Abstract
Background
Patients with severe hemophilia A (SHA) in Italy are routinely treated with standard half-life recombinant factor VIII (rFVIII) products. rFVIII Fc-fusion protein (rFVIIIFc) is an extended half-life rFVIII product that enables less frequent administration than rFVIII, which may support improved adherence. Available data indicate low breakthrough bleed rates and potentially improved long-term joint health for patients treated with rFVIIIFc prophylaxis.
Objective
This study assessed the cost effectiveness of rFVIIIFc versus rFVIII from an Italian healthcare perspective.
Methods
A Semi-Markov model was constructed to assess the lifetime costs and benefits of rFVIII and rFVIIIFc prophylaxis. rFVIII product acquisition costs from a published Italian database were included for both prophylaxis and the resolution of breakthrough bleeding. Clinical outcomes within the model were determined based on published annualized bleeding rates and literature regarding the development of target joints (TJs) as the incidence of bleeds and TJs is associated with impaired health-related quality of life. Cost effectiveness was assessed using cost per quality-adjusted life-year (QALY) gained.
Results
Compared with rFVIII, rFVIIIFc was associated with a per-patient cost saving of approximately €1.3 million and QALY gains of 0.39 over a lifetime horizon. Sensitivity analyses considering alternative efficacy, dosing, and structural assumptions each showed that rFVIIIFc dominated rFVIII (i.e., provided more QALYs at a reduced cost).
Conclusions
This cost-effectiveness analysis demonstrated that rFVIIIFc may offer a cost-effective treatment option for patients with SHA in Italy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hemophilia A (HA), caused by an inherited deficiency of factor VIII (FVIII), is the most common type of hemophilia, with an incidence of 1 in 5000 male births [1]. Patients with HA are at risk of life-threatening bleeding, particularly patients with severe disease (defined as endogenous factor VIII coagulant activity [FVIII:C] level < 1% of the normal amount), which accounts for approximately 46.2% of Italian cases [2]. More commonly, patients experience bleeding into joints (hemarthroses) and muscles, which can be severely debilitating [3, 4].
Hemarthroses and bleeding into muscles can result in the development of arthropathy (disease of the joint), which is associated with swelling, pain, and reduced movement [4]. Repeated hemarthroses in the same location leads to increased medical resource use and impaired patient health-related quality of life (HRQoL) [5]. As such, the primary treatment goals for patients with severe HA (SHA) pertain to the reduction of bleeding events and the prevention of joint health deterioration.
In Italy, the treatment of SHA most commonly involves routine prophylaxis using recombinant FVIII (rFVIII) replacement products as well as episodic rFVIII treatment for the resolution of breakthrough bleeds. Published literature suggests that 90–92% of total direct healthcare costs associated with HA patients are attributable to drug acquisition [6, 7]. Most adult Italian SHA patients are treated with rFVIII prophylaxis, and nearly all pediatric patients are expected to be treated prophylactically [2].
Compared with on-demand use only (i.e., used only for the resolution of a breakthrough bleed), prophylaxis with standard half-life (SHL) rFVIII treatment has been shown to significantly reduce the annualized bleeding rate (ABR) for SHA patients [8,9,10,11,12,13]. The most commonly used rFVIII product for prophylaxis treatment in Italian practice is Advate® (Shire Pharmaceuticals Ltd) [2, 14].
Extended half-life (EHL) FVIII Fc fusion protein (rFVIIIFc, Elocta®, Swedish Orphan Biovitrum AB) offers an alternative rFVIII treatment option. Its EHL means that patients treated at the same frequency and dose as those using SHL rFVIII products are expected to spend less time below a given targeted trough level (endogenous FVIII:C level) such that the risk of experiencing breakthrough bleeds is reduced [15, 16]. Alternatively, the EHL of rFVIIIFc may permit a reduction in administration frequency versus conventional factor therapies and consequently reduce treatment burden, which may also increase adherence to treatment and improve HRQoL [17]. rFVIIIFc prophylaxis has been studied in both adult and pediatric populations and has been shown to be associated with low ABRs as well as improved joint health outcomes (determined via the modified Hemophilia Joint Health Score [mHJHS]) over time [18,19,20].
Until recently, data regarding the association between joint health and patient HRQoL in patients with SHA were limited. In 2018, O’Hara et al. [5] published a study that provided information regarding the difference in utility between patients with and without “target joints” (TJs), and von Mackensen et al. [21] demonstrated the relationship between improved joint health (measured with the mHJHS) and HRQoL (measured using the EuroQoL 5-Dimensions [EQ-5D] and the Haem-A-QoL questionnaires). In addition, data are now available on the real-world usage of SHL and EHL rFVIII products in the prophylactic setting. Analysis of these data permit an assessment of factor consumption within a real-life setting.
This study aims to assess the cost effectiveness of rFVIIIFc versus SHL rFVIII for the prophylaxis treatment of SHA patients from an Italian healthcare perspective. This analysis makes use of recently published data regarding joint health and real-world usage of rFVIII products and thus provides an estimate of the cost effectiveness of rFVIIIFc versus rFVIII in Italian practice.
2 Methods
2.1 Model Structure
The model used to inform this cost-effectiveness analysis (CEA) built on the structure previously adopted by Henry et al. [22], who assessed the cost effectiveness of rFVIIIFc versus conventional rFVIII for Swedish SHA patients. Henry et al. [22] did not explicitly model the impact of treatment on joint health because published evidence was lacking, particularly for the Pettersson score, which is a scoring system used to reflect the extent of hemophilic arthropathy (permanent joint damage caused by hemophilia-related bleeding) and is widely used in hemophilia clinical trials [22, 23]. Without this evidence, it would not be possible to link the measured joint damage to patient-related outcomes.
While evidence concerning the relationship between patient utility and the Pettersson score remains limited, a study by O’Hara et al. [5] facilitated the specification of a CEA with health states according to TJ status. Other studies in SHA have also captured joint health, but these were primarily constructed to correspond with the availability of patient data. We considered the model structures employed within these previous studies but ultimately rejected them because of the lack of comparably robust data for rFVIII and rFVIIIFc.
The definition of a TJ has changed over time and typically refers to the frequent occurrence of bleeding into a given joint within a relatively short time [24, 25]. However, the definition of a TJ accepted by the International Society on Thrombosis and Hemostasis is a single joint into which three or more spontaneous bleeds occur within a consecutive 6-month period [26]. Patients may develop multiple TJs in different sites, the most common of which are the knee, ankle, or elbow [24, 27]. Data regarding the incidence and/or resolution of TJs are now routinely collected in SHA clinical studies, the latter of which is particularly important given the treatment goals of HA being the prevention of bleeding and joint destruction to preserve normal musculoskeletal function [25].
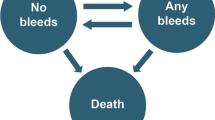
We constructed a three-state semi-Markov model in Microsoft® Excel (overview shown in Fig. 1). Upon model entry, patients are categorized by TJ status (i.e., whether they had at least one TJ). Transitions between the “alive” TJ-related health states were determined according to calculated rates of TJ development or resolution, whereas transitions to death were based on background mortality rates (which are age dependent). The impact of each treatment on patient HRQoL was determined according to the ABR (to determine the impact of a bleed event) and the presence of TJs. The costs considered by the model are related only to Factor consumption (both as prophylaxis and for the resolution of breakthrough bleeding).
In this analysis, we did not consider the probability of developing inhibitors for patients treated with either rFVIII or rFVIIIFc. No clear evidence exists of a differential risk of inhibitor development by prophylaxis regimen, so the inclusion of inhibitor development would be unlikely to affect the overall conclusions that may be made from the analysis.
Guidelines for the pharmacoeconomic evaluation of new drugs in Italy were reported by Capri et al. [28]. They recommend the presentation of outcomes in terms of costs and quality-adjusted life-years (QALYs), annual discount rates for these outcomes of 3.0%, and limiting to 30 years the time horizon over which these outcomes are considered [28]. We applied annual discount rates of 3.0% for both costs and QALYs but considered a lifetime horizon in the model base case (up to 99 years) as patients are treated with prophylaxis indefinitely (and are assumed to enter the model at the age of 1 year). While restricting the time horizon to 30 years would omit important costs and outcomes from the analysis, alternative time horizons were explored within sensitivity analysis. A model cycle length of 1 year was used, and—based on published guidance—a half-cycle correction was applied because the probability of leaving a given health state was > 5% (and the model cycle length is > 2 weeks) [29, 30].
The CEA adopts the perspective of the Italian national health service. The population considered is that of Italian patients with SHA, for which rFVIII prophylaxis represents the standard of care. In Italy, the most commonly used SHL rFVIII is Advate, hence a comparison between prophylaxis regimens with rFVIII (for which Advate is assumed to be representative of Italian practice) and rFVIIIFc is presented [2]. Use of rFVIII to resolve breakthrough bleeding episodes is also included within the analysis because, in the event of a bleed, the condition is controlled with rFVIII.
2.2 Model Inputs
2.2.1 Efficacy
Treatment efficacy was considered in two categories: (1) the impact of treatment on the number of bleeding episodes (measured with the ABR)—“bleeding-related outcomes” and (2) the impact of treatment on joint health—“joint-related outcomes”. Bleeding-related outcomes are well reported in published literature for each treatment, as the ABR is often the primary outcome of clinical trials in HA. However, joint-related outcomes are less reported, so we undertook a literature search to address data gaps.
We used published ABRs to determine the number of bleeds for patients treated with rFVIII and rFVIIIFc per model cycle (1 year), and these were subsequently used to determine the number of QALYs lost as a result of bleeding. The ABRs used for adult and pediatric patients receiving rFVIII and rFVIIIFc are summarized in Table 1 and were taken from the A-LONG, Kids A-LONG, and rAHF-PFM clinical trials (NCT01181128, NCT01458106, NCT00243386, NCT00157040) [19, 20, 31, 32]. We found no trials that provided direct comparisons between rFVIII and rFVIIIFc prophylaxis, so used unadjusted indirect comparisons within the model (as adjustment was not considered possible because of the small number of patients enrolled within each study). However, alternative ABR estimates were explored within sensitivity analysis to ascertain the impact of ABRs on cost-effectiveness results.
For joint-related outcomes, annual probabilities of developing or resolving TJs were applied for patients treated with rFVIII and rFVIIIFc. Sensitivity analyses were conducted to ascertain the impact on results of alternative assumptions regarding joint-related health.
For rFVIIIFc, the probability of TJ resolution was calculated as 99.18% based on data from the A-LONG (n = 233 of 235 resolved) and Kids A-LONG (n = 9 of 9 resolved) clinical trials [19, 33]. Data for the development of TJs were not available; however, given the high probability of TJ resolution, we assumed that no patients developed TJs while receiving rFVIIIFc. Recently available data from the A-LONG and Kids A-LONG studies showed that 95% and 100%, respectively, of TJs with ≥ 6 months of follow-up post-resolution showed no TJ reoccurrence [34]. Therefore, we conducted a scenario analysis wherein the probability of TJ development was assumed to be equal to the proportion of TJs that showed no reoccurrence. However, it should be noted that this scenario assumed that TJ reoccurrence was equivalent to the probability of patients developing a TJ and so should be interpreted with caution.
For rFVIII, the probability of TJ resolution was assumed to be 73.33% based on a study by Panicker et al. [35], wherein 11 of the 15 patients with TJs at baseline had no further TJ bleeds (with a minimum follow-up of 1 year). The study by Panicker et al. [35] was the only study identified by the authors that contained sufficient information to quantify the development of TJs for patients treated with prophylaxis. The probability of TJ development was estimated at 9.87% based on a study by Kern et al. [24] in which 15 of 16 boys developed at least one TJ while receiving episodic SHL rFVIII treatment over a 20-year period, adjusted according to the ratio of TJ-related bleeds observed in a study of once-daily and prophylaxis SHL rFVIII treatment by Manco-Johnson et al. [36].
2.2.2 Dosing
To determine the total weekly consumption of rFVIII per kilogram of body weight, the average dose per administration was multiplied by the frequency of administration per week. The average dose of rFVIII per administration and every-other-day dosing was applied based on a study by Valentino et al. [31] for adults and one by Blanchette et al. [32] for pediatrics. In the model base case, average weekly doses of 109.75 IU/kg for adults and 107.30 IU/kg−1 for pediatrics were applied. Pediatric dosing was applied within the model until 12 years of age (per the license for rFVIIIFc) [33].
For rFVIIIFc, the average reduction in weekly factor consumption versus conventional SHL rFVIII is reported in a range of published studies, identified via a targeted search of conference websites and the MEDLINE® In-Process (using PubMed.com) electronic database [37,38,39,40,41]. A random effects meta-analysis of real-world data concerning the reduction in weekly factor consumption after switching from conventional rFVIII therapy to rFVIIIFc was performed. The results of the meta-analysis are presented in a forest plot (Fig. 2) and indicate a percentage reduction in Factor consumption of 21.8%.
Meta-analysis of real-world dosing. Meta-analysis produced using the R package metafor. Sampling variance values (vi) were not reported in all studies, and so were assumed to be equal to 20% of the mean divided by the square root of the sample size (i.e., a smaller study is associated with a larger variance). CI confidence interval
In the model base case, the reduction in weekly factor consumption derived via the meta-analysis was applied for patients treated with rFVIIIFc versus rFVIII. Sensitivity analyses were conducted within the model to quantify the impact on model results of alternative relative dosing settings.
Age-specific weight data for the US HA population were identified from the recent review of emicizumab for SHA patients with inhibitors by the Institute for Clinical and Economic Review [42]. These data were adjusted using a calculated ratio of mean body weight between males from Italy and the USA of 0.92 to estimate the total Factor consumption of Italian SHA patients per year, and we assumed that weight data were comparable between the inhibitor and non-inhibitor populations [43]. Weight data were available only in 5-year intervals, so weight was assumed to be constant between age bands (e.g., ages 15–19). In sensitivity analysis, the use of unadjusted weight data for the US HA population was explored.
To resolve a bleeding episode, FVIII is required in addition to routine prophylaxis treatment. The use of rFVIII for breakthrough bleeding may require multiple infusions to successfully stop the bleeding, and—like prophylaxis—the dose required is based on patient body weight. Product consumption per year related to breakthrough bleeds was estimated using the following equation.
A summary of the data used to inform the economic model is provided as electronic supplementary material.
2.2.3 Health-Related Quality of Life
The factors affecting patient utility captured within the model were the presence of TJs and the number of bleeds experienced.
Utility values for SHA patients with and without TJs were applied within the model based on a multivariate Poisson regression analysis by O’Hara et al. [5]. The regression analysis provided estimated three-level EQ-5D (EQ-5D-3L) scores for patients with severe hemophilia and included covariates for the presence of TJs, age, and country (including Italy).
Each bleeding event was assumed to be associated with reduced HRQoL. Neufeld et al. [44] reported average utility scores for patients on a “bleed day” (0.660) and a “non-bleed day” (0.820). Assuming the duration of the impact of a bleed on utility is estimable, the number of QALYs lost per bleed may be calculated using the following equation.
Published literature suggests that a bleeding episode may last for anywhere between 1 and 509 h [45]. However, the average duration over which a bleeding episode has an impact on patient utility is unclear. In the model base case, we assumed the detrimental effects of a bleed applied for 5 days based on clinical input and the published report by the Institute for Clinical and Economic Review in which the full impact of a bleed applied for 2 days and a “half impact” applied for the remainder of the weekly model cycle [42]. This assumption was explored in sensitivity analysis. To adjust the number of QALYs lost per bleed event according to age, Italian age-specific multipliers from Scalone et al. [46] were applied to the base number of QALYs lost per bleed event [46].
2.2.4 Costs
rFVIII and rFVIIIFc are available in fixed vial sizes, so patients are often administered excess rFVIII. While this excess rFVIII consumption is considered safe (and may reduce the risk of breakthrough bleeds), the dose received per administration is important to capture within the CEA. Consequently, the average dose per administration is rounded up to the nearest whole vial (reflecting the target dose plus excess consumption due to the vial sizes).
rFVIII (Advate) and rFVIIIFc (Elocta) are available in 250 IU, 500 IU, 1000 IU, 2000 IU, and 3000 IU vials. Both are linearly priced at €0.65 per IU (based on tender prices as of May 2019) [47]. As shown via the meta-analysis (Fig. 2), the total weekly consumption used in practice is lower for rFVIIIFc than for rFVIII because of its EHL. Costs were included based on the 2019 cost year.
2.2.5 Mortality
Tagliaferri et al. [48] conducted a retrospective cohort study to investigate mortality and causes of death in Italian people with hemophilia between 1990 and 2007. They found that the life expectancy of Italian hemophilia patients has increased over time, approaching that of the male general population. This finding was attributed in part to the reduction in plasma-derived FVIII use, which historically was associated with patients contracting blood-related illnesses such as hepatitis C and HIV (the latter of which accounted for 60% of Italian hemophilia patient deaths between 1990 and 1999) [48].
Within the CEA, general population mortality was assumed to apply for Italian SHA patients, so no bleed event was assumed to be fatal. Consequently, the CEA may have underestimated the full benefits of treatment that reduces the rate of bleeding; however, the proportion of bleeds that are fatal is expected to be very small.
2.3 Model Outcomes
The primary outcome of the CEA was the cost per QALY gained (i.e., the incremental cost-effectiveness ratio [ICER]) from the health sector perspective over a lifetime horizon. Sensitivity analysis were conducted to test the robustness of the base-case cost-effectiveness results by exploring assumptions involving efficacy, dosing, and treatment benefit.
3 Results
3.1 Base-Case Results
The base-case results of the CEA are presented in Table 2. Compared with rFVIII, rFVIIIFc provided more QALYs at a reduced cost (i.e., rFVIIIFc dominates rFVIII). The reduction in costs was primarily attributable to the modelled reduction in weekly Factor consumption, and the difference in QALYs was largely related to the modelled resolution of TJs. Patients treated with rFVIII gained more QALYs in the “with TJs” health state than patients treated with rFVIIIFc, as rFVIII was associated with larger probabilities of TJ development and non-resolution.
3.2 Sensitivity Analyses
Scenario analyses were conducted to explore the impact of alternative settings, assumptions, and data sources applied within the CEA. The results of these scenarios are presented in Table 3 and discussed in turn in the following. Probabilistic sensitivity analysis (PSA) was also conducted; however, in practice, many of the model parameters will be correlated (e.g., ABRs, Factor consumption, probability of TJ development, etc.). It was not possible to quantify the correlation between these key parameters. Furthermore, the structural assumptions underpinning the CEA (e.g., the use of TJ-based health states; sources of efficacy data) were considered more relevant than parameter uncertainty to assess the robustness of the base-case CEA results. Consequently, while PSA was conducted, it does not account for the correlation between parameters (as no data are available), or the structural uncertainty, and should therefore be interpreted with caution. The results of the PSA are provided as Electronic Supplementary Material.
The impact of alternative ABRs on the CEA results was explored by using values reported in an indirect treatment comparison (ITC) conducted by Iorio et al. [49] (scenario 1) and the ASPIRE extension study of rFVIIIFc (NCT01454739) (scenario 2) [50]. In the ITC scenario, adult ABRs of 4.85 and 2.90 were applied for rFVIII and rFVIIIFc, respectively (pediatric ABRs were unchanged). In the ASPIRE scenario, ABRs of 1.80 and 1.81 were applied for adult and pediatric patients treated with rFVIIIFc, respectively (values for rFVIII remained per the base case). The results of the sensitivity analyses assuming alternative ABR values were very similar to the base-case results.
In the CEA base case, the impact of a bleed on patient utility was assumed to apply for 5 days. Sensitivity analysis explored the impact of reducing this to 2 days (scenario 3), and increasing it to 7 days, per the setting in the Henry et al. [22] CEA (scenario 4). As expected, reducing the duration of bleed impact caused a reduction in the incremental QALY gain associated with rFVIIIFc (and vice versa for the 7-day scenario), though the overall conclusion that rFVIIIFc dominates rFVIII remained unchanged.
Italian pharmacoeconomic guidelines suggest that lifetime horizon calculations should be limited to a duration of 30 years. However, given the starting age of the cohort of 1 year, the base-case analysis adopts a much longer time horizon (99 years) such that all patients have died by the end of the modelled time horizon. Henry et al. [22] considered a 70-year time horizon. Sensitivity analysis results assuming shorter time horizons of 30 years (scenario 5) and 70 years (scenario 6) provided lower estimates of total costs and QALYs across both treatment arms, with a consistent conclusion of rFVIIIFc dominating rFVIII per the model base case.
Given the limited data available regarding the resolution of TJs, sensitivity analysis was performed to establish the impact of assuming either no TJ resolution (0% probability) from baseline for both treatment arms (scenario 7), complete TJ resolution (100% probability) within the first year for both treatment arms (scenario 8), or the same probability of TJ development (9.87% probability) for both treatments (scenario 9). An additional scenario analysis was undertaken using recently published data regarding the proportion of reoccurring TJs observed within the A-LONG and Kids A-LONG studies as a proxy for TJ development in patients treated with rFVIIIFc (scenario 10) [34]. The results of these analyses illustrate consistent CEA results even when the probability of TJ resolution is varied to extreme values.
Two dosing-based sensitivity analyses were also conducted. The first considered the use of dosing data per the studies included within the ITC by Iorio et al. [49] (i.e., excluding the real-world evidence) (scenario 11). The second analysis considered the use of unadjusted weight data from the US hemophilia population (i.e., removal of the ratio to adjust US data to reflect the Italian population) (scenario 12). Higher costs were noted for patients treated with rFVIIIFc in both sensitivity analyses compared with the base case, though rFVIIIFc continued to dominate rFVIII.
Finally, a threshold analysis was conducted to ascertain what the per-unit cost of rFVIIIFc would need to be such that the total incremental costs were zero (i.e., rFVIIIFc was no longer cost saving). The result of this analysis was that rFVIIIFc would need to be at least €0.83 per IU for the total incremental costs to exceed zero.
4 Discussion
This study presents the findings of a CEA comparing the EHL rFVIII product rFVIIIFc with conventional SHL rFVIII for patients with SHA. The CEA adopted a lifetime horizon and incorporated prophylaxis and bleed-related Factor consumption as well as the impact of bleeding and impaired joint health on patient utility. Real-world evidence regarding Factor use was also included to facilitate a true-to-life estimation of Factor consumption in clinical practice. The base-case results of the CEA demonstrated that rFVIIIFc dominated (provided more QALYs at a reduced cost) rFVIII because of a modelled lower frequency of bleeding, improved joint health, and reduced Factor consumption.
A range of sensitivity analyses were conducted to explore the impact of varying assumptions, data sources, and settings on the CEA results. The overall conclusion remained unchanged across all scenarios explored, though costs were shown to be most sensitive to different model time horizons and dosing assumptions (given that these scenarios had the greatest impact on the estimation of Factor consumption costs). The incremental QALY gain for rFVIIIFc was consistently greater than zero, even when the impact of TJ resolution was omitted from the CEA.
These CEA results are aligned with the previous CEA of rFVIIIFc versus conventional factor therapies by Henry et al. [22]. In this study, the authors also demonstrated that rFVIIIFc dominated a pooled comparator of SHL rFVIII therapies in the Swedish setting. However, as previously discussed, the authors were not able to quantify the impact of joint deterioration because published evidence was lacking when the study was conducted. The consistent findings of both CEAs support the use of rFVIIIFc in clinical practice.
While the incorporation of TJ-based health states within the CEA permitted the inclusion of joint health-related utility values, it is noted that the model structure was limited to the presence or absence of at least one TJ (and does not distinguish between those with multiple TJs). O’Hara et al. [5] demonstrated within their analysis that the presence of multiple TJs was associated with increasingly impaired utility. The use of dichotomous TJ-based health states was based on currently available data to inform the development and/or resolution of TJs and is aligned with a CEA in hemophilia B by van Keep et al. [51].
Further research regarding the long-term probabilities of developing and/or resolving TJs is required to validate the findings of this study. The AHEAD study (NCT02078427) is an ongoing observational study designed to document the natural history of HA [52]. The study is expected to complete in December 2019 and may provide data regarding patient utility, bleed resolution, joint health outcomes, the development of inhibitors, and the incidence of new TJs [52].
The costs captured within the CEA are related entirely to the consumption of Factor product—either as prophylaxis or to treat breakthrough bleeding. We acknowledge that several other direct medical costs are incurred by patients with SHA in Italy, including emergency hospital admissions for severe bleeding episodes and routine monitoring appointments with a hematologist. Data to inform the estimation of non-Factor costs were unavailable for the CEA. However, omission of these costs from the study is not expected to drastically alter the findings, as the reduction in ABR for rFVIIIFc versus rFVIII is expected to lead to reduced medical resource utilization.
We also note that pricing of rFVIII products in Italy is based on a tender market. That is, static list prices do not reflect the cost of each product, and the “true” prices of each product are updated periodically. Our study aims to address fluctuations in pricing for rFVIII products through the presentation of a sensitivity analysis regarding the price of rFVIIIFc, yet we note that such fluctuations may influence the usage of different rFVIII products in Italy and therefore influence the likely cost effectiveness of rFVIIIFc versus SHL rFVIII.
An additional consideration is that, over time, SHA patients may develop an immune response to rFVIII products in the form of inhibitor antibodies. In most cases, patients are able to resolve their inhibitors through a regimen of immune-tolerance induction (ITI) therapy with rFVIII treatment. The development of inhibitors was not captured within the CEA structure. Further research is required to understand the clinical and economic implications of using EHL rFVIII therapies (such as rFVIIIFc) with respect to the development of inhibitors.
The CEA also did not capture the impact of SHA on patient caregivers in terms of both costs and health effects. Kodra et al. [7] found that 21 of the 89 Italian hemophilia patients surveyed required a carer. The authors also determined an average loss of earnings for caregivers of children with hemophilia amounting to €4099.70 per year, though how improved care may affect this figure is unclear. The impact on caregivers and family members further demonstrates the importance of effective treatment options for patients with SHA.
5 Conclusions
This study constitutes the first CEA in SHA to utilize recently published data regarding joint health and real-world dosing to establish the long-term cost effectiveness of rFVIIIFc versus conventional (SHL) rFVIII therapy in the Italian setting. The model structure adopted within this CEA is the first in SHA to distinguish between patients according to the absence or presence of TJs. Our results demonstrate that rFVIIIFc provides a cost-effective treatment option versus SHL rFVIII treatments based on the improved bleeding- and joint-related treatment outcomes predicted by the model (as well as reduced burden of administration because of its EHL). Further research is required to more accurately determine the relative improvement of rFVIIIFc versus SHL rFVIII treatments in terms of overall joint health.
Data Availability
The datasets generated and/or analyzed during the current study are proprietary of Swedish Orphan Biovitrum AB (publ) (Sobi) and Bioverativ (a Sanofi company). Access to datasets and/or the economic model is at the discretion of both Sobi and Bioverativ (a Sanofi company) and may be available from the corresponding author on reasonable request.
References
Berntorp E, Shapiro AD. Modern haemophilia care. Lancet Lond Engl. 2012;379:1447–56.
Lorenzoni V, Triulzi I, Turchetti G. Budget impact analysis of the use of extended half-life recombinant factor VIII (efmoroctocog alfa) for the treatment of congenital haemophilia a: the Italian National Health System perspective. BMC Health Serv Res. 2018;18:596.
Kuijlaars IA, Timmer MA, de Kleijn P, Pisters MF, Fischer K. Monitoring joint health in haemophilia: factors associated with deterioration. Haemophilia. 2017;23:934–40.
Knobe K, Berntorp E. Haemophilia and joint disease: pathophysiology, evaluation, and management. J Comorbidity. 2011;1:51–9.
O’Hara J, Walsh S, Camp C, Mazza G, Carroll L, Hoxer C, et al. The impact of severe haemophilia and the presence of target joints on health-related quality-of-life. Health Qual Life Outcomes. 2018;16:84.
Rocha P, Carvalho M, Lopes M, Araújo F. Costs and utilization of treatment in patients with hemophilia. BMC Health Serv Res [Internet]. 2015;15. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4624363/. Accessed 03 Jan 2019.
Kodra Y, Cavazza M, Schieppati A, De Santis M, Armeni P, Arcieri R, et al. The social burden and quality of life of patients with haemophilia in Italy. Blood Transfus. 2014;12:s567–75.
Tagliaferri A, Feola G, Molinari AC, Santoro C, Rivolta GF, Cultrera DB, et al. Benefits of prophylaxis versus on-demand treatment in adolescents and adults with severe haemophilia A: the POTTER study. Thromb Haemost. 2015;114:35–45.
Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44.
Manco-Johnson MJ, Kempton CL, Reding MT, Lissitchkov T, Goranov S, Gercheva L, et al. Randomized, controlled, parallel-group trial of routine prophylaxis vs. on-demand treatment with sucrose-formulated recombinant factor VIII in adults with severe hemophilia A (SPINART). J Thromb Haemost. 2013;11:1119–27.
Collins P, Faradji A, Morfini M, Enriquez MM, Schwartz L. Efficacy and safety of secondary prophylactic vs. on-demand sucrose-formulated recombinant factor VIII treatment in adults with severe hemophilia A: results from a 13-month crossover study. J Thromb Haemost. 2010;8:83–9.
Gringeri A, Lundin B, von Mackensen S, Mantovani L, Mannucci PM. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J Thromb Haemost JTH. 2011;9:700–10.
Khawaji M, Astermark J, Berntorp E. Lifelong prophylaxis in a large cohort of adult patients with severe haemophilia: a beneficial effect on orthopaedic outcome and quality of life. Eur J Haematol. 2012;88:329–35.
European Medicines Agency (EMA). ADVATE Summary of Product Characteristics [Internet]. 2013. https://www.ema.europa.eu/documents/product-information/advate-epar-product-information_en.pdf.
Ljung R, Fischer K, Carcao M, Santagostino E, Manco-Johnson MJ, Mathew P, et al. Practical considerations in choosing a factor VIII prophylaxis regimen: role of clinical phenotype and trough levels. Thromb Haemost. 2016;115:913–20.
Collins PW, Blanchette VS, Fischer K, Björkman S, Oh M, Fritsch S, et al. Break-through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J Thromb Haemost JTH. 2009;7:413–20.
Tiede A. Half-life extended factor VIII for the treatment of hemophilia A. J Thromb Haemost. 2015;13:S176–9.
Oldenburg J, Kulkarni R, Srivastava A, Mahlangu JN, Blanchette VS, Tsao E, et al. Improved joint health in subjects with severe haemophilia A treated prophylactically with recombinant factor VIII Fc fusion protein. Haemophilia. 2018;24:77–84.
Mahlangu J, Powell JS, Ragni MV, Chowdary P, Josephson NC, Pabinger I, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123:317–25.
Young G, Mahlangu J, Kulkarni R, Nolan B, Liesner R, Pasi J, et al. Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J Thromb Haemost JTH. 2015;13:967–77.
von Mackensen S, Eldar-Lissai A, Auguste P, Krishnan S, von Maltzahn R, Yu R, et al. Measurement properties of the Haem-A-QoL in haemophilia clinical trials. Haemophilia. 2017;23:383–91.
Henry N, Jovanović J, Schlueter M, Kritikou P, Wilson K, Myrén K-J. Cost-utility analysis of life-long prophylaxis with recombinant factor VIIIFc vs recombinant factor VIII for the management of severe hemophilia A in Sweden. J Med Econ. 2018;21:318–25.
Pettersson H, Ahlberg A, Nilsson IM. A radiologic classification of hemophilic arthropathy. Clin Orthop. 1980;149:153–9.
Kern M, Blanchette V, Stain AM, Einarson TR, Feldman BM. Clinical and cost implications of target joints in Canadian boys with severe hemophilia A. J Pediatr. 2004;145:628–34.
Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47.
Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935–9.
Lobet S, Hermans C, Lambert C. Optimal management of hemophilic arthropathy and hematomas. J Blood Med. 2014;5:207–18.
Capri S, Ceci A, Terranova L, Merlo F, Mantovani L. Guidelines for economic evaluations in italy: recommendations from the Italian Group of Pharmacoeconomic Studies. Drug Inf J. 2001;35:189–201.
Drzal R, Szmurlo D, Plisko R. Can we determine the optimal cycle length for which half-cycle correction should always be applied? Value Health. 2013;16:A27.
O’Mahony JF, Newall AT, van Rosmalen J. Dealing with time in health economic evaluation: methodological issues and recommendations for practice. PharmacoEconomics. 2015;33:1255–68.
Valentino LA, Mamonov V, Hellmann A, Quon DV, Chybicka A, Schroth P, et al. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost JTH. 2012;10:359–67.
Blanchette VS, Shapiro AD, Liesner RJ, Hernández Navarro F, Warrier I, Schroth PC, et al. Plasma and albumin-free recombinant factor VIII: pharmacokinetics, efficacy and safety in previously treated pediatric patients. J Thromb Haemost JTH. 2008;6:1319–26.
Food and Drugs Administration (FDA). ELOCTATE label [Internet]. 2014. https://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM400192.pdf.
Oldenburg J, Pasi J, Pabinger I, Nolan B, Kulkarni R, Blanchette VS, et al. Improvements in joint health during long-term use of recombinant factor VIII Fc fusion protein prophylaxis in subjects with haemophilia A. Prague: Wiley; 2019.
Panicker J, Warrier I, Thomas R, Lusher M. The overall effectiveness of prophylaxis in severe haemophilia. Haemophilia. 2003;9:272–8.
Manco-Johnson MJ, Soucie JM, Gill JC, Joint Outcomes Committee of the Universal Data Collection, US Hemophilia Treatment Center Network. Prophylaxis usage, bleeding rates, and joint outcomes of hemophilia, 1999 to 2010: a surveillance project. Blood. 1999;2017(129):2368–74.
Keepanasseril A, Stoffman J, Bouskill V, Carcao M, Iorio A, Jackson S, et al. Switching to extended half-life products in Canada—preliminary data. Haemophilia. 2017;23:e365–7.
Jimenez R, Nuñez R, Jimenez P, Rodriguez-Martorell FJ, Perez-Simon JA. Switch to extended half- life recombinant factor VIII FC in severe hemophilia A patients under prophylaxis. Experience in one centre. Madrid: Wiley; 2018.
Myren K-J, van der Sluijs M, Kritikou P, Lethagen S. Real world switching from conventional FVIII products to rFVIIIFc in France, Germany, Italy and the UK. Stockholm: Wiley; 2018.
Scott M, Xiang H, Collins PW, Hay CRM. The effect of switching to rFVIIIFc on treatment patterns and annualised bleed rate before and after: a within-patient comparison from the UK National Haemophilia Database. Glasgow: Wiley; 2018.
Tagliaferri A, Quintavalle G, Riccardi F, Matichecchia A, Benegiamo A, Rossi R, et al. Benefits of switch to FVIII-FC: experience of prophylaxis in eight patients. Madrid, Spain; 2018.
Institute for Clinical and Economic Review (ICER). Emicizumab for hemophilia A with inhibitors: effectiveness and value. Evidence report [Internet]. 2018. https://icer-review.org/wp-content/uploads/2017/08/ICER_Hemophilia_Evidence_Report_031518.pdf. Accessed 11 Jan 2019.
WorldData. Average height of men and women worldwide [Internet]. Worlddata.info. https://www.worlddata.info/average-bodyheight.php. Accessed 11 Jan 2019.
Neufeld EJ, Recht M, Sabio H, Saxena K, Solem CT, Pickard AS, et al. Effect of acute bleeding on daily quality of life assessments in patients with congenital hemophilia with inhibitors and their families: observations from the dosing observational study in hemophilia. Value Health. 2012;15:916–25.
Recht M, Neufeld EJ, Sharma VR, Solem CT, Pickard AS, Gut RZ, et al. Impact of acute bleeding on daily activities of patients with congenital hemophilia with inhibitors and their caregivers and families: observations from the dosing observational study in hemophilia (DOSE). Value Health. 2014;17:744–8.
Scalone L, Cortesi PA, Ciampichini R, Cesana G, Mantovani LG. Health related quality of life norm data of the Italian general population: results using the EQ-5D-3L and EQ-5D-5L instruments. Epidemiol Biostat Public Health [Internet]. 2015;12. https://ebph.it/article/view/11457. Accessed 03 Jan 2019.
Calabrò GE, Nicolotti N, Tamburrano A, Stojanovic J, Coretti S, Rumi F, et al. [Valutazione di Health Technology Assessment (HTA) di Efmoroctocog Alfa (Elocta) per il Trattamento dei Pazienti Affetti da Emofilia A]. VIHTALI Value Health Technol Acad Leadersh Innov Srl. 2019.
Tagliaferri A, Rivolta GF, Iorio A, Oliovecchio E, Mancuso ME, Morfini M, et al. Mortality and causes of death in Italian persons with haemophilia, 1990–2007. Haemophilia. 2010;16:437–46.
Iorio A, Krishnan S, Myrén KJ, Lethagen S, McCormick N, Yermakov S, et al. Indirect comparisons of efficacy and weekly factor consumption during continuous prophylaxis with recombinant factor VIII Fc fusion protein and conventional recombinant factor VIII products. Haemophilia. 2017;23:408–16.
Nolan B, Mahlangu J, Perry D, Young G, Liesner R, Konkle B, et al. Long-term safety and efficacy of recombinant factor VIII Fc fusion protein (rFVIIIFc) in subjects with haemophilia A. Haemophilia. 2016;22:72–80.
van Keep M, Hoxer CS, Hemstock M, Groth AV, Knight C. A new modeling approach allowing prediction and comparison of the long-term outcomes of treatments for hemophilia B. J Comp Eff Res. 2018;7:39–48.
Clinicaltrials.gov. ADVATE Hemophilia A Outcome Database (AHEAD). https://clinicaltrials.gov/ct2/show/NCT02078427. Accessed 17 Jan 2019.
Acknowledgements
The authors thank Gerardo Liguori and Cristina Teruzzi for providing Italian data inputs, and Christopher Barnowski, Louise Edvardsson, Jameel Nazir, Emmelie Persson, and Annemieke Willemze for their comments and suggestions to improve the manuscript throughout development.
Author information
Authors and Affiliations
Contributions
AB drafted the initial manuscript. AB, STM, and AJH constructed the model. KW and NL provided expert insight and validated the model inputs and results. AB and AJH performed the targeted review and subsequent meta-analysis regarding the dosing data. KW and NL reviewed the initial model and provided input into the overall structure and assumptions. Data for inclusion within the model were provided by KW and NL. All authors reviewed each version of the manuscript.
Corresponding author
Ethics declarations
Funding
This research was funded by Swedish Orphan Biovitrum AB (publ) (Sobi).
Conflict of interest
A. Bullement, S.T. McMordie, and A.J. Hatswell are employees of Delta Hat, a private consultancy that has received funding from Sobi for the research reported within this manuscript. K. Wilson is an employee of Sobi, a company involved in the development and commercialization of rFVIIIFc (Elocta). At the time this study was conducted, N. Li was an employee of Bioverativ (a Sanofi company), a company also involved in the development and commercialization of rFVIIIFc (Elocta). The views expressed are those of the author(s) and not necessarily those of the author affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bullement, A., McMordie, S.T., Hatswell, A.J. et al. Cost-Effectiveness Analysis of Recombinant Factor VIII Fc-Fusion Protein (rFVIIIFc) for the Treatment of Severe Hemophilia A in Italy Incorporating Real-World Dosing and Joint Health Data. PharmacoEconomics Open 4, 133–142 (2020). https://doi.org/10.1007/s41669-019-0158-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-019-0158-8