Abstract
Green synthesis of zero-valent copper nanoparticles successfully performed using Ficus benjamina leaves. The novel adsorbent Ficus nano-zero-valent copper (FB-nZVCu) characterized by utilizing scanning electron microscopy (SEM) and Fourier transforms infrared spectroscopy (FT-IR). The size of these nanoparticles typically ranges from 16 to 18 nm. This adsorbent investigated for removal of D-blue 60 from textile wastewater. The impact of different operating parameters, including pH, adsorbent dose, stirring rate, time, and initial dye concentration, had been tested, and optimum conditions were selected to gauge the optimum dye removal performance of the adsorbent. The maximum removal efficiency of D-blue 60 reached 87% under the following proper conditions: adsorbent dose 0.3 g L−1, time 30 min, and pH 8. The Langmuir isotherm was found to be the most appropriate system for the adsorption process (R2 = 0.9994) and (qmax = 30.03 mg g−1). The pseudo-second-order model defined the adsorption. From the data obtained and the fruitful discussion, it detected that the FB-nZVCu green adsorbent strongly recommended as a hopeful substance for removing D-blue 60 from both synthetic and actual waste samples.
Highlights
-
Green synthesis of eco-friendly FB-nZVCu nanoparticles utilizing low-cost material Ficus benjamina leaves.
-
The FB-nZVCu nanoparticles are efficient nano-adsorptive agents for removing D-blue 60 from wastewater under optimum conditions.
-
The Langmuir isotherm and PSO kinetics model provided a good fit to the adsorption data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water pollution induced by dyestuff molecules during dyeing and finishing processes in the textile industry has become the focus of intense investigation in recent years because of its serious effects on human health, creatures, and animals (Yadav et al. 2022; Kashefialasl et al. 2006; Dehghani et al. 2017). Synthetic dyes in the aquatic environment are equally detrimental, because they prevent sunlight from passing through, inhibiting water photosynthesis and disrupting the entire biological framework, and are also harmful to a human being during human exposure to dye pollutants are also harmful to humans (Yao et al. 2022).
The health of plants, animals, and soil are all negatively impacted by dye residues in the soil (Parida et al. 2011). Effective treatment is required to remove dye molecules from environmental matrices and wastewater sources. To address industrial dye pollution, a variety of wastewater treatment methods have been deployed, such as filtration (Zhijiang et al. 2018), ultrafiltration (Alventosa-deLara et al. 2012; Stolz 2001), coagulation–flocculation process (Othmani et al. 2017; Kordkandi and Forouzesh 2014; Liang et al. 2014), oxidation process (Arulmathi et al. 2019; Rameshraja and Suresh 2011), electrochemical treatment (Fadillah et al. 2019; Brillas and Martínez-Huitle 2015), reverse osmosis (Li et al. 2011; Qureshi et al. 2022), and adsorption (Jawad et al. 2020; Ayanda et al. 2017; Fahim et al. 2006).
Adsorption is a common technique, and could be seen as the most viable choice for dye discharge from wastewater due to its effectiveness, good selectivity, inexpensive, ease of use, low by-product production, and availability under a variety of trial settings (Abdel-Gawad and Abdel-Aziz 2019a; Dehghani et al. 2018a). Attempts were made to produce efficient eco-friendly adsorbents and improve their efficacy in dye removal with the use of different nanomaterials (Mahmoud et al. 2021). Presently, a large number of zero-valent metal nanoparticles have been successfully synthesized utilizing different methods and have achieved great success in the treatment of wastewater. Nano-metallic particles are notable for their high reactivity, high porosity, and large surface (Mahmoud et al. 2018; Crane and Sapsford 2019; Dehghani et al. 2018b).
Green synthesis nanoparticles are very important, because they do not require any special equipment or harmful chemicals. Flavonoids, polyphenols, and other reducing substances are abundant in plant leaves and can reduce ions to zero valences and avoid agglomeration. Many plants have received certification for use in producing green nanoparticles for wastewater treatment, since they are low-cost, non-toxic, and free of chemicals with unfavorable side effects (Abdel-Aziz et al. 2019, 2020a; Abdel-Aziz and Fayyadh 2020).
Copper nanoparticles (CuNPs) acquired a lot of attention because of their outstanding optical, electrical, catalytic, mechanical, antifungal, and antibacterial characteristics (Ismail et al. 2019; Haider et al. 2016). CuNPs offer benefits compared to other metal nanoparticles in that they are inexpensive, have extensive availability, and in comparison to the conventional adsorbent, provide superior yields and shorter reaction times under moderate reaction conditions (Kamal et al. 2016; Abdel-Aziz et al. 2021). Consequently, this is the primary motivation to develop CuNPs.
Nano zero-valent copper was prepared from mature Duranta erecta extract for the reduction of Congo red and methyl orange (Ismail et al. 2019). Nano-zero-valent copper was prepared from Camelia sinensis leaves extract and utilized to degrade dyes (Ahmed et al. 2019). For methylene blue dye adsorption, green synthetic copper nanoparticles produced from the Cynomorium coccineum extract were employed (Sebeia et al. 2020). Among the dyes, D-blue 60 (Fig. 1) was chosen as the subject of this study, because it is commonly used for dyeing polyester and cotton blended fabric dyeing and printing, as well as vinegar fiber dyeing, making it a common industrial pollutant. D-blue 60 is one of the worst pollutants in water, as it destroys the aesthetic water quality, and it prevents sunlight from penetrating and reduces dissolved oxygen, damaging the aquatic environment.
In this study, zero-valent copper (Cu0) nanoparticles were produced by a novel green method using natural materials as Ficus leaves extracts. Scanning electron microscopy and Fourier transform infrared spectroscopy applied to examine the FB-nZVCu. The target of this study is to remediate D-blue 60 from textile wastewater.
Materials and Methods
Materials
Textile dye Disperse-Blue 60 was obtained from Anil Dyes (India). All substances utilized were of the highest quality and analytical grade, including copper sulfate (CuSO4·5H2O, Sigma-Aldrich Co. Ltd.), anhydrous alcohol (C2H4OH, Fisher Scientific Co. Ltd.), and Ficus leaves (Ficus benjamina tree, Giza-Egypt). The pH value was determined using a microprocessor digital pH-meter from (3510, Jenway-England). 0.1 M HCl and 0.1 M NaOH solutions were utilized to balance the pH change. All tests conducted using bi-distilled water.
Synthesis of Copper Nanoparticles (nZVCu)
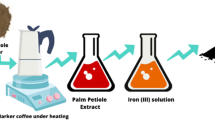
The leaves of Ficus cleaned with tap water to get rid of dust, next dipped in distilled water, and dried in an oven at 100 °C. After pulverizing into small pieces, the leaves were filtered through a 2.5 mm sieve. The leaves extract was obtained by mixing 400 g of pulverized leaves with 5 L of distilled water in a flask, boiled at (70–80) °C for 30 min, and then filtered. A copper sulfate solution was prepared by dissolving 1 g of CuSO4⋅5H2O in 100 mL double-distilled water by shaking and careful stirring. To synthesize nZVCu nanoparticles, 50 mL of Ficus benjamina leaf extract added drop by drop to 100 mL of copper sulfate solution. The mixture was agitated for 30 min to generate nanoparticles. The color of the fluid extract in the reaction vessel had changed from yellowish to brown to black, indicating the formation of FB-nZVCu nanoparticles. The FB-nZVCu nanoparticles were separated by centrifuging at 10,000 rpm for 5 min. The obtained precipitate was cleaned with distilled water and then with anhydrous alcohol. The FB-nZVCu was vacuum dried at 65 °C and stored at 4 °C in a desiccator (Abdel-Aziz et al. 2019, 2020a). The schematic for the green synthesis of FB-nZVCu nanoparticles is presented in Fig. 2.
Characterization of nZVCu
A scanning electron microscope (JEOL-JSM-5410, Japan) was used to observe the morphology of the green FB-nZVCu. Fourier transform infrared analysis of the leave extract was performed to identify the vibrational groups present.
Point of Zero Charge
7.46 g of KCl dissolved in 1000 mL of bi-distilled water. Using either 0.5 M NaOH or 0.5 M HCl, the pH of the 0.1 M KCl solution changed to a range between 2 and 12 (pHi). In 20 mL of pH-adjusted solution, 10 mg of an adsorbent Cu nanoparticle added. The samples left at room temperature for a day. The pH of each suspension was then determined (pHf). Plotting ∆pH (pHf − pHi) vs pHi value yielded the point of zero charge (Abdel-Aziz et al. 2019). Point of zero charge is a significant influence for controlling the adsorption process; pHpzc > pH > pHpzc, controls both the adsorbent surface charge and the charge of contaminant species in medium (electrostatic attraction or repulsion).
Batch Adsorption
Adsorption experiments performed to determine the dye removal efficiency. In each batch, 10 mg L−1 of dye mixed with 0.3 g L−1 of FB-nZVCu particles. Equation (1) was used to calculate the percentages of removal at λmax 203 nm (UV-1800, SHIMADZU-JAPAN)
where C0 and Ce, respectively, represent the initial and equilibrium dye concentrations (mg L−1). The adsorption capacity at equilibrium was obtained by using Eq. (2)
where V is the volume of aqueous solution (L), qe is the equilibrium adsorption capacity (mg g−1), and m is the mass of the adsorbent (g) (Soha et al. 2017; Soha and Abdel-Gawad 2018).
Adsorption Isotherm
The Freundlich and Langmuir isotherms which are the two most popular applications utilized for treating wastewater. It can be utilized to study the adsorption behavior of nanoparticles under predefined conditions of different dye concentrations of 5, 10, 15, and 20 mg L−1 with an adsorbent dose 0.3 g L−1, pH 8, contact time of 30 min, and stirring rate of 100 rpm at room temperature (Abdel-Gawad and Abdel-Aziz 2019b; Hossam and Abdel-Aziz 2020).
Freundlich Isotherm
The Freundlich isotherm used to define multi-layer heterogeneous adsorption surfaces, and it expressed by Eq. (3)
where the values of the slope and intercept of the graph of ln Ce vs ln qe can be used to calculate the Freundlich constants n (dimensionless) and Kf ((mg g−1) (mg L−1)1/n), respectively, which correspond to adsorption intensity and adsorption capacity.
Langmuir Isotherm
The Langmuir isotherm presumes that a homogeneous adsorbent surface covered in a monolayer of the adsorbate. The Langmuir isotherm is governed by Eq. (4)
where Ce (mg L−1) is the dye equilibrium dye concentration and qe (mg g−1) is the quantity of dye absorbed per mass of used adsorbent. The slope and intercept of the graph of Ce against Ce/qe used to calculate qmax (mg g−1), the maximal monolayer capacity of adsorption, and KL is the Langmuir equilibrium constant (L mg−1).
Adsorption Kinetics
Phenomena in solid–liquid systems have been described using two kinetic models, pseudo-first-order (PFO), and pseudo-second-order (PSO), at different contact times; 15, 30, 45, 69, 90, and 120 min, with an adsorbent dose of 0.3 g L−1, pH 8, and stirring rate of 100 rpm, at room temperature (Abdel-Aziz et al. 2020b).
Model for Pseudo-First Order
The PFO can be used to indicate the adsorption rate, which is described by Eq. (5)
where the dye concentration adsorption values in media at equilibrium and time t are qe and qt, respectively, and the first-order equilibrium constant K1 is determined from the plot of ln(qe − qt) against t.
Model for Pseudo-Second Order
The equation of PSO is the simplest and most commonly utilized kinetic calculation and described by Eq. (6)
where qe and qt are the capacities for adsorption at equilibrium and at time t, respectively, and K2 (g/mg min) stands for the rate constant of adsorption. K2 values calculated using the plot of t/qt against t.
Statistical Analysis Using Linear-Modeling Algorithms
Linear regression analysis with SPSS 24 applied to examine the effects of various operating parameters. These parameters are pH values of 4, 6, 8, and 10, contact times 15, 30, 45, 60, 90, and 120 min, adsorbent dose of FB-nZVCu 0.2, 0.3, 0.4, and 0.5 g L−1, stirring rate 100, 150, 200, and 250 rpm, and various dye concentrations 5, 10, 15, and 20 mg L−1. The ANOVA program showed the number of squares and the effect of the whole model.
Response Surface Methodologies
The impact of several operational factors studied, and the resulting findings corroborate the practical outcomes. If the P value is less than 0.05, the effect is deemed significant for the removal procedure. However, if the P value is greater than 0.05, the effect is regarded to be not significant. The removal equation represented by Eq. (7)
where R is the removal percentage, B (constant), X1 (pH), X2 (time), X3 (adsorbent dose), X4 (stirring rate), and X5 (dye concentration).
Artificial Neural Network
An Artificial Neural Network [ANN] using multi-layer perceptron “Multilayer Perceptron Back propagation [MLPB]” is an IBM SPSS Statistics System that devised to estimate the removal of impurities from wastewater. The multi-layer perceptron is among the most popular neural network architectures. The mean squared error [MSE], which flowed back from the output layer to the input layer to regulate the weights and biases, was calculated and used to compare the target to the output. ANN models that use back-propagation provide the link between trained and tested values. They also provide the relevance and normalized importance of each co-variable (Hossam Mohammed Abd El-Aziz and Abdel-Gawad 2020).
Results and Discussion
Characterization and Analysis of FB-nZVCu
Scanning Electron Microscopy
For identification of size and surface morphology, Fig. 3 shows an SEM image of the synthesized FB-nZVCu, with semi-spherical-shaped particles of sizes ranging from 16 to 18 nm. The huge nano-clusters visible in the SEM image may have developed during the sample's centrifugation. Because there are so many pores, pollutants can move through FB-nZVCu more easily and spread through it.
EDAX analysis indicates the synthesizing of FB-nZVCu particles. Cu peaks point to the occurrence of nano-zero-valent particles. Other peaks including C, O, Si, and S of Ficus extract, as shown in Fig. 4 (Abdel-Aziz et al. 2019).
FT-IR Measurements
Figure 5 depicts the FT-IR spectra of FB-nZVCu before the reaction in the 400–4000 cm−1 band. Polyphenol's broadband peak at 3400–3200 cm−1 relates to the vibration of –OH stretching (Nazar et al. 2018; Demirezen et al. 2019). The band at 1615 cm−1 of C=O (Demirezen et al. 2019; Yousef et al. 2018); the peak at 1539 cm−1 of Ficus amide (Zhu et al. 2018; Ramesh Kumar et al. 2014); the C=C-stretching vibration of the poly-phenolic aromatic ring at its peak, 1362 cm−1 (Zhu et al. 2018; Fathima et al. 2018). Figure 5 shows the existence and strength of phenolic compound peaks that can reduce Cu and are a reliable sign of synthesized FB-nZVCu (Abdel-Aziz et al. 2019).
Zero Charge Point
The value of the zero-charge point of FB-nZVCu was about 5.7, as shown in Fig. 6, explained by the high acidity of leaf extracts.
Effect of Operating Parameters
Effect of pH
The pH is crucial to the process of dye removal efficiency process. D-Blue 60 removal efficiency was investigated at various pH from 4 to 10. When the adsorbent dose of FB-nZVCu is 0.3 g L−1, 30 min, the dye concentration is 10 mg L−1, and the stirring rate is 100 rpm. The removal found to change with the variation of pH as 30, 55, 68, and 17%, respectively, as shown in Fig. 7.
The adsorption of dye is seen to increase with pH till it reaches pH 8 and then decreases. Ficus benjamina nano-zero-valent copper has a low zero-charge point of 5.7. At pH < pHpzc of 4, the surface of FB-nZVCu is positively charged; which leads to repulsion occurring with the blue dye, resulting in low removal efficiency. Furthermore, at a higher pH of 10, the removal greatly reduced, which is due to the precipitated part of the material in the removal process solution. Alternatively, it may be that repulsion exists between OH− and negatively charged surfaces of nanoparticles; there is steric hindrance and weak interaction. The attraction between the negatively FB-nZVCu surface and positive blue dye increased at pH 8; consequently, a high number of available vacant sites interfered between the nanoparticles and the contaminant; which led to maximum blue dye removal. Therefore, pH 8 considered optimum for maximum adsorption of the D-blue dye.
Effect of Time
The effect of time on efficient removal examined at time intervals of 15, 30, 45, 60, 90, and 120 min. For the dye concentration of 10 mg L−1, adsorbent dose of FB-nZVCu at 0.3 g L−1, pH 8, and the constant stirring rate at 100 rpm, the percentage 56, 68, 74, 76, 77, and 77%, respectively. The results are shown in Fig. 8.
It was found that as contact time increases, the percentage of dye removed rises until it reaches a nearly constant value. The number of contaminant molecules that adhere to the nanoparticles' vacant sites steadily increases with time which led to an increasing removal percentage. All of these have led to increasing the removal. The maximum percentage of removal takes place at an optimum time of 45 min.
Effect of Adsorbent Dose
The effect of adsorbent dose in the removal of dye was investigated at varying adsorbent doses of 0.2, 0.3, 0.4, and 0.5 g L−1, while the other operating conditions remained constant. As a result, the removal percentage found to be 49, 68, 84, and 98%, respectively. It was observed that the adsorption of the dye increases with an increase in the adsorbent dose until it reaches 0.3 g L−1; then, it becomes almost constant, as shown in Fig. 9.
As the FB-nZVCu dose increases, more active unoccupied adsorption sites become available; as a result, more dye is adsorbed until its concentration decreases, causing the adsorption to become constant. Hence, 0.3 g L−1 FB-nZVCu has the optimal adsorption capacity of the D-blue dye.
Effect of Stirring Rate
The impact of stirring rate was investigated at a varied stirring rate between 100 and 250 rpm, at pH 8, contact time of 30 min, and the dye concentration of 10 mg L−1. Under these conditions, the removal ratios found to be 68, 68, 69, and 69% as depicted in Fig. 10. The results showed that the stirring rate has no significant effect on the dye removal percentage.
Effect of the D-Blue Dye Concentration
The impact of the concentration of dye on its adsorption studied. The removal using FB-nZVCu was conducted on blue dye solutions with various concentrations of (5, 10, 15, and 20) mg L−1, at pH 8, time of 30 min, and an adsorbent dose of 0.3 g L−1. The removal efficiency is found to be 87, 68, 53, and 42%, as shown in Fig. 11.
From the data, it can be shown that as the dye concentration rises, the percentage of dye clearance decreases. Owing to the existence of a significant number of empty adsorption active sites in FB-nZVCu at the start of the procedure at low dye concentration, the removal ratio is high; however, as dye concentration increases, this ratio falls.
Adsorption Isotherm Studies
Freundlich and Langmuir's models are utilized to predict and evaluate the FB-nZVCu for adsorption capacity. The results obtained are shown in Fig. 12.
Langmuir model provides a better fit in both linear and non-linear as detected from the higher value of R2 (0.9994), and lower value of the Error Sum (4.5880). The maximum adsorption capacity is found to be equal to 30.03 mg g−1 (Table 1).
Kinetics Studies
According to Table 2, the PSO model provides a well fit for the data in both linear and non-linear models than the PFO, as seen in Fig. 13. The value qe [cal] = 25.67 mg g−1 is approximately the same as qe exp = 27.10 mg g−1 for D-blue dye.
Statistical Analysis
Using the entered technique, the influence of the variables on the removal process was investigated, and it was revealed that R2 = 0.946. The STD Error of the estimate is 3.60688, which indicates that the study's error rate is minimal and that the researched factors successfully control over 94% of all the variables influencing the removal operation.
When the ANOVA software used and the data provided, it was evident that the model was effective, because the P value was less than 0.05.
Response Surface Methodologies
Using the linear regression analysis, the impact of parameters investigated using SPSS Statistics, and the outcomes corroborate the practical findings.
The data obtained show that all variables without the stirring rate, which has a P value greater than 0.05, had significant effects on the removal process. It therefore ignored during the removal procedure, as shown in Table 3. By applying the B values, the removal equation derived, whereas
where R is the removal percent, B is constant, X1 is the effect of pH (4, 6, 8, and 10), X2 is the effect of time at intervals (15, 30, 45, 60, 90, and 120 min), X3 is the effect of adsorbent dose of (0.2, 0.3, 0.4, and 0.5 g L−1), X4 is the effect of stirring rate of (100, 150, 200, 250, and 300 rpm), and X5 is the effect of concentration of (5, 10, 15, and 20 mg L−1).
Artificial Neural Network
The ANN model was qualified using the multi-layer perceptron neural network model 5-1-2, for blue dye removal without discarding any result, with a total number of 22 runs with a sum of square errors of 0.130 and 0.016 for testing and training, with relative errors of 0.305 and 0.222, respectively. Figure 14 show that the normalized value and the predicted values are not exactly the same.
Figure 15 demonstrates that there is a slight deviation (− 15, 10%) between the residual value and predictive value, confirming the validity of the findings and the efficiency of the model's predictions in describing the adsorption of blue dye onto FB-nZVCu surfaces.
Figure 16 shows the importance of each co-variable in the removal efficiency, showing the most effective parameter was pH and the least effective one was stirring rate. The normalized importance is consistent with earlier discussions of how the operational parameters and RSM statistical algorithm work. The ANN results were in agreement with RSM findings and experimental data.
Finally, from the observed results obtained and the presented fruitful discussion, we can strongly say that FB-nZVCu is an eco-friendly adsorbent that is effective in removing D-blue 60 dye from textile wastewater and produces high-quality treated effluent.
Conclusions
-
1.
Green synthesis of FB-nZVCu particles utilizing low-cost Ficus benjamina leaves was successfully demonstrated.
-
2.
The formation of porous nanoparticles was confirmed through scanning electron microscopy (SEM).
-
3.
The FB-nZVCu particles are effective adsorbents for removing D-blue 60 dye from wastewater under optimum conditions.
-
4.
The maximum removal efficiency of D-blue 60 reached 87% with the following parameters: blue dye concentration of 10 mg L−1, adsorbent dose 0.3 g L−1, time 30 min, and pH 8.
-
5.
The Langmuir isotherm and pseudo-second-order kinetic models provided a good fit to the adsorption data with a high R2 value.
-
6.
The maximum adsorption capacity found to be equal to 30.03 mg g−1.
-
7.
The results of the statistical analysis using the ANOVA software showed that 94% of the variables that influence the removal process is accounted for in the experiments.
References
Abdel-Aziz HM, Farag RS, Abdel-Gawad SA (2019) Carbamazepine removal from aqueous solution by green synthesis zero-valent iron/Cu nanoparticles with Ficus benjamina leaves’ extract. Int J Environ Res 13:843–852
Abdel-Aziz HM, Fayyadh SN (2020) Removal of orange G by Fenton and Ficus benjamina nano zero valent iron. J Environ Eng Sci 1–10
Abdel-Aziz HM, Farag RS, Abdel-Gawad SA (2020a) Paracetamol removal by bimetallic zero-valent Fe/Cu with benjamina leaf extract. J Environ Eng Sci 1–11.
Abdel-Aziz HM, Farag RS, Abdel-Gawad SA (2020b) Removal of caffeine from aqueous solution by green approach using Ficus Benjamina zero-valent iron/copper nanoparticles. Adsorpt Sci Technol 5:0263617420947495
Abdel-Aziz HM, Farag RS, Abdel-Gawad SA (2021) Removal of inorganic contaminant phosphate from aqueous solutions by bimetallic Ficus-ZVFe/Cu nanoparticles. Int J Environ Eng 11:53–68
Abdel-Gawad SA, Abdel-Aziz HM (2019a) Removal of pharmaceuticals from aqueous medium using entrapped activated carbon in alginate. Air Soil Water Res 12:1178622119848761
Abdel-Gawad SA, Abdel-Aziz HM (2019b) Removal of ethinylestradiol by adsorption process from aqueous solutions using entrapped activated carbon in alginate biopolymer: isotherm and statistical studies. Appl Water Sci 9:75
Ahmed A, Usman M, Liu Q-Y, Shen Y-Q, Yu B, Cong H-L (2019) Plant mediated synthesis of copper nanoparticles by using Camelia sinensis leaves extract and their applications in dye degradation. Ferroelectrics 549:61–69
Alventosa-deLara E, Barredo-Damas S, Alcaina-Miranda M, Iborra-Clar M (2012) Ultrafiltration technology with a ceramic membrane for reactive dye removal: optimization of membrane performance. J Hazard Mater 209:492–500
Arulmathi P, Jeyaprabha C, Sivasankar P, Rajkumar V (2019) Treatment of textile wastewater by coagulation–flocculation process using gossypium herbaceum and polyaniline coagulants. Clean: Soil, Air, Water 47:1800464
Ayanda OS, Odo EA, Malomo D, Sodeinde KO, Lawal OS, Ebenezer OT, Nelana SM, Naidoo EB (2017) Accelerated decolorization of Congo red by powdered termite mound. Clean: Soil, Air, Water 45:1700537
Brillas E, Martínez-Huitle CA (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods An Updated Review. Appl Catal B Environ 166:603–643
Crane R, Sapsford D (2019) Tunable formation of copper metal, oxide, chloride and hydroxyl chloride nanoparticles from aqueous copper solutions using nanoscale zerovalent iron particles. Nanomater Nanotechnol 9:1847980419886173
Dehghani MH, Dehghan A, Najafpoor A (2017) Removing Reactive Red 120 and 196 using chitosan/zeolite composite from aqueous solutions: kinetics, isotherms, and process optimization. J Ind Eng Chem 51:185–195
Dehghani MH, Pourshabanian M, Heidarinejad Z (2018a) Experimental data on the adsorption of Reactive Red 198 from aqueous solution using Fe3O4 nanoparticles: Optimization by response surface methodology with central composite design. Data Brief 19:2126–2132
Dehghani MH, Mahdavi P, Heidarinejad Z (2018b) The experimental data of investigating the efficiency of zinc oxide nanoparticles technology under ultraviolet radiation (UV/ZnO) to remove Acid–32–Cyanine 5R from aqueous solutions. Data Brief 21:767–774
Demirezen DA, Yıldız YŞ, Yılmaz Ş, Yılmaz DD (2019) Green synthesis and characterization of iron oxide nanoparticles using Ficus carica (common fig) dried fruit extract. J Biosci Bioeng 127:241–245
Fadillah G, Saleh TA, Wahyuningsih S, Putri ENK, Febrianastuti S (2019) Electrochemical removal of methylene blue using alginate-modified graphene adsorbents. Chem Eng J 378:122140
Fahim N, Barsoum B, Eid A, Khalil M (2006) Removal of chromium(III) from tannery wastewater using activated carbon from sugar industrial waste. J Hazard Mater 136:303–309
Fathima JB, Pugazhendhi A, Oves M, Venis R (2018) Synthesis of eco-friendly copper nanoparticles for augmentation of catalytic degradation of organic dyes. J Mol Liq 260:1–8
Haider S, Kamal T, Khan SB, Omer M, Haider A, Khan FU, Asiri AM (2016) Natural polymers supported copper nanoparticles for pollutants degradation. Appl Surf Sci 387:1154–1161
Hossam SAA-G, Abdel-Aziz M (2020) Removal of sunset YellowAzo dye using activated carbon entrapped in alginate from aqueous solutions. Open Access J Sci 4:1–6
Hossam Mohammed Abd El-Aziz RSF, Abdel-Gawad SA (2020) Removal of contaminant metformin from water by using Ficus benjamina zero-valent iron/copper nanoparticles. Nanotechnol Environ Eng 4:23
Ismail M, Gul S, Khan M, Khan MA, Asiri AM, Khan SB (2019) Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange. Green Process Synth 8:135–143
Jawad AH, Abdulhameed AS, Mastuli MS (2020) Acid-factionalized biomass material for methylene blue dye removal: a comprehensive adsorption and mechanism study, Journal of Taibah University for. Science 14:305–313
Kamal T, Khan SB, Asiri AM (2016) Synthesis of zero-valent Cu nanoparticles in the chitosan coating layer on cellulose microfibers: evaluation of azo dyes catalytic reduction. Cellulose 23:1911–1923
Kashefialasl M, Khosravi M, Marandi R, Seyyedi K (2006) Treatment of dye solution containing colored index acid yellow 36 by electrocoagulation using iron electrodes. Int J Environ Sci Technol IJEST 2:365
Kordkandi SA, Forouzesh M (2014) Application of full factorial design for methylene blue dye removal using heat-activated persulfate oxidation. J Taiwan Inst Chem Eng 45:2597–2604
Li H, Lin Y, Luo Y, Yu P, Hou L (2011) Relating organic fouling of reverse osmosis membranes to adsorption during the reclamation of secondary effluents containing methylene blue and rhodamine B. J Hazard Mater 192:490–499
Liang C-Z, Sun S-P, Li F-Y, Ong Y-K, Chung T-S (2014) Treatment of highly concentrated wastewater containing multiple synthetic dyes by a combined process of coagulation/flocculation and nanofiltration. J Membr Sci 469:306–315
Mahmoud AS, EL-Tayieb MM, Ahmed NAS, Mostafa AM (2018) Algorithms and statistics for municipal wastewater treatment using nano zero valent iron (nZVI). J Environ Biotechnol Res 7:30–44
Mahmoud AS, Mostafa MK, Peters RW (2021) A prototype of textile wastewater treatment using coagulation and adsorption by Fe/Cu nanoparticles: techno-economic and scaling-up studies. Nanomater Nanotechnol 11:18479804211041180
Nazar N, Bibi I, Kamal S, Iqbal M, Nouren S, Jilani K, Umair M, Ata S (2018) Cu nanoparticles synthesis using biological molecule of P. granatum seeds extract as reducing and capping agent: growth mechanism and photo-catalytic activity. Int J Biol Macromol 106:1203–1210
Othmani A, Kesraoui A, Seffen M (2017) The alternating and direct current effect on the elimination of cationic and anionic dye from aqueous solutions by electrocoagulation and coagulation flocculation. Euro-Mediterr J Environ Integr 2:1–12
Parida K, Sahu S, Reddy K, Sahoo P (2011) A kinetic, thermodynamic, and mechanistic approach toward adsorption of methylene blue over water-washed manganese nodule leached residues. Ind Eng Chem Res 50:843–848
Qureshi SS, Shah V, Nizamuddin S, Mubarak N, Karri RR, Dehghani MH, Ramesh S, Khalid M, Rahman ME (2022) Microwave-assisted synthesis of carbon nanotubes for the removal of toxic cationic dyes from textile wastewater. J Mol Liq 356:119045
Ramesh Kumar K, Nattuthurai GP, Mariappan T (2014) Biosynthesis of silver nanoparticles from Morinda tinctoria leaf extract and their larvicidal activity against Aedes aegypti Linnaeus 1762. J Nanomed Nanotechnol 5:2
Rameshraja D, Suresh S (2011) Treatment of tannery wastewater by various oxidation and combined processes. Int J Environ Res 5:349–360
Sebeia N, Jabli M, Ghith A, Saleh TA (2020) Eco-friendly synthesis of Cynomorium coccineum extract for controlled production of copper nanoparticles for sorption of methylene blue dye. Arab J Chem 13:4263–4274
Soha HMA-A, Abdel-Gawad A (2018) Effective removal of chemical oxygen demand and phosphates from aqueous medium using entrapped activated carbon in alginate. MOJ Biol Med 3:227–236
Soha MSM, Abdel Gawad A, Abdl-Aziz HM (2017) Adsorption study for chemical oxygen demand removal from aqueous solutions using alginate beads with entrapped activated carbon. J Indian Water Resour Soc 37:461–470
Stolz A (2001) Basic and applied aspects in the microbial degradation of azo dyes. Appl Microbiol Biotechnol 56:69–80
Yadav SK, Dhakate S, Singh BP (2022) Carbon nanotube incorporated eucalyptus derived activated carbon-based novel adsorbent for efficient removal of methylene blue and eosin yellow dyes. Biores Technol 344:126231
Yao LW, Khan FSA, Mubarak NM, Karri RR, Khalid M, Walvekar R, Abdullah EC, Mazari SA, Ahmad A, Dehghani MH (2022) Insight into immobilization efficiency of Lipase enzyme as a biocatalyst on the graphene oxide for adsorption of Azo dyes from industrial wastewater effluent. J Mol Liq 354:118849
Yousef S, Tatariants M, Makarevičius V, Lukošiūtė S-I, Bendikiene R, Denafas G (2018) A strategy for synthesis of copper nanoparticles from recovered metal of waste printed circuit boards. J Clean Prod 185:653–664
Zhijiang C, Ping X, Cong Z, Tingting Z, Jie G, Kongyin Z (2018) Preparation and characterization of a bi-layered nano-filtration membrane from a chitosan hydrogel and bacterial cellulose nanofiber for dye removal. Cellulose 25:5123–5137
Zhu F, Ma S, Liu T, Deng X (2018) Green synthesis of nano zero-valent iron/Cu by green tea to remove hexavalent chromium from groundwater. J Clean Prod 174:184–190
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zayed, M.A., Abdel-Gawad, S.A., Abdel-Aziz, H.M. et al. Green Synthesis of Nano-Zero-Valent Copper for the D-Blue 60 Textile Dye Removal from Aqueous Medium. Int J Environ Res 17, 12 (2023). https://doi.org/10.1007/s41742-022-00499-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41742-022-00499-2