in presence of copper(II) at gold electrode. Applications to environmental matrices. Anal Methods 2:1784–1791
Salaün P, van den Berg CMG (2006) Voltammetric detection of mercury and copper in seawater using a gold microwire electrode. Anal Chem 78:5052–5060
Xiong W, Zhou L, Liu S (2016) Development of gold-doped carbon foams as a sensitive electrochemical sensor for simultaneous determination of Pb(II) and Cu (II). Chem Eng J 284:650–656
Zhang W, Fan S, Li X, Liu S, Duan D, Leng L, Cui C, Qu L (2019) Electrochemical determination of lead(II) and copper(II) by using phytic acid and polypyrrole functionalized metal-organic frameworks. Microchim Acta 187:69
Bonfil Y, Brand M, Kirowa-Eisner E (2002) Characteristics of subtractive anodic stripping voltammetry of Pb and Cd at silver and gold electrodes. Anal Chim Acta 464:99–114
Promphet N, Rattanarat P, Rangkupan R, Chailapakul O, Rodthongkum N (2015) An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sens Actuators B 207:526–534
Zhu G, Ge Y, Dai Y, Shang X, Yang J, Liu J (2018) Size-tunable polyaniline nanotube-modified electrode for simultaneous determination of Pb(II) and Cd(II). Electrochim Acta 268:202–210
Wang Z, Liu E, Zhao X (2011) Glassy carbon electrode modified by conductive polyaniline coating for determination of trace lead and cadmium ions in acetate buffer solution. Thin Solid Films 519:5285–5289
Chen L, Li Z, Meng Y, Zhang P, Su Z, Liu Y, Huang Zhou Y, Xie Q, Yao S (2014) Sensitive square wave anodic stripping voltammetric determination of Cd2+ and Pb2+ ions at Bi/Nafion/overoxidized 2-mercaptoethanesulfonate-tethered polypyrrole/glassy carbon electrode. Sens Actuators B 191:94–101
Yu Z, Jamal R, Zhang R, Zhang W, Yan Y, Liu Y, Ge Y, Abdiryim T (2020) PEDOT-type conducting polymers/black TiO2 composites for electrochemical determination of Cd2+ and Pb2+. J Electrochem Soc 167:067514
Chen L, Su Z, He X, Liu Y, Qin C, Zhou Y, Zhou Y, Li Z, Wang L, Xie Q, Yao S (2012) Square wave anodic stripping voltammetric determination of Cd and Pb ions at a Bi/Nafion/thiolated polyaniline/glassy carbon electrode. Electrochem Commun 15:34–37
Lo M, Diaw AKD, Gningue-Sall D, Aaron J-J, Oturan MA, Chehimi MM (2018) Tracking metal ions with polypyrrole thin films adhesively bonded to diazonium-modified flexible ITO electrodes. Environ Sci Pollut Res 25:20012–20022
Jena BK, Raj CR (2008) Gold nanoelectrode ensembles for the simultaneous electrochemical detection of ultratrace arsenic, mercury, and copper. Anal Chem 80:4836–4844
Gao XH, Wei WZ, Yang L, Yin TJ, Wang Y (2005) Simultaneous determination of lead, copper, and mercury free from macromolecule contaminants by square wave stripping voltammetry. Anal Lett 38:2327–2343
De Barros A, Constantino CJL, Bortoleto JRR, Da Cruz NC, Ferreira M (2016) Incorporation of gold nanoparticles into Langmuir–Blodgett films of polyaniline and montmorillonite for enhanced detection of metallic ions. Sens Actuators B 236:408–417
Ruecha N, Rodthongkum N, Cate DM, Volckens J, Chailapakul O, Henry CS (2015) Sensitive electrochemical sensor using a graphene–polyaniline nanocomposite for simultaneous detection of Zn(II), Cd(II), and Pb(II). Anal Chim Acta 874:40–48
Alves GMS, Magalhães JMCS, Salaün P, van den Berg CMG, Soares HMVM (2011) Simultaneous electrochemical determination of arsenic, copper, lead and mercury in unpolluted fresh waters using a vibrating gold microwire electrode. Anal Chim Acta 703:1–7
Rong R, Zhao HM, Gan XR, Chen S, Quan X (2017) An electrochemical sensor based on graphene-polypyrrole nanocomposite for the specific detection of Pb(II). NANO 12:1750008
Rehman AU, Ikram M, Kan K, Zhao YM, Zhang WJ, Zhang JW, Liu Y, Wang Y, Du LJ, Shi KY (2018) 3D interlayer nanohybrids composed of reduced grapheme scheme oxide/SnO2/PPy grown from expanded graphite for the detection of ultra-trace Cd2+, Cu2+, Hg2+ and Pb2+ ions. Sens Actuators B 274:285–295
Oularbi L, Turmine M, El Rhazi M (2017) Electrochemical determination of traces lead ions using a new nanocomposite of polypyrrole/carbon nanofibers. J Solid State Electrochem 21:3289–3300
Wang Y, Wang L, Huang W, Zhang T, Hu X, Perman JA, Ma S (2017) A metal–organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J Mater Chem A 5:8385–8393
Buica GO, Lazar IG, Saint-Aman E, Tecuceanu V, Dumitriu C, Anton IA, Stoian AB, Ungureanu EM (2017) Ultrasensitive modified electrode based on poly(1H-pyrrole-1-hexanoic acid) for Pb(II) detection. Sens Actuators B 246:434–443
Salih FE, Ouarzane A, El Rhazi M (2017) Electrochemical detection of lead (II) at bismuth/poly(1,8-diaminonaphthalene) modified carbon paste electrode. Arabian J Chem 10:596–603
Yilong Z, Dean Z, Daoliang L (2015) Electrochemical and other methods for detection and determination of dissolved nitrite: a review. Int J Electrochem Sci 10:1144–1168
Xiao Q, Feng M, Liu Y, Lu S, He Y, Huang S (2018) The graphene/polypyrrole/chitosan-modified glassy carbon electrode for electrochemical nitrite detection. Ionics 24:845–859
Arulraj AD, Sundaram E, Vasantha VS, Neppolian B (2018) Polypyrrole with a functionalized multi-walled carbon nanotube hybrid nanocomposite: a new and efficient nitrite sensor. New J Chem 42:3748–3757
Wang J, Hui N (2017) A nanocomposite consisting of flower-like cobalt nanostructures, graphene oxide and polypyrrole for amperometric sensing of nitrite. Microchim Acta 184:2411–2418
Rajesh S, Kanugula AK, Bhargava K, Ilavazhagan G, Kotamraju S, Karunakaran C (2010) Simultaneous electrochemical determination of superoxide anion radical and nitrite using Cu, ZnSOD immobilized on carbon nanotube in polypyrrole matrix. Biosens Bioelectron 26:689–695
Sahooa S, Sahoo PK, Sharma A, Satpatia AK (2020) Interfacial polymerized RGO/MnFe2O4/polyaniline fibrous nanocomposite supported glassy carbon electrode for selective and ultrasensitive detection of nitrite. Sens Actuators B 309:127763
Diarisso A, Fall M, Raouafi N (2018) Elaboration of a chemical sensor based on polyaniline and sulfanilic acid diazonium salt for highly sensitive detection nitrite ions in acidified aqueous media. Environ Sci Water Res Technol 4:1024–1034
Liu L, Cui H, An H, Zhai J, Pan Y (2017) Electrochemical detection of aqueous nitrite based on poly(aniline-co-o-aminophenol)-modified glassy carbon electrode. Ionics 23:1517–1523
Muchindu M, Waryo T, Arotiba O, Kazimierska E, Morrin A, Killard AJ, Smyth MR, Jahed N, Kgarebe B, Baker PGL, Iwuoha EI (2010) Electrochemical nitrite nanosensor developed with amine- and sulphate functionalised polystyrene latex beads self-assembled on polyaniline. Electrochim Acta 55:4274–4280
Guo M, Chen J, Li J, Tao B, Yao S (2005) Fabrication of polyaniline/carbon nanotube composite modified electrode and its electrocatalytic property to the reduction of nitrite. Anal Chim Acta 532:71–77
Jiao M, Li Z, Li Y, Cui M, Luo X (2018) Poly(3,4-ethylenedioxythiophene) doped with engineered carbon quantum dots for enhanced amperometric detection of nitrite. Microchim Acta 185:249
Gligor D, Cuibus F, Peipmann R, Bund A (2017) Novel amperometric sensors for nitrite detection using electrodes modified with PEDOT prepared in ionic liquids. J Solid State Electrochem 21:281–290
Wang G, Han R, Feng X, Li Yinan, Lin J, Luo X (2017) A glassy carbon electrode modified with poly(3,4-ethylenedioxythiophene) doped with nano-sized hydroxyapatite for amperometric determination of nitrite. Microchim Acta 184:1721–1727
Lin P, Chai F, Zhang R, Zhang R, Xu G, Fan X (2016) Electrochemical synthesis of poly(3,4-ethylenedioxythiophene) doped with gold nanoparticles, and its application to nitrite sensing. Microchim Acta 183:235–1241
Wang Junjie, Guiyun Xu, Wang Wei, Shenghao Xu, Luo Xiliang (2015) Nitrite oxidation with copper–cobalt nanoparticles on carbon nanotubes doped conducting polymer PEDOT composite. Chem Asian J 10:1892–1897
Zhang O, Wen Y, Xu J, Lu L, Duan X, Yu H (2013) One-step synthesis of poly(3,4-ethylenedioxythiophene)-Au composites and their application for the detection of nitrite. Synth Met 164:47–51
Eguilaz M, Agui L, Yanez-Sedeno P, Pingarron J (2010) A biosensor based on cytochrome c immobilization on a poly-3-methylthiophene/multi-walled carbon nanotubes hybrid-modified electrode. Application to the electrochemical determination of nitrite. J Electroanal Chem 644:30–35
Dai J, Deng D, Yuan Y, Zhang J, Deng F, He S (2016) Amperometric nitrite sensor based on a glassy carbon electrode modified with multi-walled carbon nanotubes and poly(toluidine blue). Microchim Acta 183(1553–1561):37
Rajalakshmi K, John SA (2015) Highly sensitive determination of nitrite using FMWCNTs-conducting polymer composite modified electrode. Sens Actuators B 215:119–124
Kuralay F, Dumangöz M, Tunç S (2015) Polymer/carbon nanotubes coated graphite surfaces for highly sensitive nitrite detection. Talanta 144:133–1138
Acknowledgements
The Tunisian Ministry of Higher Education and Scientific Research (Lab. LR99ES15) and the Tunisian PRF program for financial support (NanoFastResponse ref. PRF2017-D4P1 and SmartBioSens ref. PRFCOV19-D2P2) are gratefully acknowledged. The authors are grateful to the International Science Program (ISP), University of Uppsala (Sweden) for its financial support through the African Network of Electroanalytical Chemists (ANEC) and to TWAS, the World Academy of Science for the Advancement of Science in developing countries for financial and material support (TWAS RGA no. 16-499RG/CHE/AF/AC_G–FR3240293299).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Sall, M.L., Fall, B., Diédhiou, I. et al. Toxicity and Electrochemical Detection of Lead, Cadmium and Nitrite Ions by Organic Conducting Polymers: A Review. Chemistry Africa 3, 499–512 (2020). https://doi.org/10.1007/s42250-020-00157-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-020-00157-0