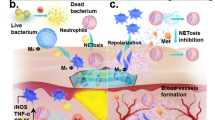
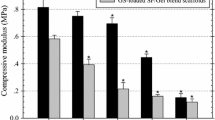
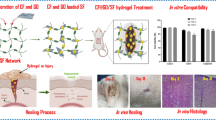
Abstract
Deep full-thickness burn wounds are prone to multi-drug resistant (MDR) infections following injury, which extends the healing time. Thus, providing a bioactive hydrogel dressing with prolonged antimicrobial activity and reduced dressing changes is quite desirable for accelerating burn wound healing and preventing scarring. To achieve this, we developed an injectable hydrogel based on silk sericin (SS), poly(vinyl alcohol) (PVA), and PVA microspheres (MSs) containing vancomycin (VA), gentamicin (GEN), or their association (VG) for the healing of infected burn wounds. The microspheres were prepared by inverse emulsion crosslinking, while the hydrogels were prepared by freeze-thawing cycles. Antibacterial studies showed that gentamicin acts synergistically with vancomycin by increasing the bacterial killing rate and enhancing the biofilm inhibition and eradication effects on methicillin-resistant Staphylococcus aureus more than on Pseudomonas aeruginosa and Escherichia coli. Findings from FESEM images showed that the microspheres were sphere-shaped with a smooth surface and their average diameter ranging from 26.22 to 32.42 μm suitable for parenteral drug delivery. The prepared hydrogel containing 10% of microspheres was more elastic than viscous, with lower tan delta values (< 1) suited for deeper injection with homogeneous tissue integration. The incorporation of VG-PVAMS in the PVA/SS hydrogel led to zero-order release kinetics and efficient antimicrobial effects. Moreover, the in vivo study using a rat full-thickness burn model showed that the VG-PVAMS@PVA/SS hydrogel displays a better therapeutic effect than drug-free PVAMS@PVA/SS hydrogel and Tegaderm™ film dressing by inducing early vascularization and collagen deposition, leading to early re-epithelialization and burn wound closure.
Graphical abstract

ToC. Development of vancomycin/gentamicin-PVA microsphere@PVA/SS hydrogel with sustained drug release, excellent mechanical, and biological properties for burn wound healing.











Similar content being viewed by others
References
Mestrallet G, Rouas-Freiss N, LeMaoult J et al (2021) Skin immunity and tolerance: focus on epidermal keratinocytes expressing HLA-G. Front Immunol 12:772516. https://doi.org/10.3389/fimmu.2021.772516
Ladhani HA, Yowler CJ, Claridge JA (2021) Burn wound colonization, infection, and sepsis. Surg Infect 22:44–48. https://doi.org/10.1089/sur.2020.346
Church D, Elsayed S, Reid O et al (2006) Burn wound infections. Clin Microbiol Rev 19:403–434. https://doi.org/10.1128/CMR.19.2.403-434.2006
Azevedo MM, Pina-Vaz C, Rodrigues AG (2021) The role of phage therapy in burn wound infections management: advantages and pitfalls. J Burn Care Res. https://doi.org/10.1093/jbcr/irab175
Chen Y-Y, Wu P-F, Chen C-S et al (2020) Trends in microbial profile of burn patients following an event of dust explosion at a tertiary medical center. BMC Infect Dis 20:193. https://doi.org/10.1186/s12879-020-4920-4
Vinaik R, Barayan D, Shahrokhi S, Jeschke MG (2019) Management and prevention of drug resistant infections in burn patients. Expert Rev Anti Infect Ther 17:607–619. https://doi.org/10.1080/14787210.2019.1648208
Shu W, Wang Y, Zhang X et al (2021) Functional hydrogel dressings for treatment of burn wounds. Front Bioeng Biotechnol 9:788461. https://doi.org/10.3389/fbioe.2021.788461
Zhang X, Shu W, Yu Q et al (2020) Functional biomaterials for treatment of chronic wound. Front Bioeng Biotechnol 8:516. https://doi.org/10.3389/fbioe.2020.00516
Moorcroft SCT, Roach L, Jayne DG et al (2020) Nanoparticle-loaded hydrogel for the light-activated release and photothermal enhancement of antimicrobial peptides. ACS Appl Mater Interfaces 12:24544–24554. https://doi.org/10.1021/acsami.9b22587
Zhou K, Wang M, Zhou Y et al (2022) Comparisons of antibacterial performances between electrospun polymer@drug nanohybrids with drug-polymer nanocomposites. Adv Compos Hybrid Mater. https://doi.org/10.1007/s42114-021-00389-9
Yu D, Wang M, Ge R (2021) Strategies for sustained drug release from electrospun multi-layer nanostructures. Wiley Interdiscip Rev Nanomed Nanobiotechnol. https://doi.org/10.1002/wnan.1772
Liu X, Xu H, Zhang M, Yu D-G (2021) Electrospun medicated nanofibers for wound healing: Review. Membranes (Basel) 11:770. https://doi.org/10.3390/membranes11100770
Zhang M, Song W, Tang Y et al (2022) Polymer-based nanofiber–nanoparticle hybrids and their medical applications. Polymers (Basel) 14:351. https://doi.org/10.3390/polym14020351
Rukavina Z, Šegvić Klarić M, Filipović-Grčić J et al (2018) Azithromycin-loaded liposomes for enhanced topical treatment of methicillin-resistant Staphyloccocus aureus (MRSA) infections. Int J Pharm 553:109–119
Chen N, Wang H, Ling C et al (2019) Cellulose-based injectable hydrogel composite for pH-responsive and controllable drug delivery. Carbohydr Polym 225:115207. https://doi.org/10.1016/j.carbpol.2019.115207
Chen X, Xu C, He H (2019) Electrospinning of silica nanoparticles-entrapped nanofibers for sustained gentamicin release. Biochem Biophys Res Commun 516:1085–1089
Kircik LH (2011) Microsphere technology: hype or help? J Clin Aesthet Dermatol 4:27–31
Wang M, Huang X, Zheng H et al (2021) Nanomaterials applied in wound healing: mechanisms, limitations and perspectives. J Control Release 337:236–247. https://doi.org/10.1016/j.jconrel.2021.07.017
Malamatari M, Charisi A, Malamataris S et al (2020) Spray drying for the preparation of nanoparticle-based drug formulations as dry powders for inhalation. Processes 8:788. https://doi.org/10.3390/pr8070788
Hiremath L, Sruti O, Aishwarya BM et al (2021) Electrospun nanofibers: characteristic agents and their applications. In: Kumar B (ed) Nanofibers - Synthesis, Properties and Applications. IntechOpen
Bayer IS (2021) A review of sustained drug release studies from nanofiber hydrogels. Biomedicines 9:1612. https://doi.org/10.3390/biomedicines9111612
Li W, Chen J, Zhao S et al (2022) High drug-loaded microspheres enabled by controlled in-droplet precipitation promote functional recovery after spinal cord injury. Nat Commun 13:1262. https://doi.org/10.1038/s41467-022-28787-7
Saini S, Kumar S, Choudhary M, Budhwar V (2018) Microspheres as controlled drug delivery system: an updated review. Int J Pharm Sci 9:1760. https://doi.org/10.13040/IJPSR.0975-8232.9(5).1760-68
Saralidze K, Koole LH, Knetsch MLW (2010) Polymeric microspheres for medical applications. Materials (Basel) 3:3537–3564. https://doi.org/10.3390/ma3063537
Mok CF, Ching YC, Muhamad F et al (2020) Adsorption of dyes using poly(vinyl alcohol) (pva) and PVA-based polymer composite adsorbents: a review. J Polym Environ 28:775–793. https://doi.org/10.1007/s10924-020-01656-4
Lamboni L, Gauthier M, Yang G, Wang Q (2015) Silk sericin: A versatile material for tissue engineering and drug delivery. Biotechnol Adv 33:1855–1867. https://doi.org/10.1016/j.biotechadv.2015.10.014
Boni BOO, Lamboni L, Bakadia BM et al (2020) Combining silk sericin and surface micropatterns in bacterial cellulose dressings to control fibrosis and enhance wound healing. Eng Sci. https://doi.org/10.30919/es8d906
Mazer-Amirshahi M, Pourmand A, May L (2017) Newly approved antibiotics and antibiotics reserved for resistant infections: Implications for emergency medicine. Am J Emerg Med 35:154–158. https://doi.org/10.1016/j.ajem.2016.10.034
Domalaon R, Idowu T, Zhanel GG, Schweizer F (2018) Antibiotic hybrids: the next generation of agents and adjuvants against gram-negative pathogens? Clin Microbiol Rev 31:e00077-e117. https://doi.org/10.1128/CMR.00077-17
Ng VWL, Chan JMW, Sardon H et al (2014) Antimicrobial hydrogels: A new weapon in the arsenal against multidrug-resistant infections. Adv Drug Deliv Rev 78:46–62. https://doi.org/10.1016/j.addr.2014.10.028
Worthington RJ, Melander C (2013) Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol 31:177–184. https://doi.org/10.1016/j.tibtech.2012.12.006
Gupta V, Datta P (2019) Next-generation strategy for treating drug resistant bacteria: Antibiotic hybrids. Indian J Med Res 149:97–106. https://doi.org/10.4103/ijmr.IJMR_755_18
Simner PJ, Patel R (2020) Cefiderocol antimicrobial susceptibility testing considerations: the achilles’ heel of the trojan horse? J Clin Microbiol 59:e00951-e1020. https://doi.org/10.1128/JCM.00951-20
Cong Y, Yang S, Rao X (2020) Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J Adv Res 21:169–176. https://doi.org/10.1016/j.jare.2019.10.005
Sidrim JJC, Teixeira CEC, Cordeiro RA et al (2015) β-Lactam antibiotics and vancomycin inhibit the growth of planktonic and biofilm Candida spp.: An additional benefit of antibiotic-lock therapy? Int J Antimicrob Agents 45:420–423. https://doi.org/10.1016/j.ijantimicag.2014.12.012
Bakadia BM, Souho T, Lamboni L et al (2021) Types and transmission routes of nosocomial antibacterial resistance. IJMSHR 5:156–168. https://doi.org/10.51505/IJMSHR.2021.5115
Ionita D, Bajenaru-Georgescu D, Totea G et al (2017) Activity of vancomycin release from bioinspired coatings of hydroxyapatite or TiO2 nanotubes. Int J Pharm 517:296–302. https://doi.org/10.1016/j.ijpharm.2016.11.062
Lamboni L, Li Y, Liu J, Yang G (2016) Silk sericin-functionalized bacterial cellulose as a potential wound-healing biomaterial. Biomacromol 17:3076–3084. https://doi.org/10.1021/acs.biomac.6b00995
Tam VH, Kabbara S, Vo G et al (2006) Comparative pharmacodynamics of gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother 50:2626–2631. https://doi.org/10.1128/AAC.01165-05
Masi M, Réfregiers M, Pos KM, Pagès JM (2017) Mechanisms of envelope permeability and antibiotic influx and efflux in Gram-negative bacteria. Nat Microbiol 2:28224989. https://doi.org/10.1038/nmicrobiol.2017.1
Muheim C, Götzke H, Eriksson AU et al (2017) Increasing the permeability of Escherichia coli using MAC13243. Sci Rep 7:1–11. https://doi.org/10.1038/s41598-017-17772-6
Ahmadi K, Hashemian AM, Bolvardi E, Hosseini PK (2016) vancomycin-resistant Pseudomonas aeroginosa in the cases of trauma. Med Arch 70:57–60. https://doi.org/10.5455/medarh.2016.70.57-60
Zhou A, Kang TM, Yuan J et al (2015) Synergistic interactions of vancomycin with different antibiotics against escherichia coli: Trimethoprim and nitrofurantoin display strong synergies with vancomycin against wild-type E. coli. Antimicrob Agents Chemother 59:276–281. https://doi.org/10.1128/AAC.03502-14
Miquel S, Lagrafeuille R, Souweine B, Forestier C (2016) Anti-biofilm activity as a health issue. Front Microbiol 7:1–14. https://doi.org/10.3389/fmicb.2016.00592
Gohil JM, Bhattacharya A, Ray P (2006) Studies on the cross-linking of poly (vinyl alcohol). J Polym Res 13:161–169. https://doi.org/10.1007/s10965-005-9023-9
Stoica-Guzun A, Stroescu M, Jipa I et al (2013) Effect of γ irradiation on poly(vinyl alcohol) and bacterial cellulose composites used as packaging materials. Radiat Phys Chem 84:200–204. https://doi.org/10.1016/j.radphyschem.2012.06.017
Thum J-Y, Sin LT, Bee S-T et al (2022) Investigation of calcination of sepia officinalis cuttlefish bone for reinforcement of polyvinyl alcohol added nano-size montmorillonite. Polymers (Basel) 14:1089. https://doi.org/10.3390/polym14061089
Danaei M, Dehghankhold M, Ataei S et al (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10:57. https://doi.org/10.3390/pharmaceutics10020057
Mao S, Guo C, Shi Y, Li LC (2012) Recent advances in polymeric microspheres for parenteral drug delivery – part 1. Expert Opin Drug Deliv 9:1161–1176. https://doi.org/10.1517/17425247.2012.709844
Enrione J, Char C, Pepczynska M et al (2020) Rheological and structural study of salmon gelatin with controlled molecular weight. Polymers (Basel) 12:1587. https://doi.org/10.3390/polym12071587
Seong Y-J, Lin G, Kim BJ et al (2019) Hyaluronic acid-based hybrid hydrogel microspheres with enhanced structural stability and high injectability. ACS Omega 4:13834–13844. https://doi.org/10.1021/acsomega.9b01475
Kim KK, Pack DW (2006) Microspheres for drug delivery. BioMEMS and Biomedical Nanotechnology. Springer, US, Boston, MA, pp 19–50
Schwendeman SP, Shah RB, Bailey BA, Schwendeman AS (2014) Injectable controlled release depots for large molecules. J Control Release 190:240–253. https://doi.org/10.1016/j.jconrel.2014.05.057
Champion JA, Walker A, Mitragotri S (2008) Role of particle size in phagocytosis of polymeric microspheres. Pharm Res 25:1815–1821. https://doi.org/10.1007/s11095-008-9562-y
Nosrati H, Aramideh Khouy R, Nosrati A et al (2021) Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis. J Nanobiotechnology 19:1. https://doi.org/10.1186/s12951-020-00755-7
Loh QL, Choong C (2013) Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev 19:485–502. https://doi.org/10.1089/ten.teb.2012.0437
Jeschke MG, van Baar ME, Choudhry MA et al (2020) Burn injury. Nat Rev Dis Prim 6:11. https://doi.org/10.1038/s41572-020-0145-5
Amalraj A, Raj KKJ, Haponiuk JT et al (2020) Preparation, characterization, and antimicrobial activity of chitosan/gum arabic/polyethylene glycol composite films incorporated with black pepper essential oil and ginger essential oil as potential packaging and wound dressing materials. Adv Compos Hybrid Mater 3:485–497. https://doi.org/10.1007/s42114-020-00178-w
Hassan CM, Peppas NA (2000) Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 33:2472–2479. https://doi.org/10.1021/ma9907587
Sirousazar M, Kokabi M, Hassan ZM (2012) Swelling behavior and structural characteristics of polyvinyl alcohol/montmorillonite nanocomposite hydrogels. J Appl Polym Sci 123:50–58. https://doi.org/10.1002/app.34437
Mofazzal Jahromi MA, Sahandi Zangabad P, Moosavi Basri SM et al (2018) Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv Drug Deliv Rev 123:33–64. https://doi.org/10.1016/j.addr.2017.08.001
Chen J, Zhang G, Zhao Y et al (2022) Promotion of skin regeneration through co-axial electrospun fibers loaded with basic fibroblast growth factor. Adv Compos Hybrid Mater. https://doi.org/10.1007/s42114-022-00439-w
Solarte David VA, Güiza-Argüello VR, Arango-Rodríguez ML et al (2022) Decellularized tissues for wound healing: towards closing the gap between scaffold design and effective extracellular matrix remodeling. Front Bioeng Biotechnol 10:821852. https://doi.org/10.3389/fbioe.2022.821852
Murphy F, Amblum J (2014) Treatment for burn blisters: debride or leave intact? Emerg Nurse 22:24–27. https://doi.org/10.7748/en2014.04.22.2.24.e1300
de Oliveira S, Rosowski EE, Huttenlocher A (2016) Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol 16:378–391. https://doi.org/10.1038/nri.2016.49
Janakiram NB, Valerio MS, Goldman SM, Dearth CL (2021) The role of the inflammatory response in mediating functional recovery following composite tissue injuries. Int J Mol Sci 22:13552. https://doi.org/10.3390/ijms222413552
Davies MJ, Hawkins CL (2020) The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease. Antioxid Redox Signal 32:957–981. https://doi.org/10.1089/ars.2020.8030
Bakadia BM, Boni BOO, Ahmed AAQ, Yang G (2021) The impact of oxidative stress damage induced by the environmental stressors on COVID-19. Life Sci 264:118653. https://doi.org/10.1016/j.lfs.2020.118653
Park SH, Cho A, Ryu HJ, Kim H (2022) Profile of vascular markers and CT enhancement of hyaline vascular type castleman’s disease. Microvasc Res 142:104357. https://doi.org/10.1016/j.mvr.2022.104357
Mathew-Steiner SS, Roy S, Sen CK (2021) Collagen in wound healing. Bioengineering 8:63. https://doi.org/10.3390/bioengineering8050063
Kuburich NA, den Hollander P, Pietz JT, Mani SA (2021) Vimentin and cytokeratin: good alone, bad together. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2021.12.006
Acknowledgements
The authors sincerely thank the Analysis and Test Center at Huazhong University of Science and Technology for the related characterizations of various samples.
Funding
This work was financially supported by the National Natural Science Foundation of China (51973076); BRICS STI Framework Programme 3rd call 2019, the National Key Research and Development Program of China (2018YFE0123700); and the Fundamental Research Funds for the Central Universities (2020kfyXJJS035).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bianza Moise Bakadia and Aimei Zhong contributed equally to this work.
Highlights
• Poly(vinyl alcohol) (PVA)/silk sericin (SS) hydrogels loaded with vancomycin–gentamicin/PVA microspheres were synthesized.
• Vancomycin and gentamicin exhibited a synergistic antibacterial effect in loaded hydrogels.
• The biological properties of the hydrogels improved obviously with microsphere loading.
• The microsphere hydrogel exhibited sustained drug release, antibacterial activity, and excellent cytocompatibility.
• The microsphere hydrogel system accelerates full-thickness burn wound healing.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bakadia, B.M., Zhong, A., Li, X. et al. Biodegradable and injectable poly(vinyl alcohol) microspheres in silk sericin-based hydrogel for the controlled release of antimicrobials: application to deep full-thickness burn wound healing. Adv Compos Hybrid Mater 5, 2847–2872 (2022). https://doi.org/10.1007/s42114-022-00467-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42114-022-00467-6