Abstract
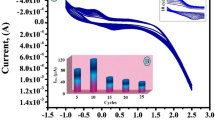
A simple poly(arginine) film-modified carbon nanotube paste electrode (PAMCNTPE) was prepared using cyclic voltammetry (CV). The devised sensor was subjected to field-emission scanning electron microscope (FESEM) and CV characterization. The sensing of 0.1 mM pyridoxine (PY) was upgraded at PAMCNTPE as compared to the bare carbon nanotube paste electrode (BCNTPE). The PAMCNTPE detects the 0.1 mM PY at a specific potential 0.727 V with a current response of 10.68 µA. In the case of BCNTPE, the PY appeared at 0.798 V with a current 2.90 µA. The proposed analytical method was optimized by prime parameters such as the impact scan rate, pH and PY concentration. Under optimal conditions, the concentration of PY is directly proportional to oxidation current (Ipa) in linear range 2–10 µM, and 10–80 µM with a detection limit (LOD) of 9.6 × 10−7 M and limit of quantification (LOQ) of 3.21 × 10−6 M. The simultaneous determination, concentration variation analysis of PY is performed with riboflavin (RF) and interference analysis in detecting PY also examined. The proposed sensor was effectively applied for the determination of PY in natural food supplement with excellent recovery.














Similar content being viewed by others
References
Jyoti TB, Nandibewoor ST. Modification of glassy carbon electrode by polybromocresol using cyclic voltammetry as a sensor and its analytical applications in determination of pyridoxine hydrochloride in commercial drinks. Anal. Bioanal. Electrochem. 2019;10(9):1144–62.
Liang T, Qingji X, Shouzhuo Y. Electrochemical and spectroelectrochemical studies on pyridoxine hydrochloride using a poly(methylene blue) modified electrode. Electroanalysis. 2004;16(19):1592–7.
Nada FA, Ahmed G, Ekram HE, Asmaa RME. Effective and facile determination of vitamin B6 in human serum with CuO nanoparticles/ionic liquid crystal carbon based sensor. J Electrochem Soc. 2017;164(13):B730–8.
Hernandez SR, Ribero GG, Goicoechea HC. Enhanced application of square wave voltammetry with glassy carbon electrode coupled to multivariate calibration tools for the determination of B6 and B12 vitamins in pharmaceutical preparations. Talanta. 2003;61(5):743–53.
Qu W, Wu K, Hu S. Voltammetric determination of pyridoxine (vitamin B6) by use of a chemically-modified glassy carbon electrode. J Pharm Biomed Anal. 2004;36(3):631–5.
Tigari G, Manjunatha JG, Raril C, Hareesha N. Determination of riboflavin at carbon nanotube paste electrodes modified with an anionic surfactant. ChemistrySelect. 2019;4(7):2168–73.
Krystian W, Boguslaw B. Voltammetric characteristics and determination of riboflavin at the different metallic bulk annular band electrodes. J Electrochem Soc. 2018;165(7):H393–8.
Selvarajan S, Suganthi A, Rajarajan M. A facile synthesis of ZnO/manganese hexacyanoferrate nanocomposite modified electrode for the electrocatalytic sensing of riboflavin. J Phys Chem Solids. 2018;121:350–9.
Vinas P, Balsalobre N, Lopez-Erroz C, Hernandez-Cordoba M. Determination of vitamin B6 compounds in foods using liquid chromatography with post-column derivatization fluorescence detection. Chromatographia. 2004;59(5–6):381–6.
Jinghe Y, Rongjiang H, Benyu SU, Cunguo L, Naixing W, Jingtian HU. Simultaneous determination of four components in composite vitamin B tablets using a square root Kalman-filter. Anal Sci. 1998;14(5):965–9.
Marti-Andres P, Escuder-Gilabert L, Martin-Biosca Y, Sagrado S, Medina-Hernandez MJ. Simultaneous determination of pyridoxine and riboflavin in energy drinks by high-performance liquid chromatography with fluorescence detection. J Chem Educ. 2015;92(5):903–6.
Devaraj M, Saravanan R, Jiaqian Q, Elumalai S, Mehmet LY, Necip A, Gracia F, Rabah B, Gracia-Pinilla MA, Gupta VK. Heterostructures of mesoporous TiO2 and SnO2 nanocatalyst for improved electrochemical oxidation ability of vitamin B6 in pharmaceutical tablets. J Colloid Interface Sci. 2019;542:45–53.
Alemu M, Saini RC, Tadese A, Rishi P. Square wave voltammetric determination of pyridoxine in pharmaceutical preparations using cobalt hexacyanoferrate modified carbon paste electrode. J. Chem. Pharm. Res. 2014;6(1):544–51.
Purvi BD, Rahul MK, Ashwini KS. Electrochemical behaviour of pyridoxine hydrochloride (vitamin B6) at carbon paste electrode modified with crown ethers. J Solid State Electrochem. 2008;12(9):1067–75.
Habibia B, Phezhhana H, Pournaghi-Azarb MH. Voltammetric determination of vitamin B6 (pyridoxine) using multi wall carbon nanotube modified carbon-ceramic electrode. J Iran Chem Soc. 2010;7(2):S103–12.
Mohammed AK, Omar AH, Ohsaka T, Awad MI. Electroanalysis of pyridoxine at copper nanoparticles modified polycrystalline gold electrode. Electroanalysis. 2016;28(3):539–45.
Jazreen HQL, Yanni Y, Rakesh G, Richard DW. Electrochemical study of pyridoxine (vitamin B6) in acetonitrile. ChemElectroChem. 2014;2(3):412–20.
Mohammadhassan M, Mohsen BS, Mahdi G, Ebrahim H. Electro-deposition of gold nanostructures on carbon paste electrode: a platform with signal amplification for voltammetric study and determination of pyridoxine (vitamin B6). Russ J Electrochem. 2016;52(5):477–87.
Beitollahi H, Fahimeh M, Somayeh T, Shohreh J. A review on the effects of introducing CNTs in the modification process of electrochemical sensors. Electroanalysis. 2019;31(7):1195–203.
Punbusayakul N. Carbon nanotubes architectures in electroanalysis. Procedia Eng. 2012;32:683–9.
Manjunatha JG. Highly sensitive polymer based sensor for determination of the drug mitoxantrone. J. Surf Sci. Technol. 2018;34(1–2):74–80.
Manjunatha JG. Poly (nigrosine) modified electrochemical sensor for the determination of dopamine and uric acid: a cyclic voltammetric study. Int. J. Chem Tech Res. 2016;9(2):136–46.
Raril C, Manjunatha JG. Cyclic voltammetric investigation of caffeine at methyl orange modified carbon paste electrode. Biomed J Sci Tech Res. 2018;9(3):1–6.
Manjunatha JG, Kumara Swamy BE, Deraman M. Electrochemical studies of dopamine, ascorbic acid and their simultaneous determination at a poly (rosaniline) modified carbon paste electrode: a cyclic voltammetric study. Anal. Bioanal. Electrochem. 2013;5(4):426–38.
Raril C, Manjunatha JG. A sensitive and selective procedure for the voltammetric determination of brilliant indigo and acid yellow 23 at surfactant modified graphene paste electrode. J. Mater. Environ. Sci. 2019;10(6):510–9.
Sangili A, Veerakumar P, Chen SM, Rajkumar C, Lin KC. Voltammetric determination of vitamin B2 by using a highly porous carbon electrode modified with palladium-copper nanoparticles. Microchim Acta. 2019;186:299.
Raril C, Manjunatha JG. Sensitive electrochemical analysis of resorcinol using polymer modified carbon paste electrode: a cyclic voltammetric study. Anal. Bioanal. Electrochem. 2018;10(4):488–98.
Manjunatha JG. A novel poly (glycine) biosensor towards the detection of indigo carmine: a voltammetric study. J. Food Drug Anal. 2018;26(1):292–9.
Barbara B, Elio D. Voltammetric determination of vitamin B6 in food samples and dietary supplements. J Food Compos Anal. 2014;33(2):155–60.
Liu G, Wang YM, Sun DM. Simultaneous determination of vitamins B2, B6 and C using silver-doped poly(l-arginine)-modified glassy carbon electrode. J Anal Chem. 2016;71(1):102–9.
Darko K, Muslim K, Eda M, Ruqia N, Naser Ramdan RA, Dalibor MS. Determination of pyridoxine (vitamin B6) in pharmaceuticals and urine samples using unmodified boron-doped diamond electrode. Diam Relat Mater. 2016;64:184–9.
Marcos FST, Glimaldo M, Edward RD, Eder TGC. Voltammetric determination of pyridoxine (vitamin B6) at a carbon paste electrode modified with vanadyl(IV)–Salen complex. Anal Chim Acta. 2004;508(1):79–85.
Ranjith Kumar D, Manoj D, Santhanalakshmi J, Shim JJ. Au–CuO core-shell nanoparticles design and development for the selective determination of vitamin B6. Electrochim Acta. 2015;176:514–22.
Moreno V, Liorent-Martinez EJ, Zougagh M, Rios A. Decoration of multi-walled carbon nanotubes with metal nanoparticles in supercritical carbon dioxide medium as a novel approach for the modification of screen-printed electrodes. Talanta. 2016;161:775–9.
Hadiseh S, Beitollahi H. Graphene/ZnO nanocomposite for voltammetric sensing of vitamin B6 using modified glassy carbon electrode. Anal. Bioanal. Electrochem. 2016;8(6):732–40.
Acknowledgements
We gratefully acknowledge the financial support from the VGST, Bangalore, under Research Project No. KSTePS/VGST—KFIST (L1)2016–2017/GRD-559/2017-18/126/333, 21/11/2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
About this article
Cite this article
Tigari, G., Manjunatha, J.G. Electrochemical Preparation of Poly(arginine)-Modified Carbon Nanotube Paste Electrode and its Application for the Determination of Pyridoxine in the Presence of Riboflavin: An Electroanalytical Approach. J. Anal. Test. 3, 331–340 (2019). https://doi.org/10.1007/s41664-019-00116-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-019-00116-w