Abstract
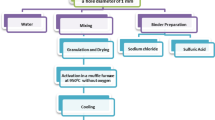
Fe-loaded activated carbon (AC) has high surface acidity and more active sites, while manganese-loaded AC has high oxygen content. Coconut husk AC modified by Fe–Mn was studied with the aim of revealing the modification mechanism. First, HNO3/AC was prepared using the nitric acid immersion method. Second, Fe–Mn/AC was prepared using the Fe(NO3)3 and Mn(NO3)2 sequential immersion. The effects of HNO3, Fe(NO3)3, and Mn(NO3)2 on the pore texture and surface chemical characteristics of carbon materials were examined by scanning electron microscopy, Brunauer–Emmett–Teller (BET) analysis, X-ray diffraction and Fourier-transform infrared spectroscopy. The surface topography, pore structure, active material, and functional groups of AC, HNO3/AC, and Fe–Mn/AC were systematically studied. The following results were obtained. The surface of HNO3/AC has more ditches and air voids; the micropores of HNO3/AC are deformed and flattened compared to those of AC. The surface of Fe–Mn/AC exhibits an accumulation phenomenon. MnFe2O4 and FeMn2O4 formed more pore structures. AC and HNO3/AC have numerous micropores. The higher loading quantity of Fe–Mn results in bigger specific surface. The active components of Fe–Mn/AC-1, Fe–Mn/AC-2, Fe–Mn/AC-3, and Fe–Mn/AC-4 are MnFe2O4, Mn0.43Fe2.57O4, Mn3O4, and α-Fe2O3, respectively. The surface functional groups of AC and HNO3/AC are oxygen-containing functional groups. The effect of Fe–Mn modifying conditions on functional group species is rare; however, Fe/AC has more oxygen-containing functional groups. These research findings can aid in the desulfurization and denitrification of the Fe–Mn/AC catalyst.







Similar content being viewed by others
References
M.E. de Oliveira Ferreira, B.G. Vaz, C.E. Borba, C.G. Alonso, I.C. Ostroski, Microporous Mesoporous Mater. 277 (2019) 208–216.
Z.B. Zhang, X.Y. Liu, D.W. Li, Y.Q. Lei, T.T. Gao, B.G. Wu, J.W. Zhao, Y.K. Wang, G.Y. Zhou, H.M. Yao, Ultrason. Sonochem. 51 (2019) 206–213.
C. Nieto-Delgado, J. Gutiérrez-Martínez, J.R. Rangel-Méndez, J. Environ. Sci. 76 (2019) 403–414.
Y.H. Cao, K.L. Wang, X.M. Wang, Z.R. Gu, W. Gibbons, H. Vu, Bioresour. Technol. 196 (2015) 525–532.
N. Wang, B. Han, J.B. Wen, M.W. Liu, X.J. Li, Colloids Surf. A Physicochem. Eng. Asp. 567 (2019) 313–318.
Y. Ma, B.B. Wang, Q. Wang, S.T. Xing, Chem. Eng. J. 354 (2018) 75–84.
S.S.R. Putluru, L. Schill, A.D. Jensen, B. Siret, F. Tabaries, R. Fehrmann, Appl. Catal. B 165 (2015) 628–635.
S.L. Yin, B.Z. Zhu, Y.L. Sun, Z.H. Zi, Q.L. Fang, G.B. Li, C. Chen, T.Y. Xu, J.X. Li, Asia-Pac. J. Chem. Eng. 13 (2018) e2231.
F.T. You, G.W. Yu, Z.J. Xing, J. Li, S.Y. Xie, C.X. Li, G. Wang, H.Y. Ren, Y. Wang, Appl. Surf. Sci. 471 (2019) 633–644.
J.K. Du, J.G. Bao, Y. Liu, S.H. Kim, D.D. Dionysiou, Chem. Eng. J. 376 (2019) 119193.
S. Nagamuthu, S. Vijayakumar, S.H. Lee, K.S. Ryu, Appl. Surf. Sci. 390 (2016) 202–208.
M.J. Akhtar, M. Younas, Solid State Sci. 14 (2012) 1536–1542.
B. Sajjadi, J.W. Broome, W.Y. Chen, D.L. Mattern, N.O. Egiebor, N. Hammer, C.L. Smith, Ultrason. Sonochem. 51 (2019) 20–30.
Z. Liu, Z.J. Wang, S.J. Qing, N.N. Xue, S.P. Jia, L. Zhang, L. Li, N. Li, L.Y. Shi, J.Z. Chen, Appl. Catal. B 232 (2018) 86–92.
J.H. Yu, J.H. So, Chem. Phys. Lett. 716 (2019) 237–246.
L.L. Deng, B.Q. Lu, J.L. Li, G.Q. Lv, S.J. Du, J.Y. Shi, Y.X. Yang, Fuel 200 (2017) 54–61.
Fan, J. Sun, L. Chu, L. Cui, G. Quan, J. Yan, Q. Hussain, M. Iqbal, Chemosphere 207 (2018) 33–40.
Y.H. Cao, Y. Gu, K.L. Wang, X.M. Wang, Z.R. Gu, T. Ambrico, M.A. Castro, J. Lee, W. Gibbons, J.A. Rice, J. Taiwan Inst. Chem. Eng. 66 (2016) 347–356.
L.T. Lin, Y.R. Li, Z.C. Xu, J. Xiong, T.Y. Zhu, Fuel 223 (2018) 312–323.
J. Palomo, J.J. Ternero-Hidalgo, J.M. Rosas, J. Rodríguez-Mirasol, T. Cordero, Fuel Process. Technol. 156 (2017) 438–445.
S.D. Lakshmi, P.K. Avti, G. Hegde, Nano-Struct. Nano-Objects 16 (2018) 306–321.
Acknowledgements
The authors are grateful for Open Fund of Key Laboratory of Ministry of Education for metallurgical emission reduction and comprehensive utilization of resources (JKF19-08), General Project of Science and Technology Plan of Yunnan Science and Technology Department (2019FB077 and 202001AT070029) and the Open Fund of Key Laboratory for Ferrous Metallurgy and Resources Utilization of Ministry of Education (Grant No. FMRUlab-20-4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Long, Hm., Huang, Bf., Shi, Z. et al. Physicochemical properties of coconut husk activated carbon modified by Fe(NO3)3 and Mn(NO3)2. J. Iron Steel Res. Int. 28, 530–537 (2021). https://doi.org/10.1007/s42243-021-00599-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-021-00599-x