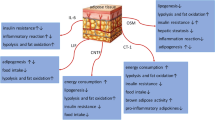
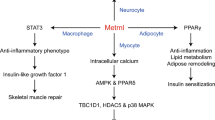
Abstract
The suppressors of cytokine signaling (SOCS) are a group of eight proteins responsible for preventing excessive cytokine signaling. Among this protein family, SOCS3 has received special attention. SOCS3 expression is important to control certain allergy autoimmune diseases. Furthermore, SOCS3 expression is elevated in obesity and it is involved in the inhibition of leptin and insulin signaling, two important hormones involved in the control of energy metabolism. Therefore, increased SOCS3 expression in obese individuals is associated with several metabolic disorders, including reduced energy expenditure, increased food intake and adiposity, and insulin and leptin resistance. In addition, recent studies found that SOCS3 expression regulates energy and glucose homeostasis in several metabolic conditions, such as pregnancy, caloric restriction, and refeeding. Importantly, attenuation of SOCS3 expression in most cases improves leptin and insulin sensitivity, leading to beneficial metabolic effects. This review aims to discuss the role of SOCS3 in the control of blood glucose levels as well as in energy homeostasis. The development of pharmacological compounds to inhibit SOCS3 activity and/or expression may represent a promising therapeutic approach to treat type 2 diabetes mellitus, obesity, and other metabolic imbalances.

Similar content being viewed by others
References
Yoshimura A, Naka T, Kubo M (2007) SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 7(6):454–465. https://doi.org/10.1038/nri2093
Krebs DL, Hilton DJ (2001) SOCS proteins: negative regulators of cytokine signaling. Stem Cells 19(5):378–387. https://doi.org/10.1634/stemcells.19-5-378
Howard JK, Flier JS (2006) Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab 17(9):365–371. https://doi.org/10.1016/j.tem.2006.09.007
Yin Y, Liu W, Dai Y (2015) SOCS3 and its role in associated diseases. Hum Immunol 76(10):775–780. https://doi.org/10.1016/j.humimm.2015.09.037
Wong PK, Egan PJ, Croker BA, O'Donnell K, Sims NA, Drake S, Kiu H, McManus EJ, Alexander WS, Roberts AW, Wicks IP (2006) SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J Clin Invest 116(6):1571–1581. https://doi.org/10.1172/JCI25660
Shouda T, Yoshida T, Hanada T, Wakioka T, Oishi M, Miyoshi K, Komiya S, Kosai K, Hanakawa Y, Hashimoto K, Nagata K, Yoshimura A (2001) Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J Clin Invest 108(12):1781–1788. https://doi.org/10.1172/JCI13568
Calegari VC, Bezerra RM, Torsoni MA, Torsoni AS, Franchini KG, Saad MJ, Velloso LA (2003) Suppressor of cytokine signaling 3 is induced by angiotensin II in heart and isolated cardiomyocytes, and participates in desensitization. Endocrinology 144(10):4586–4596. https://doi.org/10.1210/en.2003-0046
Ferrario CM (2006) Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J Renin-Angiotensin-Aldosterone Syst 7(1):3–14. https://doi.org/10.3317/jraas.2006.003
Agha M, Agha R (2017) The rising prevalence of obesity: part a: impact on public health. International journal of surgery Oncology 2(7):e17. https://doi.org/10.1097/IJ9.0000000000000017
Upadhyay J, Farr O, Perakakis N, Ghaly W, Mantzoros C (2018) Obesity as a disease. Med Clin North Am 102(1):13–33. https://doi.org/10.1016/j.mcna.2017.08.004
Palanivel R, Fullerton MD, Galic S, Honeyman J, Hewitt KA, Jorgensen SB, Steinberg GR (2012) Reduced Socs3 expression in adipose tissue protects female mice against obesity-induced insulin resistance. Diabetologia 55(11):3083–3093. https://doi.org/10.1007/s00125-012-2665-3
Pedroso JA, Buonfiglio DC, Cardinali LI, Furigo IC, Ramos-Lobo AM, Tirapegui J, Elias CF, Donato J Jr (2014) Inactivation of SOCS3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Mol Metab 3(6):608–618. https://doi.org/10.1016/j.molmet.2014.06.001
Zampieri TT, da Silva TE, de Paula Romeu D, Torrao AS, Donato J Jr (2016) SOCS3 expression within leptin receptor-expressing cells regulates food intake and leptin sensitivity but does not affect weight gain in pregnant mice consuming a high-fat diet. Physiol Behav 157:109–115. https://doi.org/10.1016/j.physbeh.2016.01.039
Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS (1998) Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1(4):619–625
Santillan-Benitez JG, Mendieta-Zeron H, Gomez-Olivan LM, Quiroz AO, Torres-Juarez JJ, Gonzalez-Banales JM (2014) JAK2, STAT3 and SOCS3 gene expression in women with and without breast cancer. Gene 547(1):70–76. https://doi.org/10.1016/j.gene.2014.06.025
Ahima RS, Stanley TL, Khor VK, Zanni MV, Grinspoon SK (2011) Estrogen sulfotransferase is expressed in subcutaneous adipose tissue of obese humans in association with TNF-alpha and SOCS3. J Clin Endocrinol Metab 96(7):E1153–E1158. https://doi.org/10.1210/jc.2010-2903
Xu K, Zhang X, Wang Z, Hu Y, Sinha R (2018) Epigenome-wide association analysis revealed that SOCS3 methylation influences the effect of cumulative stress on obesity. Biol Psychol 131:63–71. https://doi.org/10.1016/j.biopsycho.2016.11.001
Zhou Y, Rui L (2013) Leptin signaling and leptin resistance. Front Med 7(2):207–222. https://doi.org/10.1007/s11684-013-0263-5
Lubis AR, Widia F, Soegondo S, Setiawati A (2008) The role of SOCS-3 protein in leptin resistance and obesity. Acta Med Indones 40(2):89–95
Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E (2001) SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem 276(51):47944–47949. https://doi.org/10.1074/jbc.M104602200
Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E (2000) SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem 275(21):15985–15991
Klok MD, Jakobsdottir S, Drent ML (2007) The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev 8(1):21–34. https://doi.org/10.1111/j.1467-789X.2006.00270.x
Shimizu H, Oh IS, Okada S, Mori M (2007) Leptin resistance and obesity. Endocr J 54(1):17–26
El-Haschimi K, Lehnert H (2003) Leptin resistance - or why leptin fails to work in obesity. Exp Clin Endocrinol Diabetes 111(1):2–7. https://doi.org/10.1055/s-2003-37492
Guo S (2014) Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol 220(2):T1–T23. https://doi.org/10.1530/JOE-13-0327
Rui L, Yuan M, Frantz D, Shoelson S, White MF (2002) SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem 277(44):42394–42398. https://doi.org/10.1074/jbc.C200444200
Zampieri TT, Ramos-Lobo AM, Furigo IC, Pedroso JA, Buonfiglio DC, Donato J Jr (2015) SOCS3 deficiency in leptin receptor-expressing cells mitigates the development of pregnancy-induced metabolic changes. Molecular metabolism 4(3):237–245. https://doi.org/10.1016/j.molmet.2014.12.005
Pedroso JA, Silveira MA, Lima LB, Furigo IC, Zampieri TT, Ramos-Lobo AM, Buonfiglio DC, Teixeira PD, Frazao R, Donato J Jr (2016) Changes in leptin signaling by SOCS3 modulate fasting-induced Hyperphagia and weight regain in mice. Endocrinology 157(10):3901–3914. https://doi.org/10.1210/en.2016-1038
Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A (2004) Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10(7):739–743. https://doi.org/10.1038/nm1071
Jorgensen SB, O'Neill HM, Sylow L, Honeyman J, Hewitt KA, Palanivel R, Fullerton MD, Oberg L, Balendran A, Galic S, van der Poel C, Trounce IA, Lynch GS, Schertzer JD, Steinberg GR (2013) Deletion of skeletal muscle SOCS3 prevents insulin resistance in obesity. Diabetes 62(1):56–64. https://doi.org/10.2337/db12-0443
Ortega FB, Lavie CJ, Blair SN (2016) Obesity and cardiovascular disease. Circ Res 118(11):1752–1770. https://doi.org/10.1161/CIRCRESAHA.115.306883
Lipek T, Igel U, Gausche R, Kiess W, Grande G (2015) Obesogenic environments: environmental approaches to obesity prevention. J Pediatr Endocrinol Metab 28(5–6):485–495. https://doi.org/10.1515/jpem-2015-0127
Myers MG Jr, Olson DP (2012) Central nervous system control of metabolism. Nature 491(7424):357–363. https://doi.org/10.1038/nature11705
Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG (2000) Central nervous system control of food intake. Nature 404(6778):661–671. https://doi.org/10.1038/35007534
Munzberg H, Myers MG, Jr. (2005) Molecular and anatomical determinants of central leptin resistance. Nat Neurosci 8 (5):566–570. doi:https://doi.org/10.1038/nn1454
Friedman JM, Halaas JL (1998) Leptin and the regulation of body weight in mammals. Nature 395(6704):763–770. https://doi.org/10.1038/27376
Zhang YY, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homolog. Nature 372(6505):425–432. https://doi.org/10.1038/372425a0
Kwon O, Kim KW, Kim MS (2016) Leptin signalling pathways in hypothalamic neurons. Cell Mol Life Sci 73(7):1457–1477. https://doi.org/10.1007/s00018-016-2133-1
Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM (1997) Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A 94(16):8878–8883
Reed AS, Unger EK, Olofsson LE, Piper ML, Myers MG Jr, Xu AW (2010) Functional role of suppressor of cytokine signaling 3 upregulation in hypothalamic leptin resistance and long-term energy homeostasis. Diabetes 59(4):894–906. https://doi.org/10.2337/db09-1024
Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle JN, Yoshimura A (1999) The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J 18 (5):1309–1320. doi:DOI 10.1093/emboj/18.5.1309
Fruhbeck G (2006) Intracellular signalling pathways activated by leptin. Biochem. J. 393(Pt 1):7–20. https://doi.org/10.1042/BJ20051578
Krol E, Speakman JR (2007) Regulation of body mass and adiposity in the field vole, Microtus agrestis: a model of leptin resistance. J Endocrinol 192(2):271–278. https://doi.org/10.1677/JOE-06-0074
Norman JE, Reynolds RM (2011) The consequences of obesity and excess weight gain in pregnancy. Proc Nutr Soc 70(4):450–456. https://doi.org/10.1017/S0029665111003077
Ladyman SR, Fieldwick DM, Grattan DR (2012) Suppression of leptin-induced hypothalamic JAK/STAT signalling and feeding response during pregnancy in the mouse. Reproduction 144(1):83–90. https://doi.org/10.1530/REP-12-0112
Nagaishi VS, Cardinali LI, Zampieri TT, Furigo IC, Metzger M, Donato J Jr (2014) Possible crosstalk between leptin and prolactin during pregnancy. Neuroscience 259:71–83. https://doi.org/10.1016/j.neuroscience.2013.11.050
Ayyad C, Andersen T (2000) Long-term efficacy of dietary treatment of obesity: a systematic review of studies published between 1931 and 1999. Obes Rev 1(2):113–119
Mann T, Tomiyama AJ, Westling E, Lew AM, Samuels B, Chatman J (2007) Medicare's search for effective obesity treatments: diets are not the answer. Am Psychol 62(3):220–233. https://doi.org/10.1037/0003-066X.62.3.220
Hambly C, Duncan JS, Archer ZA, Moar KM, Mercer JG, Speakman JR (2012) Repletion of TNFalpha or leptin in calorically restricted mice suppresses post-restriction hyperphagia. Dis Model Mech 5(1):83–94. https://doi.org/10.1242/dmm.007781
Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS (2004) Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10(7):734–738. https://doi.org/10.1038/nm1072
Briancon N, McNay DE, Maratos-Flier E, Flier JS (2010) Combined neural inactivation of suppressor of cytokine signaling-3 and protein-tyrosine phosphatase-1B reveals additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes 59(12):3074–3084. https://doi.org/10.2337/db10-0481
Liu ZJ, Bian J, Zhao YL, Zhang X, Zou N, Li D (2011) Lentiviral vector-mediated knockdown of SOCS3 in the hypothalamus protects against the development of diet-induced obesity in rats. Diabetes Obes Metab 13(10):885–892. https://doi.org/10.1111/j.1463-1326.2011.01419.x
de Backer MW, Brans MA, van Rozen AJ, van der Zwaal EM, Luijendijk MC, Garner KG, de Krom M, van Beekum O, la Fleur SE, Adan RA (2010) Suppressor of cytokine signaling 3 knockdown in the mediobasal hypothalamus: counterintuitive effects on energy balance. J Mol Endocrinol 45(5):341–353. https://doi.org/10.1677/JME-10-0057
Matarazzo V, Schaller F, Nedelec E, Benani A, Penicaud L, Muscatelli F, Moyse E, Bauer S (2012) Inactivation of Socs3 in the hypothalamus enhances the hindbrain response to endogenous satiety signals via oxytocin signaling. J Neurosci 32(48):17097–17107. https://doi.org/10.1523/JNEUROSCI.1669-12.2012
Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS (2006) Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab 4(2):123–132. https://doi.org/10.1016/j.cmet.2006.06.010
Zhang R, Dhillon H, Yin H, Yoshimura A, Lowell BB, Maratos-Flier E, Flier JS (2008) Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight. Endocrinology 149(11):5654–5661. https://doi.org/10.1210/en.2008-0805
Pedroso JAB, de Mendonca POR, Fortes MAS, Tomaz I, Pecorali VL, Auricino TB, Costa IC, Lima LB, Furigo IC, Bueno DN, Ramos-Lobo AM, Lotfi CFP, Donato J Jr (2017) SOCS3 expression in SF1 cells regulates adrenal differentiation and exercise performance. J Endocrinol 235(3):207–222. https://doi.org/10.1530/JOE-17-0255
Ramos-Lobo AM, Teixeira PDS, Furigo IC, Donato J Jr (2017) SOCS3 ablation in SF1 cells causes modest metabolic effects during pregnancy and lactation. Neuroscience 365:114–124. https://doi.org/10.1016/j.neuroscience.2017.09.048
Ramos-Lobo AM, Furigo IC, Teixeira PDS, Zampieri TT, Wasinski F, Buonfiglio DC, Donato J Jr (2018) Maternal metabolic adaptations are necessary for normal offspring growth and brain development. Phys. Rep. 6(5). https://doi.org/10.14814/phy2.13643
Torisu T, Sato N, Yoshiga D, Kobayashi T, Yoshioka T, Mori H, Iida M, Yoshimura A (2007) The dual function of hepatic SOCS3 in insulin resistance in vivo. Genes Cells 12(2):143–154. https://doi.org/10.1111/j.1365-2443.2007.01044.x
Sachithanandan N, Fam BC, Fynch S, Dzamko N, Watt MJ, Wormald S, Honeyman J, Galic S, Proietto J, Andrikopoulos S, Hevener AL, Kay TW, Steinberg GR (2010) Liver-specific suppressor of cytokine signaling-3 deletion in mice enhances hepatic insulin sensitivity and lipogenesis resulting in fatty liver and obesity. Hepatology 52(5):1632–1642. https://doi.org/10.1002/hep.23861
Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS (1999) The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem 274(42):30059–30065
Ladyman SR, Augustine RA, Grattan DR (2010) Hormone interactions regulating energy balance during pregnancy. J Neuroendocrinol 22(7):805–817. https://doi.org/10.1111/j.1365-2826.2010.02017.x
Ladyman SR, Grattan DR (2005) Suppression of leptin receptor messenger ribonucleic acid and leptin responsiveness in the ventromedial nucleus of the hypothalamus during pregnancy in the rat. Endocrinology 146(9):3868–3874. https://doi.org/10.1210/en.2005-0194
Ladyman SR, Grattan DR (2016) Central effects of leptin on glucose homeostasis are modified during pregnancy in the rat. J Neuroendocrinol 28(10). https://doi.org/10.1111/jne.12431
Beauloye V, Willems B, de Coninck V, Frank SJ, Edery M, Thissen JP (2002) Impairment of liver GH receptor signaling by fasting. Endocrinology 143(3):792–800. https://doi.org/10.1210/endo.143.3.8692
Ueki K, Kondo T, Kahn CR (2004) Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 24(12):5434–5446. https://doi.org/10.1128/MCB.24.12.5434-5446.2004
Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA (2003) Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 278(16):13740–13746. https://doi.org/10.1074/jbc.M210689200
Shi H, Tzameli I, Bjorbaek C, Flier JS (2004) Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. J Biol Chem 279(33):34733–34740. https://doi.org/10.1074/jbc.M403886200
Yang Z, Hulver M, McMillan RP, Cai L, Kershaw EE, Yu L, Xue B, Shi H (2012) Regulation of insulin and leptin signaling by muscle suppressor of cytokine signaling 3 (SOCS3). PLoS One 7(10):e47493. https://doi.org/10.1371/journal.pone.0047493
Tups A, Benzler J, Sergi D, Ladyman SR, Williams LM (2017) Central regulation of glucose homeostasis. Compr Physiol 7(2):741–764. https://doi.org/10.1002/cphy.c160015
Donato J Jr (2012) The central nervous system as a promising target to treat diabetes mellitus. Curr Top Med Chem 12(19):2070–2081
Havrankova J, Roth J, Brownstein M (1978) Insulin receptors are widely distributed in the central nervous system of the rat. Nature 272(5656):827–829
Obici S, Zhang BB, Karkanias G, Rossetti L (2002) Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8(12):1376–1382. https://doi.org/10.1038/nm798
Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR (2000) Role of brain insulin receptor in control of body weight and reproduction. Science 289(5487):2122–2125
Denroche HC, Huynh FK, Kieffer TJ (2012) The role of leptin in glucose homeostasis. J Diabetes Investig 3(2):115–129. https://doi.org/10.1111/j.2040-1124.2012.00203.x
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F (1995) Effects of the obese gene product on body weight regulation in Ob/Ob mice. Science 269(5223):540–543
Hedbacker K, Birsoy K, Wysocki RW, Asilmaz E, Ahima RS, Farooqi IS, Friedman JM (2010) Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell Metab 11(1):11–22. https://doi.org/10.1016/j.cmet.2009.11.007
Hidaka S, Yoshimatsu H, Kondou S, Tsuruta Y, Oka K, Noguchi H, Okamoto K, Sakino H, Teshima Y, Okeda T, Sakata T (2002) Chronic central leptin infusion restores hyperglycemia independent of food intake and insulin level in streptozotocin-induced diabetic rats. FASEB J 16(6):509–518. https://doi.org/10.1096/fj.01-0164com
Meek TH, Morton GJ (2012) Leptin, diabetes, and the brain Indian journal of endocrinology and metabolism. 16(Suppl 3):S534–S542. https://doi.org/10.4103/2230-8210.105568
Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L (2005) Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54(11):3182–3189. https://doi.org/10.2337/diabetes.54.11.3182
Seufert J (2004) Leptin effects on pancreatic beta-cell gene expression and function. Diabetes 53(Suppl 1):S152–S158
Scheller EL, Hankenson KD, Reuben JS, Krebsbach PH (2011) Zoledronic acid inhibits macrophage SOCS3 expression and enhances cytokine production. J Cell Biochem 112(11):3364–3372. https://doi.org/10.1002/jcb.23267
Whitaker M, Guo J, Kehoe T, Benson G (2012) Bisphosphonates for osteoporosis--where do we go from here? N Engl J Med 366(22):2048–2051. https://doi.org/10.1056/NEJMp1202619
He L, Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5(7):522–531. https://doi.org/10.1038/nrg1379
Soriano A, Jubierre L, Almazan-Moga A, Molist C, Roma J, de Toledo JS, Gallego S, Segura MF (2013) microRNAs as pharmacological targets in cancer. Pharmacol Res 75:3–14. https://doi.org/10.1016/j.phrs.2013.03.006
Li Y, Luo T, Wang L, Wu J, Guo S (2016) MicroRNA-19a-3p enhances the proliferation and insulin secretion, while it inhibits the apoptosis of pancreatic beta cells via the inhibition of SOCS3. Int J Mol Med 38(5):1515–1524. https://doi.org/10.3892/ijmm.2016.2748
Bao L, Fu X, Si M, Wang Y, Ma R, Ren X, Lv H (2015) MicroRNA-185 targets SOCS3 to inhibit beta-cell dysfunction in diabetes. PLoS One 10(2):e0116067. https://doi.org/10.1371/journal.pone.0116067
Davalos A, Suarez Y (2013) MiRNA-based therapy: from bench to bedside. Pharmacol Res 75:1–2. https://doi.org/10.1016/j.phrs.2013.06.010
Nybo L (2003) CNS fatigue and prolonged exercise: effect of glucose supplementation. Med Sci Sports Exerc 35(4):589–594. https://doi.org/10.1249/01.MSS.0000058433.85789.66
Fujikawa T, Castorena CM, Lee S, Elmquist JK (2017) The hypothalamic regulation of metabolic adaptations to exercise. J Neuroendocrinol 29(10). https://doi.org/10.1111/jne.12533
Beltowski J, Wojcicka G, Marciniak A, Jamroz A (2004) Oxidative stress, nitric oxide production, and renal sodium handling in leptin-induced hypertension. Life Sci 74(24):2987–3000. https://doi.org/10.1016/j.lfs.2003.10.029
Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI (1997) Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest 100(2):270–278. https://doi.org/10.1172/JCI119532
Zhang DY, Anderson AS (2014) The sympathetic nervous system and heart failure. Cardiol Clin 32(1):33–45, vii. https://doi.org/10.1016/j.ccl.2013.09.010
Acknowledgements
We would like to thank the São Paulo Research Foundation (FAPESP, Brazil) for supporting this study (grant numbers: 2013/25032-2, 2014/11752-6 and 2017/02983-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pedroso, J.A., Ramos-Lobo, A.M. & Donato, J. SOCS3 as a future target to treat metabolic disorders. Hormones 18, 127–136 (2019). https://doi.org/10.1007/s42000-018-0078-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42000-018-0078-5