Abstract
Having been extensively studied in the past five decades, lithium–sulfur (Li–S) batteries possess a high theoretical energy density (~ 2600 Wh kg−1), offering the potential to power advanced twenty-first-century technologies such as electric vehicles and drones. However, a surprisingly complex engineering challenge remains in the application of these batteries: the identification of appropriate electrolytes that are compatible with both sulfur cathodes and lithium metal anodes. Non-aqueous liquid electrolytes, typically consisting of a lithium salt dissolved in an organic solvent, cannot themselves demonstrate effective electrochemical performances. Researchers have found that functional electrolytes offer unique possibilities to engineer the surface chemistries of sulfur cathodes and lithium anodes to enable long-term cycling. In this article, recent progresses in the development of functional non-aqueous liquid electrolytes in Li–S batteries are reviewed, including novel co-solvent solutions, lithium salts, additives, redox mediators, and ionic liquids. Characterization techniques and interpretations are cited to elucidate the effects of these components on the kinetics of sulfur redox reactions, lithium passivation, and cell performance. The information presented and the studies highlighted in this review will provide guidance for future optimized electrolyte designs.
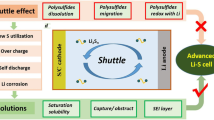
Graphical Abstract










Similar content being viewed by others
References
Blomgren, G.E.: The development and future of lithium ion batteries. J. Electrochem. Soc. 164, A5019–A5025 (2017)
Etacheri, V., Marom, R., Elazari, R., et al.: Challenges in the development of advanced Li-ion batteries: a review. Energy Environ. Sci. 4, 3243–3262 (2011)
Nitta, N., Wu, F., Lee, J.T., et al.: Li-ion battery materials: present and future. Mater. Today 18, 252–264 (2015)
Bruce, P., Freunberger, S.: Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11, 19–30 (2012)
Manthiram, A., Fu, Y., Chung, S.H., et al.: Rechargeable lithium–sulfur batteries. Chem. Rev. 114, 11751–11787 (2014)
Pang, Q., Liang, X., Kwok, C.Y., et al.: Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 1, 16132 (2016)
Cheon, S.E., Cho, J.H., Ko, K.S., et al.: Structural factors of sulfur cathodes with poly(ethylene oxide) binder for performance of rechargeable lithium sulfur batteries. J. Electrochem. Soc. 149, A1437 (2002)
Cheon, S.E., Choi, S.S., Han, J.S., et al.: Capacity fading mechanisms on cycling a high-capacity secondary sulfur cathode. J. Electrochem. Soc. 151, A2067–A2073 (2004)
Song, J., Xu, T., Gordin, M.L., et al.: Nitrogen-doped mesoporous carbon promoted chemical adsorption of sulfur and fabrication of high-areal-capacity sulfur cathode with exceptional cycling stability for lithium–sulfur batteries. Adv. Funct. Mater. 24, 1243–1250 (2014)
Yin, Y.X., Xin, S., Guo, Y.G., et al.: Lithium–sulfur batteries: electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. Engl. 52, 13186–13200 (2013)
Akridge, J.: Li/S fundamental chemistry and application to high-performance rechargeable batteries. Solid State Ionics 175, 243–245 (2004)
Mikhaylik, Y.V., Akridge, J.R.: Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc. 151, A1969–A1976 (2004)
Yu, X., Pan, H., Zhou, Y., et al.: Direct observation of the redistribution of sulfur and polysufides in Li–S batteries during the first cycle by in situ X-ray fluorescence microscopy. Adv. Energy Mater. 5, 1500072 (2015)
Lu, Y.C., He, Q., Gasteiger, H.A.: Probing the lithium–sulfur redox reactions: a rotating-ring disk electrode study. J. Phys. Chem. C 118, 5733–5741 (2014)
Barchasz, C., Molton, F., Duboc, C., et al.: Lithium/sulfur cell discharge mechanism: an original approach for intermediate species identification. Anal. Chem. 84, 3973–3980 (2012)
Hagen, M., Schiffels, P., Hammer, M., et al.: In-situ Raman investigation of polysulfide formation in Li–S cells. J. Electrochem. Soc. 160, A1205–A1214 (2013)
See, K.A., Leskes, M., Griffin, J.M., et al.: Ab initio structure search and in situ 7Li NMR studies of discharge products in the Li–S battery system. J. Am. Chem. Soc. 136, 16368–16377 (2014)
Pascal, T.A., Wujcik, K.H., Velasco-Velez, J., et al.: X-ray absorption spectra of dissolved polysulfides in lithium–sulfur batteries from first-principles. J. Phys. Chem. Lett. 5, 1547–1551 (2014)
Wang, Q., Zheng, J., Walter, E., Zuo, P.: Direct observation of sulfur radicals as reaction media in lithium sulfur batteries. J. Electrochem. Soc. 162, A474–A478 (2015)
Zou, Q., Lu, Y.C.: Solvent-dictated lithium sulfur redox reactions: An operando UV-vis spectroscopic study. J Phys Chem Lett. 7, 1518–1525 (2016)
Cuisinier, M., Hart, C., Balasubramanian, M., et al.: Radical or not radical: revisiting lithium–sulfur electrochemistry in nonaqueous electrolytes. Adv. Energy Mater. 5, 1401801 (2015)
Xu, R., Belharouak, I., Zhang, X., et al.: Insight into sulfur reactions in Li–S batteries. ACS Appl. Mater. Interfaces. 6, 21938–21945 (2014)
Lin, D., Liu, Y., Cui, Y.: Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017)
Guo, Y., Li, H., Zhai, T.: Reviving lithium–metal anodes for next-generation high-energy batteries. Adv. Mater. 29, 1–25 (2017)
Yamin, H., Gorenshtein, A., Penciner, J., et al.: Lithium sulfur battery oxidation/reduction mechanisms of polysulfides in THF solutions. J. Electrochem. Soc. 135, 1045–1048 (1988)
Bieker, G., Wellmann, J., Kolek, M., et al.: Influence of cations in lithium and magnesium polysulphide solutions: dependence of the solvent chemistry. Phys. Chem. Chem. Phys. 19, 11152–11162 (2017)
Barchasz, C., Leprêtre, J.C., Patoux, S., et al.: Electrochemical properties of ether-based electrolytes for lithium/sulfur rechargeable batteries. Electrochim. Acta 89, 737–743 (2013)
Schneider, H., Gollub, C., Weiss, T., et al.: On the electrode potentials in lithium–sulfur batteries and their solvent-dependence. J. Electrochem. Soc. 161, A1399–A1406 (2014)
Pan, H., Wei, X., Henderson, W.A., et al.: On the way toward understanding solution chemistry of lithium polysulfides for high energy Li–S redox flow batteries. Adv. Energy Mater. 5, 1–7 (2015)
Fan, F.Y., Pan, M.S., Lau, K.C., et al.: Solvent effects on polysulfide redox kinetics and ionic conductivity in lithium–sulfur batteries. J. Electrochem. Soc. 163, A3111–A3116 (2016)
Pan, H., Chen, J., Cao, R., et al.: Non-encapsulation approach for high-performance Li–S batteries through controlled nucleation and growth. Nat. Energy. 2, 813–820 (2017)
Qu, C., Chen, Y., Yang, X., et al.: LiNO3-free electrolyte for Li–S battery: a solvent of choice with low Ksp of polysulfide and low dendrite of lithium. Nano Energy 39, 262–272 (2017)
Kim, S., Jung, Y., Lim, H.S.: The effect of solvent component on the discharge performance of Lithium–sulfur cell containing various organic electrolytes. Electrochim. Acta 50, 889–892 (2004)
Barchasz, C., Leprêtre, J.C., Patoux, S., et al.: Revisiting TEGDME/DIOX binary electrolytes for lithium/sulfur batteries: importance of solvation ability and additives. J. Electrochem. Soc. 160, A430–A436 (2013)
Aurbach, D., Pollak, E., Elazari, R., et al.: On the surface chemical aspects of very high energy density, rechargeable Li–sulfur batteries. J. Electrochem. Soc. 156, A694 (2009)
Huang, F., Ma, G., Wen, Z., et al.: Enhancing metallic lithium battery performance by tuning the electrolyte solution structure. J. Mater. Chem. A. 6, 1612–1620 (2018)
Azimi, N., Weng, W., Takoudis, C., et al.: Improved performance of lithium–sulfur battery with fluorinated electrolyte. Electrochem. Commun. 37, 96–99 (2013)
Zu, C., Azimi, N., Zhang, Z., et al.: Insight into lithium–metal anodes in lithium–sulfur batteries with a fluorinated ether electrolyte. J. Mater. Chem. A. 3, 14864–14870 (2015)
Azimi, N., Xue, Z., Bloom, I., Gordin, M.L.: Understanding the effect of a fluorinated ether on the performance of lithium–sulfur batteries. ACS Appl. Mater. Interfaces. 7, 9169–9177 (2015)
Chen, S., Yu, Z., Gordin, M.L., et al.: A fluorinated ether electrolyte enabled high performance prelithiated graphite/sulfur batteries. ACS Appl. Mater. Interfaces 9, 6959–6966 (2017)
Gordin, M.L., Dai, F., Chen, S., et al.: Bis(2,2,2-trifluoroethyl) ether as an electrolyte co-solvent for mitigating self-discharge in lithium–sulfur batteries. ACS Appl. Mater. Interfaces 6, 8006–8010 (2014)
Talian, S.D., Jeschke, S., Vizintin, A., et al.: Fluorinated ether based electrolyte for high-energy lithium–sulfur batteries: Li+ solvation role behind reduced polysulfide solubility. Chem. Mater. 29, 10037–10044 (2017)
Gu, S., Qian, R., Jin, J., et al.: Suppressing the dissolution of polysulfides with cosolvent fluorinated diether towards high-performance lithium sulfur batteries. Phys. Chem. Chem. Phys. 18, 29293–29299 (2016)
Yim, T., Park, M.S., Yu, J.S., et al.: Effect of chemical reactivity of polysulfide toward carbonate-based electrolyte on the electrochemical performance of Li–S batteries. Electrochim. Acta 107, 454–460 (2013)
Liang, X., Wen, Z., Liu, Y., et al.: Improved cycling performances of lithium sulfur batteries with LiNO3-modified electrolyte. J. Power Sour. 196, 9839–9843 (2011)
Zhang, S.S., Read, J.A.: A new direction for the performance improvement of rechargeable lithium/sulfur batteries. J. Power Sour. 200, 77–82 (2012)
Xiong, S., Xie, K., Diao, Y., et al.: Characterization of the solid electrolyte interphase on lithium anode for preventing the shuttle mechanism in lithium–sulfur batteries. J. Power Sour. 246, 840–845 (2014)
Cheng, X.B., Yan, C., Chen, X., et al.: Implantable solid electrolyte interphase in lithium–metal batteries. Chemistry 2, 258–270 (2017)
Li, W., Yao, H., Yan, K., et al.: The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 6, 7436 (2015)
Diao, Y., Xie, K., Xiong, S., et al.: Shuttle phenomenon the irreversible oxidation mechanism of sulfur active material in Li–S battery. J. Power Sour. 235, 181–186 (2013)
Zhang, S.S.: Role of LiNO3 in rechargeable lithium/sulfur battery. Electrochim. Acta 70, 344–348 (2012)
Rosenman, A., Elazari, R., Salitra, G., et al.: The effect of interactions and reduction products of LiNO3, the anti-shuttle agent, in Li–S battery systems. J. Electrochem. Soc. 162, A470–A473 (2015)
Wu, F., Lee, J.T., Nitta, N., et al.: Lithium iodide as a promising electrolyte additive for lithium–sulfur batteries: mechanisms of performance enhancement. Adv. Mater. 27, 101–108 (2015)
Jozwiuk, A., Berkes, B.B., Weiß, T., et al.: The critical role of lithium nitrate in the gas evolution of lithium–sulfur batteries. Energy Environ. Sci. 9, 2603–2608 (2016)
Xiong, S., Xie, K., Diao, Y., et al.: Oxidation process of polysulfides in charge process for lithium–sulfur batteries. Ionics 18, 867–872 (2012)
Wu, F., Qian, J., Chen, R., et al.: An effective approach to protect lithium anode and improve cycle performance for Li–S batteries. ACS Appl. Mater. Interfaces 6, 15542–15549 (2014)
Fan, L., Zhuang, H.L., Gao, L., et al.: Regulating Li deposition at artificial solid electrolyte interphases. J. Mater. Chem. A. 5, 3483–3492 (2017)
Zhang, X.Q., Chen, X., Xu, R., et al.: Columnar lithium metal anodes. Angew. Chemie. Int. Ed. 56, 14207–14211 (2017)
Lin, D., Liu, Y., Chen, W., et al.: Conformal lithium fluoride protection layer on three-dimensional lithium by nonhazardous gaseous reagent freon. Nano Lett. 17, 3731–3737 (2017)
Xu, R., Zhang, X.Q., Cheng, X.B., et al.: Artificial soft–rigid protective layer for dendrite-free lithium metal anode. Adv. Funct. Mater. 28, 1–7 (2018)
Liang, X., Pang, Q., Kochetkov, I.R., et al.: A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 2, 17119 (2017)
Wu, F., Thieme, S., Ramanujapuram, A., et al.: Toward in situ protected sulfur cathodes by using lithium bromide and pre-charge. Nano Energy 40, 170–179 (2017)
Bron, P., Johansson, S., Zick, K., et al.: Li10SnP2S12: an affordable lithium superionic conductor. J. Am. Chem. Soc. 135, 15694–15697 (2013)
Kanno, R., Murayama, M.: Lithium ionic conductor thio-LISICON: the Li2S–GeS2P2S5 system. J. Electrochem. Soc. 148, A742 (2001)
Yamane, H., Shibata, M., Shimane, Y., et al.: Crystal structure of a superionic conductor, Li7P3S11. Solid State Ionics 178, 1163–1167 (2007)
Lin, Z., Liu, Z., Fu, W., et al.: Phosphorous pentasulfide as a novel additive for high-performance lithium–sulfur batteries. Adv. Funct. Mater. 23, 1064–1069 (2013)
Pang, Q., Liang, X., Shyamsunder, A., et al.: An in vivo formed solid electrolyte surface layer enables stable plating of Li metal. Joule 1, 871–886 (2017)
Wang, J., Lin, F., Jia, H., et al.: Towards a safe lithium–sulfur battery with a flame-inhibiting electrolyte and a sulfur-based composite cathode. Angew. Chem. Int. Ed. Engl. 53, 10099–10104 (2014)
Lin, F., Wang, J., Jia, H., et al.: Nonflammable electrolyte for rechargeable lithium battery with sulfur based composite cathode materials. J. Power Sour. 223, 18–22 (2013)
Meini, S., Elazari, R., Rosenman, A., et al.: The use of redox mediators for enhancing utilization of Li2S cathodes for advanced Li–S battery systems. J. Phys. Chem. Lett. 5, 915–918 (2014)
Chen, S., Dai, F., Gordin, M.L., et al.: Functional organosulfide electrolyte promotes an alternate reaction pathway to achieve high performance in lithium–sulfur batteries. Angew. Chemie. Int. Ed. 55, 4231–4235 (2016)
Chen, S., Gao, Y., Yu, Z., et al.: High capacity of lithium–sulfur batteries at low electrolyte/sulfur ratio enabled by an organosulfide containing electrolyte. Nano Energy 31, 418–423 (2017)
Li, G., Gao, Y., He, X., et al.: Organosulfide-plasticized solid-electrolyte interphase layer enables stable lithium metal anodes for long-cycle lithium–sulfur batteries. Nat. Commun. 8, 850 (2017)
Trofimov, B.A., Markova, M.V., Morozova, L.V., et al.: Protected bis(hydroxyorganyl) polysulfides as modifiers of Li/S battery electrolyte. Electrochim. Acta 56, 2458–2463 (2011)
Chang, C.H., Chung, S.H., Han, P., et al.: Oligoanilines as a suppressor of polysulfide shuttling in lithium–sulfur batteries. Mater. Horiz. 4, 908–914 (2017)
Wu, H.L., Shin, M., Liu, Y.M., et al.: Thiol-based electrolyte additives for high-performance lithium–sulfur batteries. Nano Energy 32, 50–58 (2017)
Wu, H.L., Huff, L.A., Gewirth, A.A.: In situ Raman spectroscopy of sulfur speciation in lithium–sulfur batteries. ACS Appl. Mater. Interfaces 7, 1709–1719 (2015)
Chen, Y., Zhang, H., Xu, W., et al.: Polysulfide stabilization: a pivotal strategy to achieve high energy density Li–S batteries with long cycle life. Adv. Funct. Mater. 28, 1704987 (2018)
Liu, M., Ren, Y.X., Jiang, H.R., et al.: An efficient Li2S-based lithium-ion sulfur battery realized by a bifunctional electrolyte additive. Nano Energy 40, 240–247 (2017)
Ren, Y.X., Zhao, T.S., Liu, M., et al.: A self-cleaning Li–S battery enabled by a bifunctional redox mediator. J. Power Sources 361, 203–210 (2017)
Kim, K.R., Lee, K.S., Ahn, C.Y., et al.: Discharging a Li–S battery with ultra-high sulphur content cathode using a redox mediator. Sci. Rep. 6, 32433 (2016)
Li, J., Yang, L., Yang, S., et al.: The application of redox targeting principles to the design of rechargeable Li–S flow batteries. Adv. Energy Mater. 5, 1501808 (2015)
Li, J., Yang, L., Yuan, B., et al.: Combined mediator and electrochemical charging and discharging of redox targeting lithium–sulfur flow batteries. Mater. Today Energy 5, 15–21 (2017)
Choi, W., Im, D., Park, M.S., et al.: Keggin-type polyoxometalates as bidirectional redox mediators for rechargeable batteries. Electrochemistry 84, 882–886 (2016)
Frischmann, P.D., Gerber, L.C.H., Doris, S.E., et al.: Supramolecular perylene bisimide-polysulfide gel networks as nanostructured redox mediators in dissolved polysulfide lithium–sulfur batteries. Chem. Mater. 27, 6765–6770 (2015)
Gerber, L.C.H., Frischmann, P.D., Fan, F.Y., et al.: Three-dimensional growth of Li2S in lithium–sulfur batteries promoted by a redox mediator. Nano Lett. 16, 549–554 (2016)
Seddon, K.R.: Ionic liquids for clean technology. J. Chem. Technol. Biotechnol. 68, 351–356 (1997)
Watanabe, M., Thomas, M.L., Zhang, S., et al.: Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 117, 7190–7239 (2017)
Armand, M., Endres, F., MacFarlane, D.R., et al.: Ionic–liquid materials for the electrochemical challenges of the future. Nat. Mater. 8, 621–629 (2009)
Park, J.W., Ueno, K., Tachikawa, N., et al.: Ionic liquid electrolytes for lithium–sulfur batteries. J. Phys. Chem. C 117, 20531–20541 (2013)
Ma, G., Wen, Z., Jin, J., et al.: The enhanced performance of Li–S battery with P14YRTFSI-modified electrolyte. Solid State Ionics 262, 174–178 (2014)
Eshetu, G.G., Grugeon, S., Gachot, G., et al.: LiFSI vs. LiPF6 electrolytes in contact with lithiated graphite: Comparing thermal stabilities and identification of specific SEI-reinforcing additives. Electrochim. Acta 102, 133–141 (2013)
Wang, L., Byon, H.R.: N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide-based organic electrolyte for high performance lithium–sulfur batteries. J. Power Sour. 236, 207–214 (2013)
Xiong, S., Scheers, J., Aguilera, L., et al.: Role of organic solvent addition to ionic liquid electrolytes for lithium–sulphur batteries. RSC Adv. 5, 2122–2128 (2015)
Wang, L., Liu, J., Yuan, S., et al.: To mitigate self-discharge of lithium–sulfur batteries by optimizing ionic liquid electrolytes. Energy Environ. Sci. 9, 224–231 (2016)
Barghamadi, M., Best, A.S., Bhatt, A.I., et al.: Effect of LiNO3 additive and pyrrolidinium ionic liquid on the solid electrolyte interphase in the lithium–sulfur battery. J. Power Sour. 295, 212–220 (2015)
Zu, C., Manthiram, A.: Stabilized lithium–metal surface in a polysulfide-rich environment of lithium–sulfur batteries. J. Phys. Chem. Lett. 5, 2522–2527 (2014)
Liang, X., Hart, C., Pang, Q., et al.: A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 6, 5682 (2015)
Conder, J., Bouchet, R., Trabesinger, S., et al.: Direct observation of lithium polysulfides in lithium–sulfur batteries using operando X-ray diffraction. Nat. Energy 2, 17069 (2017)
Choi, J.W., Cheruvally, G., Kim, D.S., et al.: Rechargeable lithium/sulfur battery with liquid electrolytes containing toluene as additive. J. Power Sour. 183, 441–445 (2008)
Wu, H.L., Haasch, R.T., Perdue, B.R., et al.: The effect of water-containing electrolyte on lithium–sulfur batteries. J. Power Sour. 369, 50–56 (2017)
Ismail, I., Noda, A., Nishimoto, A., et al.: XPS study of lithium surface after contact with lithium-salt doped polymer electrolytes. Electrochim. Acta 46, 1595–1603 (2001)
Acknowledgements
SX and JS would like to thank the National Natural Science Foundation of China (No. 51602250) and Thousand Youth Talents Plan Project of China for their funding support. MR and DW would like to acknowledge the Office of Vehicle Technologies of the U.S. Department of Energy under Contract No. DE-EE0007795 for its support of this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xiong, S., Regula, M., Wang, D. et al. Toward Better Lithium–Sulfur Batteries: Functional Non-aqueous Liquid Electrolytes. Electrochem. Energ. Rev. 1, 388–402 (2018). https://doi.org/10.1007/s41918-018-0015-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41918-018-0015-y