Abstract
Background
A range of treatments for patients with severe hemophilia A (HA) have been developed over the last decade, allowing for reduced frequency of administration and improved outcomes (joint health and breakthrough bleeding rates). While clinically effective, the cost effectiveness of these treatments has not been established.
Objective
This study presents a cost-effectiveness analysis of contemporary rFVIII treatments for severe HA patients without inhibitors.
Methods
A published semi-Markov model was used to compare three different prophylaxis regimens: (1) extended half-life (EHL) recombinant Factor VIII (rFVIII) Fc-fusion protein (rFVIIIFc, Eloctate®, Sanofi), (2) EHL PEGylated rFVIII (PEG-rFVIII, Adynovate®, Takeda), and (3) standard half-life (SHL) rFVIII (antihemophilic factor [recombinant], Advate®, Takeda), used as a proxy for all SHL rFVIII treatments. Acquisition costs were included based on published dosing and weight data. Benefits were incorporated through published annualized bleeding rates, rates of target joint development/resolution, and improvements in the modified hemophilia joint health score. Results were presented as total, discounted costs, and quality-adjusted life-years (QALYs).
Results
rFVIIIFc was shown to provide the most QALYs (27.922) compared with both PEG-rFVIII (27.454) and SHL rFVIII (27.071), at lower costs. Discounted lifetime costs were estimated at US$18.235m (rFVIIIFc), US$20.198m (PEG-rFVIII), and US$18.285m (SHL rFVIII), and were predominantly affected by model settings related to acquisition costs, patient weight, and dosing.
Conclusions
rFVIIIFc may offer a cost-effective option for severe HA patients. Uncertainties owing to the limited evidence base is the main limitation of the study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Prophylaxis with recombinant Factor VIII (rFVIII) has been shown to lead to substantial improvement in outcomes for patients with hemophilia A compared with on-demand use, though rFVIII consumption comprises the majority of costs in the management of this condition. |
rFVIIIFc, an extended half-life rFVIII product, may provide cost savings and improve quality of life versus another extended half-life rFVIII product (PEG-rFVIII) and standard half-life rFVIII products, yet the differences in costs and effects versus these therapies remain uncertain. |
This study illustrates that the key determinants of cost effectiveness in hemophilia A are related to product acquisition costs and how impacts on joint health translate to changes in health-related quality of life. |
1 Introduction
Hemophilia A (HA) is a rare genetic disorder caused by deficiencies in the clotting protein Factor VIII (FVIII). There are approximately 20,000 people in the US living with HA, with approximately 56.6% of cases termed ‘severe’ (defined as patients with an endogenous factor VIII coagulant activity [FVIII:C] level <1% of the ‘normal’ amount) [1, 2]. As HA is caused by mutation of the X chromosome, nearly all patients are male; however, in rare cases HA may also occur in females.
Patients with HA are at risk of life-threatening bleeding, particularly those with severe disease [2, 3]. Due to lower FVIII levels compared with healthy individuals, patients with severe HA (SHA) bleed for longer periods of time—both externally (for example, due to trauma) or internally (predominantly into joints and muscles). Soft tissue (muscle) bleeding and hemarthroses (joint bleeding) in particular can be severely debilitating for patients with SHA, and are associated with impaired mobility, a risk of developing arthropathy (disease of the joint), and reduced quality of life [2,3,4,5].
Konkle et al. (2019) recently reviewed a range of clinical studies in hemophilia with the goal of identifying which outcomes are of most importance to patients and providers [6]. The authors of this study noted that traditional endpoints related to bleeding are of clear importance to both providers and patients, especially when considering the link between joint bleeds and the preservation of joint health. However, bleeding episodes alone do not reflect the full impact of SHA on patients, and other measures (such as the development of complications and ability to perform usual activities) are also important to capture within contemporary trials.
The standard of care for patients with SHA in the US currently comprises prophylaxis using recombinant FVIII (rFVIII) replacement products for the prevention of bleeding episodes, plus episodic rFVIII treatment for the resolution of breakthrough bleeds.
Some patients may develop alloantibodies against exogenous FVIII (typically referred to as ‘inhibitors’), rendering rFVIII therapies ineffective, and so alternative treatments such as bypassing agents and other non-factor therapies may be used. However, the preferred outcome is to eliminate the inhibitor, for which immune tolerance induction may be needed.
Some patients may be treated with higher doses of rFVIII based upon the individual patient’s age, disease history, and level of physical activity. In spite of the different treatment options that may be used, the primary goals of treatment for patients with HA are twofold: first, to prevent or reduce the frequency of bleeding episodes; and second, to minimize the complications of bleeding, namely, to preserve joint health (i.e., to avoid further deterioration due to hemarthroses and/or soft tissue bleeding) [6,7,8,9].
Since the introduction of rFVIII therapies in the US in the 1990s, the treatment landscape for patients with SHA has evolved markedly. More recent rFVIII products have been described as having an extended half-life (EHL) compared with previously developed, ‘standard’ half-life (SHL) rFVIII products. EHL products may be administered less frequently while achieving similar FVIII:C levels compared with an SHL product. Alternatively, with EHL rFVIII products, patients may be treated at the same frequency and dose as with SHL rFVIII products, but are expected to spend less time below a given targeted endogenous FVIII:C level such that the risk of experiencing breakthrough bleeds is reduced.
The first FDA-approved EHL product was rFVIII Fc-fusion protein (rFVIIIFc, Eloctate®, Sanofi). The pivotal A-LONG study allowed patients to be treated with an individualized regimen, every 3–5 days, in order to maintain appropriate FVIII:C levels (ClinicalTrials.gov identifier: NCT01181128, further information reported by Mahlangu et al. [10]). Individualized regimens can be tailored to a number of patient-specific considerations, such as bleeding risk, pharmacokinetic profile, joint status, and physical activity/lifestyle [11].
Since the initial approval of rFVIIIFc in 2014 [12], other EHL FVIII products, such as PEGylated rFVIII (PEG-rFVIII, Adynovate®, Takeda) have been approved and shown to be associated with benefits in terms of reduced frequency of administration and lower annualized bleed rates (ABRs) versus SHL rFVIII treatment [10, 13]. The use of SHL versus EHL rFVIII products for the treatment of SHA patients over time was considered by Croteau et al. [14]. In this study, the authors found that over an 18-month period from mid-2016 to late 2017, EHL rFVIII use more than doubled, increasing from approximately 13% to 28% of patients [14].
To establish the relative value of treatments, cost-effectiveness analysis (CEA) may be performed in order to compare the costs and effects attributable to each treatment. This study presents the results of a CEA of potential rFVIII-based treatments for severe HA adult patients without inhibitors in the US. The aim of the study was to determine the cost effectiveness of these treatments, and to establish the key drivers of CEA results in this patient population. The CEA adopts a US healthcare payer perspective.
2 Methods
2.1 Analysis Scope
The population considered within the analysis was patients with severe HA without inhibitors, initiating prophylaxis from the age of 1 year. Three prophylaxis treatments were compared: (i) rFVIIIFc (EHL), (ii) PEG-rFVIII (EHL), and (iii) SHL rFVIII. For the purpose of this analysis, antihemophilic factor (recombinant) (Advate®, Takeda) was assumed to represent a proxy for a range of SHL rFVIII products used in clinical practice. Alternative costs associated with other SHL rFVIII products were explored with sensitivity analyses.
Outcomes of the analysis were expressed as total costs and quality-adjusted life-years (QALYs). The QALY is a composite measure that aims to incorporate both the health-related quality and length of life. From these outcomes, the cost per QALY gained may be calculated (i.e., the incremental cost-effectiveness ratio [ICER]). Different treatments are expected to provide similar clinical benefits, which may lead to a difference in QALYs close to zero; however, costs are expected to differ.
In line with the reference case used in the Institute for Clinical and Economic Review’s assessment of emicizumab in the inhibitor population, the CEA adopted a lifetime horizon, with a discount rate of 3% applied for both costs and health outcomes (i.e. QALYs). A model cycle length of 1 year was used, with a half-cycle correction applied based on published guidance [15, 16].
2.2 Model Structure and Patient Population
The model used to inform this CEA builds on a previously used structure to assess the cost effectiveness of rFVIIIFc for Italian SHA patients [17]. The model structure was chosen based on the ability to capture the impact of treatment on joint health and translate this into both costs and outcomes within the model. The previous study did not include a comparison with PEG-rFVIII, yet the structure is aligned with commonly reported outcomes that are documented within published clinical studies (i.e., bleeding events and capturing the impact of joint disease) [6].
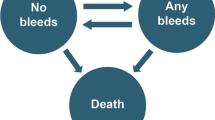
A three-state semi-Markov model was constructed in Microsoft® Excel (Fig. 1). Patients were categorized by target jointFootnote 1 (TJ) status at baseline, and transitions between health states were based on calculated rates of TJ development or resolution. Transitions to death were based on age-dependent background mortality rates.
Patient health-related quality of life (HRQoL) was captured within the model according to the number of bleeds per year (ABR), and whether or not the patient had TJ(s). Only treatment acquisition costs were included within the model (prophylaxis and episodic treatment), as all other costs were assumed to be similar between treatments.
All patients were assumed to be male, and at baseline 68.5% were assumed to have pre-existing TJs (aligned with the A-LONG study population). The starting age of the cohort was set at 1 year, in line with the licensed indications for rFVIIIFc, PEG-rFVIII, and SHL rFVIII. In specifying a starting age of 1 year, differences in the longer-term costs and effects of treatment were captured as patients age.
2.3 Efficacy Inputs
The impact of treatment of patient outcomes was captured as a combination of impacts due to the frequency of breakthrough bleeding episodes, and the impact on joint health. To capture the impact of bleeding episodes, ABRs were identified from published literature. The values used to inform the base-case analyses are presented in Table 1, with alternative values explored within sensitivity analysis. For consistency between the evidence sources, the median ABR associated with all reported bleeds was used for each regimen.
To capture the impact of treatments on joint health, probabilities were included within the model relating to the development or resolution of TJs. For simplicity, it was assumed that patients could not develop further TJs while receiving either rFVIIIFc or PEG-rFVIII, based on very few reports of TJ development in patients receiving EHL rFVIII treatment. However, for SHL rFVIII, a 9.87% probability of developing TJs per year was used [17].
For the resolution of TJs, further data are available. The probabilities used within the economic model (per annual model cycle) are
-
rFVIIIFc: 99.15%, based on data from A-LONG (n = 233 of 235 of TJs at baseline were resolved over a 12-month period) [19]
-
PEG-rFVIII: 85.53%, based on data from Mullins et al., where n = 10 of 14 pediatric patients with TJs at baseline resolved one or more TJs within a 6-month period [20]
-
SHL rFVIII: 73.33%, based on data from Panicker et al., which showed that 11 of 15 patients with TJs at baseline had no further TJ bleeds after at least 1 year of follow up [21]
As per the base-case settings for bleeding outcomes, alternative settings for joint outcomes were explored in sensitivity analysis. In reality, some bleeding episodes may be fatal, though this is expected to be a very small proportion of bleeding events, thus mortality effects were omitted from the analysis. The model therefore assumes all deaths are informed via background mortality rates (US life tables) [22].
2.4 Quality of Life Inputs
To incorporate changes in quality of life within the CEA, HRQoL data were sourced to inform the model. Health state utility values were incorporated via a multivariate Poisson regression analysis by O’Hara et al. [23]. The regression included covariates for the presence of TJs, age, and country (the UK, France, Germany, Spain, and Italy). For the purpose of this analysis, patients from Germany were assumed to serve as a proxy for the US, and the impact on results when considering each of the other four countries was explored within sensitivity analysis. As age and TJ status influence utility, there are no fixed health state utility values provided within the model. However, for every year a patient ages, utility is expected to fall by 0.005, and TJ status is associated with a decrement of 0.110.
Separate to the health state utilities, breakthrough bleeding episodes were associated with a utility decrement. A utility decrement of 0.16 was estimated from Neufeld et al., and applied for an assumed bleed duration of 5 days per the published report by the Institute for Clinical and Economic Review of emicizumab for severe HA patients with inhibitors [24, 25]. The utility decrement per bleed was adjusted according to age, using age-specific multipliers [26].
Treatment with Eloctate® has been shown to be associated with an improvement in modified hemophilia joint health score (mHJHS) of 4.1 points [9]. To capture the impact of utility on changes in mHJHS, a persistent utility benefit of 0.012 (i.e., 0.01 per unit increase in the mHJHS) was assigned to patients treated with Eloctate® (and set to 0 for all other treatments) [27]. This was included to capture utility benefits associated with joint health outside of resolving TJs alone.
2.5 Cost Inputs
Only product acquisition costs (including excess factor product consumption) were included within the analysis, as all other medical resource use was expected to be reasonably similar between alternative treatments (consistent with the approach taken in other previous economic evaluations of rFVIII therapies in the SHA population [17, 28]). Healthcare utilization costs were captured in the Institute for Clinical and Economic Review’s assessment of emicizumab for patients with inhibitors, yet these were assumed equal between prophylaxis regimens and therefore not considered within the current analysis.
As all included treatments are dosed according to patient weight, age-specific weight data for the US HA population were used to inform the model. These data were taken from the aforementioned Institute for Clinical and Economic Review report. To determine the total weekly consumption of each product, the average dose per administration was multiplied by the frequency of administration per week and patient weight.
The average dose per administration for each product used to inform the analysis is presented in Table 2, alongside the cost per international unit (IU) and the available vial sizes/potencies. Unit costs were taken from AnalySource® [29]. Excess consumption was costed by rounding each administration to the nearest 250-IU vial. Costs were taken for the year 2020.
To resolve breakthrough bleeding episodes, episodic rFVIII treatment may be required. All bleeds were assumed to be treated (as treated versus untreated bleeds were not explicitly reported in each of the clinical trials used to inform the model). Breakthrough bleeds were assumed to be treated with the prophylaxis regimen used. The dose of rFVIII to resolve each bleed was assumed to be 50.0 IU per kg for all prophylaxis regimens.
3 Results
3.1 Headline Model Results
Table 3 presents the headline results of the CEA. Compared with PEG-rFVIII, rFVIIIFc provided more QALYs (27.922 versus 27.454) at a lower cost (2020 US$18.235 million versus US$20.198 million). This means that rFVIIIFc dominates PEG-rFVIII (i.e., provides more QALYs at a reduced cost). The key driver of the difference in QALYs was the improvement in the mHJHS and modeled ABR (given that TJ resolution was similar across both treatment arms); whereas the difference in the costs of each prophylaxis regimen led to large cost savings associated with rFVIIIFc.
Compared with SHL rFVIII, rFVIIIFc also provided more QALYs (27.922 versus 27.071) at similar, though slightly lower costs (US$18.235 million versus US$18.285 million). The QALY gain in this comparison is larger owing to the relatively greater improvement in terms of ABR, as well as the increased risk of TJ development for patients treated with SHL rFVIII. Costs were similar in this comparison owing to the trade-off between the per-unit cost of each product, versus the number of units required per week.
3.2 Sensitivity Analyses
Owing to the correlation between several key model parameters that were estimated in isolation from each other (e.g., ABR values for separate interventions), a traditional one-way sensitivity analysis (OWSA) was not performed. Instead, a series of deterministic scenario analyses were undertaken to more appropriately explore the impact of varying key model settings and assumptions on the results (presented in Table 4).
Sensitivity analyses explored the impact of reducing the duration of a bleed from 5 to 2 days, and increasing it to 7 days. Reducing the duration led to an increase in total QALYs for all arms (and vice versa for the 7-day scenario), however the overall conclusions remained unchanged. Similar findings were shown when varying the magnitude of the disutility, instead of varying the duration over which it was applied.
In the base-case analysis, Germany was selected as a proxy country to inform estimation of utility values. In scenario analyses, the region coefficient for the other four countries was applied, which caused the total QALY gains to vary markedly; however, the incremental QALY gain between treatments was essentially unchanged.
Limited data are available to inform the model transition probabilities (i.e., the development or resolution of TJs), and so sensitivity analysis was performed to establish the impact of varying these assumptions. The scenarios explored included assuming no TJ resolution (0% probability) for all arms, complete TJ resolution (100% probability) within the first year for all arms, or an assumed probability of TJ development (1% probability) for all arms per year. As with the other scenarios explored, the findings of these analyses were also consistent with the base-case results.
Additional scenarios were considered varying the per-unit price of SHL rFVIII therapies, reflective of the range of options currently available to patients in practice. When considering the lower bound price (equivalent to US$1.69 per IU), rFVIIIFc was associated with incremental costs in the region of US$373,000. However, when using the upper bound cost of US$1.77 per IU, the cost savings estimated in the base-case analysis increased further to US$473,000.
Finally, a scenario analysis was conducted to explore the impact in results if rFVIIIFc and PEG-rFVIII were administered once per week (instead of twice weekly per the base-case analysis), but with the same overall factor consumption. Both of these products may be used with a relatively longer time period (versus SHL rFVIII) between administrations, owing to their EHL. The reduced frequency of administration leads to further cost savings due to less excess rFVIII consumption.
4 Discussion
This study presents the results of a CEA (based on a previously published economic model) comparing three alternative rFVIII prophylaxis treatments for adults with severe HA without inhibitors. The results of the base-case analysis demonstrate that rFVIIIFc is expected to provide more QALYs than its comparators, and is associated with cost savings.
In the non-inhibitor severe HA population, previously published studies have explored the cost effectiveness of alternative rFVIII regimens, though typically these have comprised a single comparison [17, 28, 33]. However, to date (and to the authors’ knowledge) no formal cost-utility comparisons between EHL rFVIII prophylaxis regimens have been conducted, and therefore this was the focus of our study.
Sensitivity analyses were conducted to explore the impact of alternative model settings and assumptions, as well as the choice of data source(s) on results. In general, the overall findings of the base-case analysis were unchanged when varying assumptions. However, costs were found to be most sensitive to acquisition costs, weight, and dosing assumptions; with QALYs most sensitive to the application of improved joint health via the mHJHS.
The lack of available data regarding joint health is a key limitation of this analysis. Until recently, prevention of joint damage was not considered a primary goal of treatment, as historical treatment strategies focused predominantly on the prevention of bleeding episodes. Following the success of routine prophylaxis in preventing breakthrough bleeds, preservation of joint health has become an achievable goal of treatment, and therefore is also an increasingly relevant outcome measure in contemporary trials. Further evidence collection is planned concerning long-term joint health outcomes, which may facilitate an update to this CEA to include other aspects of joint health (for instance, cost savings through avoided surgeries). Such data collection may, in principle, also allow for the development of a more complex model structure (e.g., a simulation model), which is not possible to robustly develop with current data.
In April 2018, the Institute for Clinical and Economic Review published its review of emicizumab (Hemlibra®, Roche) for the treatment of severe HA patients with inhibitors [24]. The review found emicizumab use led to cost savings with improved clinical outcomes versus prophylaxis with bypassing agents in the severe HA population of patients with inhibitors. However, the scope of the review was restricted to the inhibitor population only (in line with the license for emicizumab at the time this review was conducted).
More recently, in August 2020, the Institute for Clinical and Economic Review published its draft assessment of valoctocogene roxaparvovec (Roctavian®, BioMarin) and emicizumab for the non-inhibitor population [34]. However, conducting a comparison between valoctocogene roxaparvovec, emicizumab, and rFVIII products is challenging, in light of varying product profiles and the relatively limited evidence on these products for a robust and balanced comparison between these treatment options. Therefore, a comparison between rFVIII products and other non-rFVIII products was not considered in this study, but may be possible to consider in the future.
Medical resource costs not related to product acquisition were omitted from the CEA as there are no major differences expected between the management of patients treated with alternative treatments. However, patients with a lower ABR would theoretically have a lower medical resource use cost, and so the cost effectiveness of treatments that reduce ABR may be underestimated by our analysis (due to medical resource use cost savings not captured by the model).
A further limitation of the study is that the acquisition costs for each product may be subject to a number of confidential pricing agreements, and were therefore not captured within the CEA. In lieu of average sales prices (ASPs) to inform the CEA, wholesale acquisition costs (WACs) were used. Consequently, the ‘true’ cost effectiveness of alternative treatments may be affected if the CEA is informed by the ‘true’ acquisition costs (i.e., ASPs instead of WACs). This remains a key limitation of the findings of this study, and indeed of other CEAs conducted in the severe HA population based on publicly available data.
The available data to inform the CEA is predominantly based on studies in adult patients. While there are pediatric studies for rFVIIIFc and PEG-rFVIII, at the time of the analyses there were no available studies for emicizumab or valoctocogene roxaparvovec (though emicizumab is licensed for use in ages newborn and older) [35]. In practice, pediatric patients are expected to be monitored more closely than adults, given that effective management in the early years of life is associated with substantially reduced risk of longer-term complications (e.g., joint damage). The focus of this CEA was on comparisons where pediatric data were available, in order to estimate the lifetime costs and effects associated with alternative treatments. Further evidence collection is required in order to establish the cost effectiveness of other treatments.
The impact of breakthrough bleeding episodes is expected to also affect caregivers—both in terms of financial burden and quality of life. Impacts on caregivers were not captured within the CEA for the same reason that medical resource costs were not included (i.e., no major differences expected between treatments), and also in acknowledgement of pediatric patients being the primary recipients of informal care. Nevertheless, the burden of sub-optimal management for severe HA patients on caregivers is an important consideration when determining the most appropriate treatment for a given patient.
5 Conclusions
This study builds upon a previously published CEA in SHA to facilitate comparisons of contemporary rFVIII treatment options for adults with severe HA, without the presence of inhibitors. The model utilizes available data concerning joint health, dosing, and rates of breakthrough bleeding in order to capture multiple aspects of the disease and its impact on patients. Based on the findings of this analysis, rFVIIIFc may offer a clinically and cost-effective option for patients with severe HA, especially compared with PEG-rFVIII, with cost savings in the region of US$2 million over a lifetime horizon.
The key limitation of the CEA is a lack of data availability to fully capture changes in joint health over time, and how products compare within a head-to-head trial setting. Further to this, like many other previously developed economic evaluations, the model does not capture the development of inhibitors. Further research that encompass the full range of impacts on joint health and the risk of inhibitor development, especially within the context of a pediatric population, is warranted to expand upon the current CEA analysis.
Notes
The definition of a TJ has changed over time, but typically refers to the frequent occurrence of bleeding into a given joint within a relatively short time period. The International Society on Thrombosis and Hemostasis (ISTH)-accepted definition of a TJ is considered to be a single joint into which three or more spontaneous bleeds occur within a consecutive 6-month period.
References
National Hemophilia Foundation. Hemophilia A [Internet]. Natl. Hemoph. Found. 2014. https://www.hemophilia.org/Bleeding-Disorders/Types-of-Bleeding-Disorders/Hemophilia-A. Accessed 21 Apr 2020.
Soucie JM, Wang C, Siddiqi A, Kulkarni R, Recht M, Konkle BA, et al. The longitudinal effect of body adiposity on joint mobility in young males with Haemophilia A. Haemoph Off J World Fed Hemoph. 2011;17:196–203.
Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1-47.
Kuijlaars Ia R, Timmer MA, de Kleijn P, Pisters MF, Fischer K. Monitoring joint health in haemophilia: factors associated with deterioration. Haemophilia. 2017;23:934–40.
Knobe K, Berntorp E. Haemophilia and joint disease: pathophysiology, evaluation, and management. J Comorbidity. 2011;1:51–9.
Konkle BA, Skinner M, Iorio A. Hemophilia trials in the twenty-first century: defining patient important outcomes. Res Pract Thromb Haemost. 2019;3:184–92.
Puthenveetil G, Nugent D. Hemophilia—impact of recent advances on management. Indian J Pediatr. 2020;87:134–40.
Rodriguez-Merchan EC. Articular bleeding in hemophilia. Cardiovasc Hematol Disord Drug Targets. 2016;16:21–4.
Oldenburg J, Kulkarni R, Srivastava A, Mahlangu JN, Blanchette VS, Tsao E, et al. Improved joint health in subjects with severe haemophilia A treated prophylactically with recombinant factor VIII Fc fusion protein. Haemophilia. 2018;24:77–84.
Mahlangu J, Powell JS, Ragni MV, Chowdary P, Josephson NC, Pabinger I, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123:317–25.
Poon M-C, Lee A. Individualized prophylaxis for optimizing hemophilia care: can we apply this to both developed and developing nations? Thromb J. 2016;14:65–71.
Food and Drugs Administration (FDA). ELOCTATE® Prescribing Information [Internet]. 2014. https://www.fda.gov/media/88746/download. Accessed 22 Apr 2020.
Konkle BA, Stasyshyn O, Chowdary P, Bevan DH, Mant T, Shima M, et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126:1078–85.
Croteau SE, Cheng D, Cohen AJ, Holmes CE, Malec LM, Silvey M, et al. Regional variation and cost implications of prescribed extended half-life factor concentrates among U.S. Haemophilia Treatment Centres for patients with moderate and severe haemophilia. Haemoph Off J World Fed Hemoph. 2019;25:668–75.
Drzal R, Szmurlo D, Plisko R. Can we determine the optimal cycle length for which half-cycle correction should always be applied? Value Health. 2013;16:A27.
O’Mahony JF, Newall AT, van Rosmalen J. Dealing with time in health economic evaluation: methodological issues and recommendations for practice. Pharmacoeconomics. 2015;33:1255–68.
Bullement A, McMordie ST, Hatswell AJ, Li N, Wilson K. Cost-effectiveness analysis of recombinant factor VIII Fc-fusion protein (rFVIIIFc) for the treatment of severe hemophilia A in Italy incorporating real-world dosing and joint health data. Pharmacoeconomics. 2019. https://doi.org/10.1007/s41669-019-0158-8.
Nolan B, Mahlangu J, Pabinger I, Young G, Konkle BA, Barnes C, et al. Recombinant factor VIII Fc fusion protein for the treatment of severe haemophilia A: final results from the ASPIRE extension study. Haemoph Off J World Fed Hemoph. 2020;26:494–502.
Wang M, Pasi KJ, Pabinger I, Kerlin BA, Kulkarni R, Nolan B, et al. Long-term efficacy and quality of life with recombinant factor VIII Fc fusion protein (rFVIIIFc) prophylaxis in pediatric, adolescent, and adult subjects with target joints and severe hemophilia A. Blood. 2016;128:3791–3791.
Mullins ES, Stasyshyn O, Alvarez-Román MT, Osman D, Liesner R, Engl W, et al. Extended half-life pegylated, full-length recombinant factor VIII for prophylaxis in children with severe haemophilia A. Haemoph Off J World Fed Hemoph. 2017;23:238–46.
Panicker J, Warrier I, Thomas R, Lusher M. The overall effectiveness of prophylaxis in severe haemophilia. Haemophilia. 2003;9:272–8.
Center for Disease Control and Prevention (CDC). Life table for males: United States, 2017 [Internet]. 2019. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/NVSR/68_07/. Accessed 22 Apr2020.
O’Hara J, Walsh S, Camp C, Mazza G, Carroll L, Hoxer C, et al. The impact of severe haemophilia and the presence of target joints on health-related quality-of-life. Health Qual Life Outcomes. 2018;16:84.
Institute for Clinical and Economic Review (ICER). Emicizumab for Hemophilia A with Inhibitors: Effectiveness and Value. Evidence Report [Internet]. 2018. https://icer-review.org/wp-content/uploads/2017/08/ICER_Hemophilia_Evidence_Report_031518.pdf. Accessed 11 Jan 2019.
Neufeld EJ, Recht M, Sabio H, Saxena K, Solem CT, Pickard AS, et al. Effect of acute bleeding on daily quality of life assessments in patients with congenital hemophilia with inhibitors and their families: observations from the dosing observational study in hemophilia. Value Health. 2012;15:916–25.
Szende A, Janssen B, Cabases J, editors. Self-Reported Population Health: An International Perspective based on EQ-5D [Internet]. Springer Netherlands; 2014. https://www.springer.com/gp/book/9789400775954. Accessed 22 Apr 2020.
Li N, Bullement A, McMordie S, Hatswell AJ, Wilson K. RO4: cost-effectiveness analysis of rFVIIIFc, Pegylated rFVIII, and emicizumab for the prophylactic treatment of severe hemophilia A patients without inhibitors in the United States. Value Health. 2019;22:S389.
Henry N, Jovanović J, Schlueter M, Kritikou P, Wilson K, Myrén K-J. Cost-utility analysis of life-long prophylaxis with recombinant factor VIIIFc vs recombinant factor VIII for the management of severe hemophilia A in Sweden. J Med Econ. 2018;21:318–25.
First Databank. AnalySource® as reprinted with permission by First Databank Inc. All rights reserved. © 2020. https://www.fdbhealth.com/. Accessed 8 Oct 2020.
Schwartz EL, Epstein J, Xiong Y, Hafeman A, Valentino LA. Utilization predictability for BAX 855 vs. BIIB031: a descriptive comparison from pivotal trials. In: Presented at the international society on thrombosis and haemostasis, June 20–25 2015, Toronto, Canada; 2015.
Valentino LA, Mamonov V, Hellmann A, Quon DV, Chybicka A, Schroth P, et al. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost JTH. 2012;10:359–67.
Blanchette VS, Shapiro AD, Liesner RJ, Hernández Navarro F, Warrier I, Schroth PC, et al. Plasma and albumin-free recombinant factor VIII: pharmacokinetics, efficacy and safety in previously treated pediatric patients. J Thromb Haemost JTH. 2008;6:1319–26.
Cook K, Forbes SP, Adamski K, Ma JJ, Chawla ALPG Jr. Assessing the potential cost-effectiveness of a gene therapy for the treatment of hemophilia A. J Med Econ. 2020;23:501–12.
Institute for Clinical and Economic Review (ICER). Valoctocogene Roxaparvovec and Emicizumab for Hemophilia A: Effectiveness and Value. Draft Evidence Report [Internet]. 2020. https://icer-review.org/wp-content/uploads/2019/12/ICER_Hemophilia-A_Draft-Evidence-Report_082620-1.pdf. Accessed 3 Sep 2020.
Food and Drugs Administration (FDA). HEMLIBRA® Prescribing Information [Internet]. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761083s002s004lbl.pdf. Accessed 22 Apr 2020.
Acknowledgements
The authors thank Koo Wilson, Nanxin Li, Anthony Hatswell, and Samuel McMordie for their input into the cost-effectiveness analysis from which this analysis builds upon. In addition, the authors thank the patients and investigators involved in the A-LONG clinical study of rFVIIIFc, as well as those involved in the other supporting clinical studies, without which this cost-effectiveness analysis would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Sanofi. Editorial support in the preparation of this publication was provided by Ash Bullement and Emma S. Knowles of Delta Hat Ltd and funded by Sanofi.
Conflict of interest
AB and ESK are employees of Delta Hat Limited, a private consultancy that has received funding from Sanofi for the research reported within this manuscript. RP and PD are employees and stockholders of Sanofi. TA was an employee of Sanofi at the time of study and is currently an employee of uniQure N.V. The views expressed are those of the author(s) and not necessarily those of the author affiliations.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Availability of data and material
The key model inputs and assumptions used to parameterize the model are available within the article, with supporting information (such as background mortality rates) cited.
Code availability
Please see declaration regarding availability of data and material.
Author contributions
All authors contributed to the study concept, design, and data analysis and interpretation. RP conceived the idea for the manuscript, with input from AB, PDasM, and TA. AB and EK conducted the analysis and wrote the draft manuscript. RP, PDasM, and TA critically reviewed the draft analysis and manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Bullement, ., Knowles, .S., DasMahapatra, . et al. Cost-Effectiveness Analysis of rFVIIIFc Versus Contemporary rFVIII Treatments for Patients with Severe Hemophilia A Without Inhibitors in the United States. PharmacoEconomics Open 5, 625–633 (2021). https://doi.org/10.1007/s41669-021-00283-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-021-00283-6