Abstract
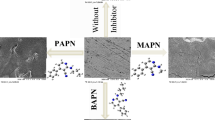
1-[(5-Phenyl-1,3, 4-oxadiazol-2-yl)thio] acetone (POTA) was synthesized and tested as a new corrosion inhibitor for low carbon steel in 1 M hydrochloric acid. The diagnosis of POTA was carried out by FTIR and NMR analysis. Inhibitor performance was investigated using mass loss technique. POTA was acted as a moderate corrosion inhibitor for low carbon steel in 1 M hydrochloric acidic solution with efficiency more than 70%. The inhibitor performance was attributed to the formation of an adsorption layer on the low carbon steel surface. The mechanism of inhibitor adsorption on the low carbon steel surface was according to the Langmuir adsorption isotherm. The value of adsorption heat was within the range of chemical adsorption. Quantum chemical studies were adopted as a theoretical tool to clarify the mechanism of inhibition and to support the experimental part.











Similar content being viewed by others
References
Verma C, Olasunkanmi LO, Obot IB, Ebenso EE, Quraishi MA (2016) 2,4-Diamino-5-(phenylthio)-5H-chromeno[2, 3-b]pyridine-3-carbonitriles as green and effective corrosion inhibitors: gravimetric, electrochemical, surface morphology and theoretical studies. RSC Adv 6:53933–53948
Sasikumar Y, Adekunle AS, Olasunkanmi LO, Bahadur Baskar IR, Kabanda MM, Obot IB (2015) Ebenso EE Experimental, quantum chemical and Monte Carlo simulation studies on the 15 corrosion inhibition of some alkyl imidazolium ionic liquids containing tetrafluoroborate anion on MSin acidic medium. J Mol Liq 211:105–118
Khadiri K, Saddik R, Bekkouche K, Aouniti A, Hammouti B, Benchat N, Bouachrine M, Solmaz R (2016) Gravimetric, electrochemical and quantum chemical studies of some pyridazine derivatives as corrosion inhibitors for mild steel in 1 M HCl solution. J Taiwan Inst Chem Eng 58:552–564
Khadom AA, Yaro AS, AlTaie AS, Kadum AAH (2009) Electrochemical, activations and adsorption studies for the corrosion inhibition of low carbon steel in acidic media. Portugaliae Electrochimica Acta 27(6):699–712
Kadhum AAH, Mohamad AB, Takriff MS, Daud AR, Kamarudin SK (2010) On the inhibition of mild steel corrosion by 4-amino-5-phenyl-4H-1, 2,4-trizole-3-thiol. Corros Sci 52(2):526–533
Khadom AA, Musa AY, Kadhum AAH, Mohamad AB, Takriff MS (2010) Adsorption kinetics of 4-amino-5-phenyl-4H-1,2, 4-triazole-3-thiol on mild steel surface. Portugaliae Electrochimica Acta 28(4):221–230
Alaneme KK, Daramola YS, Olusegun SJ, Afolabi AS (2015) Corrosion inhibition and adsorption characteristics of rice husk extracts on mild steel immersed in 1 M H2SO4 and HCl solutions. Int J Electrochem Sci 10:3553–3567
Noor EA, Al-Moubaraki Aisha H (2008) Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4(-X)-styryl pyridinium iodides/hydrochloric acid systems. Mater Chem Phys 110:145–154
Bentiss F, Lebrini M, Lagrenee M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros Sci 47:2915–2931
Popova A, Christov M, Zwetanova A (2007) Effect of the molecular structure on the inhibitor properties of azoles on mild steel corrosion in 1 M hydrochloric acid. Corros Sci 49:2131–2143
Musa AY, Amir A, Kadhum H, Mohamad AB, Takriff MS (2010) Corros Sci 52:3331–3340
Bhrara K, Kim H, Singh G (2008) Corros Sci 50:2747–2754
Sudheer MA (2014) Quraishi. Ind Eng Chem Res 53:2851–2859
Nwanonenyi SC, Obasi HC, Chukwujike IC et al (2018) Chemistry Africa. https://doi.org/10.1007/s42250-018-00035-w
Raghavendra N (2019) Chem Afr. https://doi.org/10.1007/s42250-019-00061-2
Nwanonenyi SC, Obasi HC, Eze IO (2019) Chem Afr. https://doi.org/10.1007/s42250-019-00062-1
Ahmed SK, Ali WB, Khadom AA (2019) Int J Ind Chem 10:159. https://doi.org/10.1007/s40090-019-0181-8
Khadom A, Abd A, Ahmed N (2018) Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: kinetics and mathematical studies. S Afr J Chem Eng 25:13–21
Khadom A, Abd A, Ahmed N (2018) Potassium iodide as a corrosion inhibitor of mild steel in hydrochloric acid: kinetics and mathematical studies. J Bio Tribo Corros 4(17):2–10
Hassan K, Khadom A, Kurshed N (2016) Citrus aurantium leaves extracts as a sustainable corrosion inhibitor of mild steel in sulfuric acid. S Afr J Chem Eng 22:1–5
Arun K (2012) Gupta, sumeet prachand, arpit patel and sanjay jain, synthesis of some 4-Amino-5-(substituted-phenyl)-4H-[1,2,4] triazole-3-thiol derivatives and Antifungal activity. Int J Pharm Life Sci 3:1848–1857
Aly AA, Brown AB, El-Emary TI, Mohamed Ewas AM, Mohamed R (2009) Hydrazinecarbothioamide group in the synthesis of heterocycles. Arkivoc I:150–197
Salih Hadi Kadhim (2017) Ibtihal qahtan abd-alla and thikra jawad hashim, synthesis and characteristic study of Co(II), Ni(II) and Cu(II) complexes of new schiff base derived from 4-amino antipyrine. Int J Chem Sci 15:107–115
Khadom AA (2015) Kinetics and Synergistic effect of halide ion and naphthylamin for inhibition of corrosion reaction of mild steel in hydrochloric acid. React Kinet Mech Catal 115:463–481
Khadom AA, Yaro AS (2011) Protection of low carbon steel in phosphoric acid by potassium iodide. Prot Met Phys Chem Surf 47:662–669
Hassan Karim H, Khadom Anees A, Kurshed Noor H (2016) Citrus aurantium leaves extracts as a sustainable corrosion inhibitor of mild steel in sulfuric acid. S Afr J Chem Eng 22:1–5
Khadom AA, Abdul-Hadi AA (2014) Kinetic and mathematical approaches to the corrosion of mild steel in nitric acid. React Kinet Mech Catal 112:15–26
Yaro Aprael S, Al-Jendeel Haidar, Khadom Anees A (2011) Cathodic protection system of copper–zinc–saline water in presence of bacteria. Desalination 270:193–198
Khadom AA, Abd AN, Ahmed NA (2018) Xanthiumstrumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: Kinetics and mathematical studies. South Af J Chem Eng 25:13–21
Saady A, El-Hajjaji F, Taleb M, Alaoui KI, El Biache A, Mahfoud A, Quraishi MA (2018) Experimental and theoretical tools for corrosion inhibition study of mild steel in aqueous hydrochloric acid solution by new indanones derivatives. Mater Discov 12:30–42
Yaro AS, Khadom AA (2011) Hadeel F. Ibraheem, peach juice as an anti-corrosion inhibitor of mild steel, anti-corrosion methods and materials 58(3):116–124
Ehteram A (2009) Noor, evaluation of inhibitive action of some quaternary N-heterocyclic compounds on the corrosion of Al–Cu alloy in hydrochloric acid. Mater Chem Phys 114:533–541
Umoren SA, Ebenso EE (2007) The synergistic effect of polyacrylamide and iodide ions on the corrosion inhibition of mild steel in H2SO4. Mater Chem Phys 106:387–393
Obot IB, Obi-Egbedi NO (2010) An interesting and efficient green corrosion inhibitor for aluminium from extracts of Chlomolaena odorata L. in acidic solution. J Appl Electrochem 40:1977–1984
Amar H, Benzakour J, Derja A, Villemin D, Moreau B, Braisaz T (2006) Piperidin-1-yl-phosphonic acid and (4-phosphono-piperazin-1-yl) phosphonic acid: a new class of iron corrosion inhibitors in sodium chloride 3% media. Appl Surf Sci 252:6162
Li XH, Deng SD, Fu H, Mu GN (2010) Synergistic inhibition effect of rare earth cerium(IV) ion and sodium oleate on the corrosion of cold rolled steel inphosphoric acid solution. Corros Sci 52:1167–1178
Guo L, Zhu S, Zhang S, He Q, Li W (2014) Theoretical studies of three triazolederivatives as corrosion inhibitors for mild steel in acidic medium. Corros Sci 87:366–375
Yadav M, Behera D, Kumar S, Sinha RR (2013) J Ind Eng Chem Res 52(19):6318–6328
Hu K, Zhuang J, Ding J, Ma Z, Wang F, Zeng X (2017) Influence of biomacromolecule DNA corrosion inhibitor on carbon steel. Corros Sci 125:68–76
Mourya P, Singh P, Tewari AK, Rastogi RB, Singh MM (2015) Relationship between structure and inhibition behaviour of quinolinium salts for mild steel corrosion: experimental and theoretical approach. Corros Sci 95:71–87
Pearson RG (1988) Absolute electronegativity and hardness: application to inorganic chemistry. Inorg Chem 27:734–740
Lukovits I, Lalman E, Zucchi F (2001) Corrosion inhibitors—correlation between electronic structure and efficiency. Corrosion 57:3
Zhang D, Tang Y, Qi S, Dong D, Cang H, Lu G (2016) The inhibition performance of long-chain alkyl-substituted benzimidazole derivatives for corrosion of mild steel in HCl. Corros Sci 102:517–522
Qiang Y, Guo L, Zhang S, Li W, Yu S, Tan J (2016) Synergistic effect of tartaric acidwith 2,6-diaminopyridine on the corrosion inhibition of mild steel in 0.5 M HCl. Sci Rep 6:33305
Li LJ, Zhang XP, Lei JL, He JX, Zhang ST, Pan FS (2012) Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel. Corros Sci 63:82–90
Yüce AO, Mert BD, Kardaş G, Yazıcı B (2014) Electrochemical and quantum chemical studies of 2-amino-4-methyl-thiazole as corrosion inhibitor for mild steel in HCl solution. Corros Sci 83:310–316
Zheng X, Zhang S, Li W, Gong M, Yin L (2015) Experimental and theoretical studiesof two imidazolium-based ionic liquids as inhibitors for mild steel in sulfuricacid solution. Corros Sci 95:168–179
Acknowledgements
Special thanks to the Department of Chemistry – College of Science – University of University of Thi-Qar and Department of Chemical Engineering – College of Engineering – University Diyala for continuous support and facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
There are no conflicts of interest arising from the involvement of other parties either internal or external to the University.
Rights and permissions
About this article
Cite this article
Mahood, H.B., Sayer, A.H., Mekky, A.H. et al. Performance of Synthesized Acetone Based Inhibitor on Low Carbon Steel Corrosion in 1 M HCl Solution. Chemistry Africa 3, 263–276 (2020). https://doi.org/10.1007/s42250-019-00104-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-019-00104-8