Abstract
Objective
The aim of this study was to evaluate the cost effectiveness of tofacitinib versus other treatment options currently available in Colombia in naïve to biologics (first-line) and exposed to biologics (second-line) patients with moderate to severe active ulcerative colitis (UC).
Methods
A Markov model was constructed with 8-week cycles, simulating a cohort of patients in a 5-year time horizon. The health states included remission, treatment response, active UC, and colectomy. The transition probabilities for the induction and maintenance phase were obtained from a network meta-analysis, and effectiveness was measured using quality-adjusted life-years (QALYs). Unit costs were derived from official national sources.
Results
For first line, the incremental cost-effectiveness ratio (ICER) per QALY was $883 for tofacitinib and $3619 for infliximab, compared with adalimumab. Sensitivity analysis showed that tofacitinib is cost effective in 45% of the iterations, adalimumab in 5%, and infliximab in 50%. Meanwhile, the ICER of adalimumab was $14,927 compared with tofacitinib in second-line treatment. In the sensitivity analysis, tofacitinib was cost effective in 64% of the iterations, followed by adalimumab in 36%. Infliximab and golimumab were not included due to data limitations in the network meta-analysis of second-line treatment.
Conclusion
The analysis suggests that in Colombia, treatment with tofacitinib for patients with moderate-to-severe UC is a cost-effective option in both lines compared with other treatment options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ulcerative colitis as chronic condition can have a profound impact on quality of life. Biologics and tofacitinib have the potential to improve health-related quality of life in ulcerative colitis patients. |
These results are relevant for periods of financial crisis, budget constraints and sustainability issues in health care system. The policymakers may carefully consider balance investment against the far-reaching benefits of those treatments. |
Tofacitinib for moderate-to-severe ulcerative colitis patients is a cost-effective option in both lines, compare with other treatment options available in Colombia. |
1 Introduction
Ulcerative colitis (UC) is a chronic condition characterized by intermittent and unpredictable flares and remissions, and can be accompanied by the formation of ulcers that eventually produce pus and mucous [1, 2]. UC has an unpredictable natural history [3] and the symptoms include bloody diarrhea, rectal bleeding, passage of mucus, tenesmus, and abdominal pain [4]. In patients with UC, the lesions usually remain superficial and extend proximally; colectomy is required for 10–30% of patients [2].
The prevalence of UC in Colombia has been estimated to be approximately 51.77/100,000 individuals between 2010 and 2014, and is most often prevalent among women older than 40 years of age, with a similar reported prevalence in developing countries [5] and predominance of UC over Crohn’s disease (3.9:1) [6]. One study in Colombia estimated a period of 9.2 months to establish a diagnosis of UC after the onset of symptoms [7]. According to observational studies in Colombia [6, 8, 9], the majority of UC patients receive 5-aminosalicylates, systemic or topical corticosteroids, azathioprine (immunosuppressants), and biological drugs such as antitumor necrosis factor (anti-TNF) agents (infliximab, adalimumab and golimumab). Anti-adhesion molecules (vedolizumab) are the most commonly used and surgery is considered in a few clinical scenarios [6]. These treatment strategies reflect the current clinical guidelines [10]. Another treatment option that has recently become available in Colombia is tofacitinib, a Janus kinase (JAK) inhibitor approved in 2020 as an alternative treatment for patients with UC.
UC can have a profound impact on quality of life [11, 12]; however, it has been estimated that biologics and tofacitinib have the potential to improve health-related quality of life in UC patients [13, 14].Patients with moderate-to-severe UC have been approved for treatment in Colombia, but there are no data on the costs or technology assessment. As stated above, the cost effectiveness of new treatment options becomes more relevant for decision makers in the Colombian setting. Therefore, the aim of this study was to examine the cost effectiveness of the treatment options available for patients with moderate-to-severe UC in Colombia, by line of treatment.
2 Methods
To assess the cost effectiveness of tofacitinib as first- and second-line therapy versus other treatment options currently available in Colombia, Markov cohort models were developed. The main outcome in this analysis was incremental cost per additional quality-adjusted life-year (QALY). The analysis was performed using the TreeAge Pro Healthcare software package.
This evaluation was conducted from the perspective of the Colombian health care system, which covers the treatment costs of UC. The Colombian health care system has two main regimens—contributory and subsidized; the contributory regimen covers salaried workers, pensioners, and independent workers, whereas the subsidized regimen covers individuals receiving the minimum salary or less, who do not contribute. The health care system has reported a broad coverage, i.e. 95% to May 2022 [15]. However, there are social inequities that mean that despite the existence of coverage and access to health care, the health care system is limited by socioeconomic, social, and geographical barriers.
The patients, structure of the model, and inputs (transition probabilities, utilities and costs) are described in detail below.
2.1 Patients and Comparators
The population consisted of patients with moderate-to-severely active UC (defined by a Mayo score of 6–12 and an endoscopic subscore of ≥2) following an inadequate response to conventional treatment. In the first-line model, tofacitinib was compared with adalimumab, infliximab, golimumab, and vedolizumab in patients who were naïve to biologics, while in the second-line model, tofacitinib was compared with adalimumab and vedolizumab in patients who had not responded to previous biologics. Other treatments were not considered due to a lack of evidence in this population. Ustekinumab was not incorporated as a treatment strategy, mainly because this indication was not approved and intravenous infusion was not commercially available in Columbia, but efficacy data were extracted.
-
Adalimumab: 160 mg subcutaneous injection at week 0, 80 mg injection at week 2, and 40 mg injection every 2 weeks thereafter.
-
Infliximab: 5 mg/kg intravenous infusions at weeks 0, 2, and 6, and every 8 weeks thereafter.
-
Golimumab: 100 mg subcutaneous injection at week 0, 50 mg injection at weeks 2 and 6, and 50 mg injection every 4 weeks thereafter.
-
Tofacitinib: 10 mg twice daily for 8 weeks, then 5 mg twice daily thereafter.
-
Vedolizumab: 300 mg intravenous infusions at weeks 0, 2, and 6, and every 8 weeks thereafter.
2.2 Model Structure
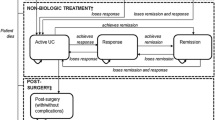
A Markov model was constructed with 8-week cycles, matching the reporting period of the clinical trials with two groups of patients: first-line (naïve to biologics) and second-line (exposed to biologics). The model was designed to reflect the clinical practice and disease progression of UC and simulate a cohort of patients with moderate-to-severe UC from the Colombian health care system perspective over a 5-year time horizon as the base-case scenario. The health states included remission, treatment response (without remission), active UC, colectomy, and post-colectomy. The model design was based on that presented in previous studies [16,17,18], one of which was a systematic review of the economic models. The model was validated by clinical experts familiar with clinical practices in Colombia.
Patients entered the model with active UC (Mayo score 6–12), and response was monitored during the induction phase based on the treatment evaluated. Patients who did not respond during the induction period switched to another biologic treatment, which was validated by clinical experts. Those who responded remained in the maintenance phase depending on their clinical response (decrease in Mayo score of 3 units or at least 30%, and decrease in rectal bleeding of 1 unit or absolute value between 0 and 1), remission (Mayo score 0–2), or response without remission (Mayo score 3–5). Patients could transition among the health states during treatment until loss of response, at which point they were switched to another treatment. Additionally, patients could die from any cause and have a colectomy because those endpoints were not assessed in the trials but corresponded to a natural history of UC (Fig. 1).
2.3 Clinical Efficacy
Information on efficacy was obtained through a systematic review of the evidence [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. A search was conducted in the MEDLINE and EMBASE databases, and snowball searching was used from two previously published systematic reviews [34, 35]. This strategy involved other possible publications inside of the references used in selected articles. Databases were last searched on 10 November 2020. The search included Medical Subject Heading (MeSH) terms and terms related to disease condition and treatment for UC, adalimumab, golimumab, infliximab, ustekinumab, tofacitinib, and vedolizumab. MeSH or EMTREE terms were used, as well as related terms combined with Boolean operators. The complete search strategy is described in electronic supplementary material (ESM) 1.
Due to the lack of head-to-head clinical trial data to estimate response and remission for each treatment, a network meta-analysis (NMA) was performed using a Bayesian approach with WinBugs software (version 1.4). The NMA was conducted separately for the induction and maintenance phases, clinical outcomes (remission, response [without remission], and sustained response), and by type of treatment line (first or second). Using the Markov chain Monte Carlo method with three chains of 90,000 after a burn-in phase of 30,000, the analysis parameters were estimated. The fit to the data, both fixed-effect and random-effect approaches, was evaluated according to the lowest value of the deviance information criterion (DIC). In this model, the distribution was 0.00001 for a normal distribution and 0.5 for a non-normal distribution.
The transition probabilities among the health states during the maintenance phase were conditional on the results in the induction phase. Therefore, during extraction of the data, the number of patients in remission or response in the induction phase was considered to adjust the probabilities in the maintenance phase. Additionally, the definitions and the report of outcomes in the clinical trials were considered to estimate the probabilities required for the different health states. Because some transition probabilities were not available using NMA, the method reported by Hernandez et al. [36] was used.
The data from the maintenance trials provided the probabilities of remission and response by the end of the year. For this reason, the probabilities beyond the first year of treatment were assumed to be the same as those estimated for the first year of treatment. NMA could not be performed for infliximab and golimumab in patients who were exposed to biologics, due to limitations in the data. The results of NMA were reported in risk ratios (RRs) with 95% credible intervals (95% CrIs). The transition probabilities for each group of patients, by treatment, are shown in Table 1, and the estimates for response and remission for each treatment, from the meta-analysis and trials, are included in ESM 2–4.
Mortality was assumed to be similar to that in the general population, which increases over time as patients age, as was reported by the National Administrative Department of Statistics (DANE) [37]. Furthermore, the probability of colectomy was dependent on the time since UC diagnosis obtained from published literature [38]. The mean age was 41 years and the mean time since UC diagnosis was 3 years, which were estimated based on the pooled patient populations of trials included in the NMA.
2.4 Utility
The utility weights were presented by health state and were obtained from OCTAVE trials for each health state (remission, response without remission, and no response). They were captured using the EuroQoL 5-dimensions (EQ-5D) questionnaire and those scores were converted into utilities—the utility index scores at week 8 for the health states in the induction phase, and at week 52 for the health states in the maintenance phase [39]. The utility weights were stratified by phase [40]. Colectomy was extracted from previously published models [16, 41]. The utility values used in the model are presented in Table 2.
2.5 Costs
The model included costs associated with drug and administration, medical costs associated with each health state, and colectomy. All costs were expressed or updated to 2021 using the Consumer Price Index (IPC) for Colombia (Historic Annual IPC, Department of National Statistics [DANE]) at the average exchange rate of $3693 Colombian Peso/US Dollars [42] discounted at a rate of 5% per year for both costs and outcomes, in accordance with the Institute of Health Technology (IETS) assessment in Colombia recommendations [43].
The costs of treatments used were calculated based on the average doses used in the clinical trials and were costed based on the information on prices of medicines system (Sistema de Información de Medicamentos [SISMED]) [44] and weighted average by the number of reported units incorporating the branded and biosimilar versions; the cost of administering an intravenous infusion was assumed to be $13 per infusion and a mean body weight of 65 kg for adult patients with UC (Table 3). The costs of care of UC and colonoscopy were taken from the local tariff manual, the Mandatory Traffic Accident Service (i.e., SOAT) [45], and complications of colonoscopy were derived from published local studies [46,47,48,49] (Table 2).
2.6 Sensitivity Analyses
One-way sensitivity analyses were performed over the specified ranges for each variable and were conducted over multiple time horizons in which the maximum duration of time for treatment varied among 1, 5, and 10 years. The lifetime horizon was not modeled due to extrapolating results to those that did not adequately capture treatment outcomes and cost impacts. Furthermore, the patient will not continue under the treatments evaluated in that time horizon based on the availability of the primary data.
In addition, a probabilistic sensitivity analysis (PSA) was performed running 1000 independent simulations, according to variable-specific distributions that vary simultaneously and randomly in the model by each line of treatment. Beta and triangular distributions were used for transition probabilities and utilities, and gamma distributions were used for costs. The results are presented graphically as acceptability curves, and are considered cost effective at one and three times the gross domestic product (GDP) [$5391–$16,174] [50] per QALY threshold or willingness-to-pay (WTP) value. The ranges and distribution are described in ESM 5.
3 Results
3.1 Base-Case Scenario
Table 4 shows the results of the base-case scenario for each line of treatment over the 5-year time horizon of the model. In the first line, tofacitinib had an incremental cost-effectiveness ratio (ICER) of $883 per QALY versus adalimumab, and infliximab had an ICER of $3619 per QALY versus tofacitinib, being a cost-effective treatment with a WTP threshold of one GDP in Colombia. Vedolizumab and golimumab were not cost effective (higher costs and lower QALYs), and it was projected that those patients treated with infliximab had slightly improved outcomes but had higher costs than tofacitinib and adalimumab. Tofacitinib was associated with a higher proportion (~20%) of patients in time spent in treatment, response, and remission than other biologics.
In the second-line group, the resulting ICER for adalimumab versus tofacitinib was $14,927 per QALY, while vedolizumab was not a cost-effective strategy. Assuming a WTP threshold of three times the GDP of Colombia, tofacitinib was cost effective in both lines of treatment. A greater proportion of patients treated with adalimumab spent longer times in response and remission than tofacitinib and vedolizumab (5.4% and 7.5%, respectively) but at a higher cost than tofacitinib.
3.2 One-Way Sensitivity Analysis
The one-way sensitivity analyses suggested that for first-line treatments, the ICERs were sensitive to the cost of treatment of infliximab and probability of remission for infliximab and tofacitinib, and other parameters had minor impacts on the ICER. For second-line treatment, in all simulations, tofacitinib remained cost effective under a one GDP threshold, suggesting that the results were robust to changes in a range of input parameters. When employed over multiple time horizons, for 1 year to 10 years, the extension of the time horizon yielded more favorable ICERs. In 1 year, the treatments remained in the efficiency frontier as the base-case scenario for first- and second-line treatments. The tornado analyses are described in ESM 6.
3.3 Probabilistic Sensitivity Analysis (PSA)
The cost-effectiveness acceptability curve is shown in Fig. 2. At a WTP of one GDP for each additional QALY gained, 50% of simulations with infliximab were likely cost effective, 45% and 5% of simulations were cost effective for tofacitinib and adalimumab, respectively, for first-line treatment, and regardless of the threshold (one or three times the GDP), tofacitinib had a 64% probability of being cost effective. Adalimumab had a 36% probability of being effective for second-line treatment. The scatterplots are described in ESM 7.
4 Discussion
The cost-effectiveness analysis of tofacitinib for moderate to severe active UC in Colombia showed that both first- and second-line patients had slight clinical differences compared with other treatments at an incremental cost per QALY within the thresholds for Colombia. Therefore, the analysis suggested that tofacitinib was cost effective for first- and second-line patients. This uncertainty analysis suggested an important likelihood that it is cost effective compared with other treatments. In the univariate sensitivity analysis, the cost of infliximab and probability of remission for infliximab and tofacitinib, could affect the ICER. The PSA revealed that the probability of tofacitinib being cost effective was 45% in the first-line setting and 64% in the second-line setting for a WTP threshold of one GDP per QALY.
UC has a significant impact on quality of life, work productivity, and out-of-pocket expenses, and has an important and significant role in disease burden [51]. The indirect cost was not incorporated in the analysis; a systematic literature analysis reported 35% of total UC costs were associated with indirect costs [52]. Alternatively, injection or intravenous infusion therapies exposed patients to potential out-of-pocket expenses (i.e., transportation) and led to a greater chance of infection or discomfort [53].
Previous analyses analyzed the cost effectiveness of tofacitinib in moderate-to-severe active UC patients in different settings. In Great Britain, tofacitinib was likely to be cost effective [18]; similar scenarios were found in Germany [16], Greece [54] and Canada, and tofacitinib was found to be the optimal therapy in second-line patients [40] and in middle-income China [55]. No other cost-effectiveness analyses exist for Colombia, hence it was not possible to compare the results of this study with other publications. This lack of results indicates a need for further health economic research in this area to identify cost-effective options, given that those analyses are policy mechanisms that improve the efficient use of resources with a combination of global clinical inputs and local costs.
Contrary to other cost-effectiveness models where surgery and post-surgery complications were included in the model to estimate the impact on QALYs and costs [36, 55, 56], in this economic model surgery was only included to reflect the natural history of the disease; in Colombian clinical guidelines, surgery was considered an alternative in a few cases. Therefore, the impact of this state was not significant in the model, as discussed by Hernandez et al. [36].
Ustekinumab was included in the NMA to estimate the RR for the different states of the model for all analyzed treatments, given that clinical trials are available, other economic models are included, and the NMA included information about the placebo arm, used as reference treatment, in the clinical trials of ustekinumab, increasing the power of the analysis. However, ustekinumab has no regulatory approval for UC in Colombia and the intravenous dosage form was not available at the time, therefore cost-effectiveness results were not incorporated.
This model only followed patients until inadequate response at the first biologic or for tofacitinib, both first- and second-line treatments, which the authors consider more conservative for the comparisons and to show the true value of one specific treatment being useful for physicians and decision makers. Other cost-effectiveness models have assessed treatment sequences with a global approach; however, this could be more restrictive for clinicians given that the results of the model are limited to combinations of treatments.
The current analyses have several strengths. The trials used in the NMA included studies included in other systematic reviews and the most recent studies published for new treatments for this disease, such as tofacitinib and ustekinumab, clinical trial head-to-head comparisons, such as Varsity studies [26], and post hoc analyses, to obtain accurate information on biologically naïve and biologically exposed populations. This type of patient was treated separately to decrease the heterogeneity in the population; however, the analysis did not attempt to capture additional lines of therapy after patients did not respond to anti-TNF therapy, to accurately reflect the efficacy results. In this analysis, adjustment over re-randomization after the induction phase was not conducted, maintaining the clinical results reported in the studies. Similar trends in the NMA were obtained for other indirect comparisons of clinical remission and response, regardless of the treatment and phase included. Compared with the other treatments, tofacitinib has similar efficacy in terms of response and remission in the induction and maintenance phases for first- and second-line treatments. Singh et al. focused on biologic-naïve patients and biologically exposed patients in the induction phase [57]. Systematic reviews have been conducted that analyzed the same group of patients in the induction and maintenance phases [18, 34, 58]; however, sustained remission and response were not analyzed in other systematic reviews.
Exhaustive searching of post hoc analyses from published clinical trials was conducted to include more information for the different phases and health states; however, there were challenges in obtaining the transition probabilities between response, remission, and active disease, which were conditional on results in the induction phase for the cost-effectiveness model for UC due to limitations on the reporting of clinical trials. However, in some cases, this information was not available.
Certainly, some limitations in this analysis should be noted. No data were available beyond the primary data, but the assumptions employed to address this absence of data were reviewed and validated by clinical experts. Another related limitation was data were missing for the efficacy of infliximab and golimumab in patients who were exposed to second-line biologics. Nevertheless, infliximab is the most widely used biological medicine in first-line UC in Colombia [59]. The model did not include biologics or tofacitinib in combination with other treatments, such as azathioprine, which is only recommended for patients with severe disease [10]. Due to the lack of information reported in the clinical trials, the NMA cannot provide information for certain transition probabilities (i.e., remission to response only, or only response to no response), requiring estimation from other combinations of health states.
The Colombian health care system faces budgetary constraints where health management organizations (HMOs) are responsible for handling the funds assigned by the Minister of Health. Treatments recommended by Colombian guidelines for UC [10] are financed by the health care system. An update of the benefit plan in Colombia was made at the beginning of this year. As previous changes are expected that represent greater access to new technologies, measured through greater use of new technologies [60]. However, they require additional administrative processes compared with other treatments for other diseases. This cost-effectiveness analysis allows HMOs to optimize the budget for the management of UC, including new treatments such as tofacitinib.
5 Conclusion
This analysis suggests that in Colombia, treatment with tofacitinib for patients with moderate-to-severe UC is a cost-effective option in both first- and second-line treatment compared with other available treatment options. However, additional clinical studies are required to evaluate the long-term outcomes for all treatments in order to have more confidence in the results.
References
Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014;7(1):113–20. https://pubmed.ncbi.nlm.nih.gov/25075198/
Cosnes J, Gowerrousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785-1794.e4.
Danese S, Allez M, Van Bodegraven AA, et al. Small and large bowel: systematic review and meta-analysis unmet medical needs in ulcerative colitis: an expert group consensus. 2016 https://www.karger.com/ddi. Accessed 10 Sep 2021.
Cottone M, Scimeca D, Mocciaro F, et al. Clinical course of ulcerative colitis. Dig Liver Dis. 2008;40(Suppl 2):S247–52.
Juliao F, Calixto O. Prevalencia de enfermedad de crohn y colitis ulcerativa en poblacion adulta en colombia: analisis del sistema integral de informacion de proteccion social (SISPRO). acadi2017.gastrocol.com. 2017. http://acadi2017.gastrocol.com/abstract_app/Premio_mejor_poster_ACADI_2017/107_poster_final_acadi_17_prevalencia_eii_identificado.pdf. Accessed 9 Sep 2021.
Juliao-Baños F, Puentes F, López R, et al. Caracterización de la enfermedad inflamatoria intestinal en Colombia: resultados de un registro nacional. Rev Gastroenterol México. 2021;86(2):153–62.
Juliao-Baños F, Vélez M, Arango J, et al. Fenotipo e historia natural de la enfermedad inflamatoria intestinal en un centro de referencia en Medellín-Colombia. 2010. https://www.redalyc.org/pdf/3377/337731598003.pdf. Accessed 10 Sep 2021.
Juliao Baños F, Agudelo Zapata Y, Yepes DC. Survey results regarding variations in care of patients with inflammatory bowel disease. Rev Colomb Gastroenterol. 2014;29(1):11–8.
Ciapponi A, Virgilio SA, Berrueta M, et al. Epidemiology of inflammatory bowel disease in Mexico and Colombia: analysis of health databases, mathematical modelling and a case-series study. PLoS ONE. 2020;15(1): e0228256.
Juliao-Baños F, Torres-Amaya M, Otero-Regino W, et al. Guidelines for the management of ulcerative colitis in the adult population (update). Rev Colomb Gastroenterol. 2020;35:2–62. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-99572020000600002&lng=en&nrm=iso&tlng=en. https://doi.org/10.22516/25007440.636.
Casellas F, Arenas JI, Baudet JS, et al. Impairment of health-related quality of life in patients with inflammatory bowel disease: a Spanish multicenter study. Inflamm Bowel Dis. 2005;11(5):488–96.
Reinisch W, Sandborn WJ, Bala M, et al. Response and remission are associated with improved quality of life, employment and disability status, hours worked, and productivity of patients with ulcerative colitis. Inflamm Bowel Dis. 2007;13(9):1135–40.
LeBlanc K, Mosli M, Parker C, et al. The impact of biological interventions for ulcerative colitis on health-related quality of life. Cochrane Database Syst Rev. 2015;2015(9):CD008655. https://pubmed.ncbi.nlm.nih.gov/26393522/.
Panés J, Vermeire S, Lindsay JO, et al. Tofacitinib in patients with ulcerative colitis: health-related quality of life in phase 3 randomised controlled induction and maintenance studies. J Crohn’s Colitis. 2018;12(2):145–56.
Ministerio de Salud y Protección Social. Cifras de aseguramiento en salud con corte a mayo de 2022. 2022. https://www.minsalud.gov.co/proteccionsocial/Paginas/cifras-aseguramiento-salud.aspx.
Sardesai A, Dignass A, Quon P, et al. Cost-effectiveness of tofacitinib compared with infliximab, adalimumab, golimumab, vedolizumab and ustekinumab for the treatment of moderate to severe ulcerative colitis in Germany. J Med Econ. 2021;24(1):279–90. https://doi.org/10.1080/13696998.2021.1881323.
Archer R, Tappenden P, Ren S, Martyn-St James M, Harvey R, Basarir H, et al. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262):clinical effectiveness systematic review and economic model. Health Technol Assess. 2016;20(39):1–326.
Lohan C, Diamantopoulos A, LeReun C, et al. Tofacitinib for the treatment of moderately to severely active ulcerative colitis: a systematic review, network meta-analysis and economic evaluation. BMJ Open Gastroenterol. 2019;6(1):302. https://doi.org/10.1136/bmjgast-2019-000302/pmc/articles/PMC6673763/.
Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710.
Sandborn WJ, Su C, Sands BE, D’Haens GR, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–36. https://doi.org/10.1056/nejmoa1606910#:~:text=In%20conclusion%2C%20among%20patients%20with,of%20remission%20and%20mucosal%20healing.
Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):96-109.e1.
Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–14. https://doi.org/10.1056/NEJMoa1900750.
Suzuki Y, Motoya S, Hanai H, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol. 2013;49(2):283–94.
Hirayama Y, Watanabe O, Nakamura M, et al. P584. Infliximab therapy for Japanese patients with ulcerative colitis: efficacy, safety, and association between serum infliximab levels and early response in a randomized, double-blind, placebo-controlled study. J Crohn’s Colitis. 2015;9(Suppl 1):S372–3.
Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–7.
Hibi T, Imai Y, Senoo A, et al. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: a phase 3, double-blind, randomized, placebo-controlled study (PURSUIT-J study). J Gastroenterol. 2017;52(10):1101–11.
Jiang XL, Cui HF, Gao J, et al. Low-dose infliximab for induction and maintenance treatment in Chinese patients with moderate to severe active ulcerative colitis. J Clin Gastroenterol. 2015;49(7):582–8.
Motoya S, Watanabe K, Ogata H, et al. Vedolizumab in Japanese patients with ulcerative colitis: a Phase 3, randomized, double-blind, placebo-controlled study. PLoS ONE. 2019;14(2):e0212989. https://doi.org/10.1371/journal.pone.0212989.
Rutgeerts P, Feagan BG, Marano CW, et al. Randomised clinical trial: a placebo-controlled study of intravenous golimumab induction therapy for ulcerative colitis. Aliment Pharmacol Ther. 2015;42(5):504–14.
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2009;353(23):2462–76.
Sands BE, Peyrin-Biroulet L, Edward V, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381(13):1215–26.
Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;13(2):70–1.
Sandborn WJ, Van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257-265.e3.
Welty M, Mesana L, Padhiar A, Naessens D, Diels J, van Sanden S, et al. Efficacy of ustekinumab vs advanced therapies for the treatment of moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Curr Med Res Opin. 2020;36(4):595–606.
Bonovas S, Lytras T, Nikolopoulos G, Peyrin-Biroulet L, Danese S. Systematic review with network meta-analysis: comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment Pharmacol Ther. 2018;47(4):454–65.
Hernandez L, Kuwabara H, Shah A, et al. Cost-effectiveness analysis of vedolizumab compared with other biologics in anti-TNF-naïve patients with moderate-to-severe ulcerative colitis in Japan. Pharmacoeconomics. 2020;38(1):69–84.
DANE. Proyecciones de la población 2005–2020. https://www.dane.gov.co/files/investigaciones/poblacion/proyepobla06_20/8Tablasvida1985_2020.pdf. Accessed 16 Apr 2018.
Targownik L, Singh H, Nugent Z, et al. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. Am J Gastroenterol. 2012;107(8):1228–35.
PFIZER. Adhoc analysis of OCTAVE Sustain (1096 DB Maintenance) [data on file]. Study report output for PRJA392 submission (ibd_pub) Protocol (SCSA3920202a).
Canadian Agency for Drugs and Technologies in Health (CADTH). Tofacitinib (Xeljanz). Pharmacoeconomic review report. CADTH; 2019. https://www.ncbi.nlm.nih.gov/books/NBK572213/
Canadian Agency for Drugs and Technologies in Health (CADTH). Golimumab (Simponi), pharmacoeconomic review report. CADTH; 2014. https://www.ncbi.nlm.nih.gov/books/NBK263293/.
Banco de la República de Colombia. Tasa representativa del mercado dólares. 2021. http://www.banrep.gov.co/es/-estadisticas.
Instituto de Evaluación Tecnológica en Salud. Manual para la elaboración de evaluaciones económicas en salud. Bogotá: IETS; 2014.
Ministerio de Salud y Protección Social de Colombia. Sistema de información de precios de medicamentos SISMED. 2021. https://web.sispro.gov.co/WebPublico/Consultas/ConsultarCNPMCadenaComercializacionCircu2yPA_028_2_2.aspx.
Consejo Nacional de Seguridad Social en Salud. Manual Tarifario SOAT. 2021.
Hernández F, Lasalvia P, Garzón J, et al. Cost-effectiveness of ceftolozane/tazobactam for the treatment of complicated intraabdominal and urinary tract infections in Colombia. Infection. 2020;24(1):9–14. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0123-93922020000100009&lng=en&nrm=iso&tlng=en.
Pinzón-Espitia OL, Chicaiza-Becerra L, García-Molina M, et al. El caso del soporte nutricional enteral por sonda en Colombia: problemas de coordinación institucional. Nutr Hosp. 2015;32(1):222–30. https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-16112015000700033&lng=es&nrm=iso&tlng=es.
Restrepo LA, Suárez JC, Martínez ME. Costo de las infecciones del sitio operatorio en una institución de alta complejidad. Medellín–Colombia, 2008-2009 (Cost of operative site infections in an institution of high complexity. Medellinn–Colombia, 2008–2009). https://revistas.ces.edu.co/index.php/ces_salud_publica/article/view/1997.
Gil-Rojas Y, Garzón A, Lasalvia P, et al. Cost-effectiveness of bariatric surgery compared with nonsurgical treatment in people with obesity and comorbidity in Colombia. Value Heal Reg Issues. 2019;20:79–85.
DANE—Dirección de Síntesis y Cuentas Nacionales y Banco de la República EECB de la R-CF. PIB. Banco de la República (Banco Central de Colombia). 2021. http://www.banrep.gov.co/es/pib.
Kawalec P. Indirect costs of inflammatory bowel diseases: Crohn’s disease and ulcerative colitis. A systematic review. Arch Med Sci. 2016;12(2):295–302.
Constantin J, Atanasov P, Wirth D, et al. Indirect costs associated with ulcerative colitis: a systematic literature review of real-world data. BMC Gastroenterol. 2019;19(1):179.
Augustovski F, Beratarrechea A, Irazola V, et al. Patient preferences for biologic agents in rheumatoid arthritis: a discrete-choice experiment. Value Health. 2013;16(2):385–93.
Vellopoulou K, Stefanou G, Tzanetakos C, et al. Cost-effectiveness of tofacitinib for the treatment of moderate to severe active ulcerative colitis in Greece. Eur J Gastroenterol Hepatol. 2021;33(3):325–33. https://pubmed.ncbi.nlm.nih.gov/32976189/.
Wu B, Zhang Z, Qiang W. Cost-effectiveness of different strategies for the treatment of moderate-to-severe ulcerative colitis. Inflamm Bowel Dis. 2018;24(11):2291–302.
Wilson MR, Bergman A, Chevrou-Severac H, et al. Cost-effectiveness of vedolizumab compared with infliximab, adalimumab, and golimumab in patients with ulcerative colitis in the United Kingdom. Eur J Health Econ. 2018;19(2):229–40.
Singh S, Murad MH, Fumery M, Dulai PS, Sandborn W, et al. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol. 2020;18(10):2179-2191.e6.
Trigo-Vicente C, Gimeno-Ballester V, García-López S, López-Del VA. Systematic review and network meta-analysis of treatment for moderate-to-severe ulcerative colitis. Int J Clin Pharm. 2018;40(6):1411–9.
Reyes G, Gil F, Carvajal G. Enfermedad inflamatoria intestinal: características de fenotipo y tratamiento en un hospital universitario de Bogotá, Colombia. Rev Colomb Gastroenterol. 2018;33(2):117–26.
Romero-Prada M, Marrugo-Figueroa R, Acero G, et al. Impact on the access to medication in Colombian population after the benefit plan update in 2012. Rev Gerencia Polit Salud. 2014;13(7):228–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
Juan M. Reyes, Luisa Amador, and Natalia Castano are paid employees of Pfizer S.A.S. Fabio Gil and Fabian Juliao-Baños have received speakers fees from several pharmaceutical companies.
Funding
This work was funded by Pfizer S.A.S.
Author contributions
JMR and NC had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept, design, analysis, or interpretation of data: FG, FJ-B, LA, NC, JMR. Drafting of the manuscript: JMR, NC. Critical revision of the manuscript for important intellectual content: FG, FJ-B, LA.
Ethics approval
No ethics approval is required for this economic evaluation. As only previously published studies were included and reported in this research, no additional formal ethical assessment was required.
Consent to participate
As only previously published studies were included and reported in this research, no informed consent was required.
Consent for publication (from patients/participants)
As only previously published studies were included and reported in this research, no informed consent for publication was required.
Availability of data and material
The data supporting the findings of this study are available within the article and its electronic supplementary materials.
Code availability
Code sharing is not applicable to this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gil, F., Juliao-Baños, F., Amador, L. et al. Cost Effectiveness of Tofacitinib for the Treatment of Moderate to Severe Active Ulcerative Colitis in Colombia. PharmacoEconomics Open 6, 837–846 (2022). https://doi.org/10.1007/s41669-022-00360-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-022-00360-4